Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 1, p 35-40
DOI 10.2478/v10136-012-0003-1
Phagocytosis of insect haemocytes as a new alternative model
Josef Berger, Martina Jurcova
Address: Josef Berger, Department of Clinical and Preclinical Studies, Faculty of Health and Social Studies, University of South Bohemia, Emy Destinove 46, 370 05 Ceske Budejovice, Czech Republic
berger@jcu.cz
Received 14th October 2011.
Revised 9th December 2011.
Published online 5th January 2012.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
References
SUMMARY
Phagocytosis is an important function of both insect haemocytes and mammalian blood cells. Linden bugs and cotton leaf worms have been suggested as new alternative models for ecological and drug toxicology but no data on their haemocyte physiology have been published. Our assays with particle ingestion of the NBT test were carried out on prohaemocytes, granulocytes, plasmatocytes and spherulocytes of adult linden bug and cotton leaf worm larvae. We found that phagocytic activity is on average 10% in the linden bug, and 50% in cotton leaf worm haemocytes: the phagocytic index is 3.5 in both species and nitroblue tetrazolium reduction is 0.5 in the linden bug and 3.2 in the cotton leaf worm. Phagocytic charactersitics of the prohaemocytes and granulocytes in the cotton leaf worm are closed to mammalian neutrophil physiology. Our data suggest that cotton leaf worm haemocytes may be a new potential alternative model for screening of phagocytosis.
KEY WORDS
alternative model; phagocytosis; particle ingestion; NBT; linden bug; cotton leaf worm
INTRODUCTION
Studies of phagocytosis in various mammalian
species focusing on the clinical or toxicological
background revealed a wide diversity depending on
the dominant environment. For example, the
granucocytes of rats, with a higher infection risk,
have higher phagocytic potential than human
granulocytes (Vetvicka et al. 1982, Berger 1988).
The most frequent examinations of phagocytosis
in mammalian haematotoxicology cover the ingestion
of various particles (phagocytic activity and index)
and the production of reactive oxygen species as part
of the oxidative killing of invading pathogens (Wang
and Schwarz 1959, Sanchez et al. 2010, Pohanka et
al. 2011, Steevels and Meyaard 2011). Phagocytic
activity and index reflect the first stage of
phagocytosis. There are various particles which can
be used as yeast cells, Escherichia coli, cadmium and
HEMA particles. The examination of oxidative killing
reflects the last phase, evaluated by the nitroso blue
tetrazolium reduction test (NBT; Johnston and
Baehner 1971).
Insect haemocytes participate in immunity
reactions (Strand 2008). The innate cellular defences
against infection include haemocyte-mediated
responses like phagocytosis, microaggregation,
nodulation and encapsulation (Stanley and Miller
2006, Marmaras and Lampropoulou 2009).
Phagocytosis is an evolutionarily conserved cellular
response in all eukaryotic phyla (Yutin et al. 2009)
but most of the available data refer to mammalian
circulating white blood cells and macrophages. Too
little is known about phagocytosis in insects as it is
difficult to conduct experiments on insect haemocytes
and identify these cells in some species.
Both linden bug (Pyrrhocoris apterus) adults and
cotton leaf worm (Spodoptera littoralis) larvae are
used as "laboratory" insects in physiology research,
and their insectaria are standardized in ways similar
to the more well-known rodent vivaria (Berger 2009a,
Picmonova and Berger 2012). Their haemocyte
morphology has been described and these species are
suggested as applicable biomodels for toxicological
screening elsewhere (Gelbic et al. 2006, Berger and
Slavickova 2008, Berger 2009b, 2010). Nevertheless,
the functional characteristics of haemocytes in these
two insect species are unknown. The aim of this study
is to evaluate them as potential alternative models for
the screening of phagocytosis in vivo using methods
which are standardized in mammalian physiology.
MATERIAL AND METHODS
Insects
We used both males and females of the adult linden
bug, Pyrrhocoris apterus (L.) (Heteroptera), and the
larvae of the cotton leaf worm, Spodoptera littoralis
(Boisd.) (Lepidoptera), in the last instar. Insects were
kept at a constant temperature of 25±1 °C, in
75%±10% relative humidity under an artificial
photoperiod of 16 h of light and 8 h of dark.
Adults of the linden bug were fed with lime seeds,
the larvae of cotton leaf worm with an artificial
Stonefly Heliothis diet (prepared by Wards,
Rochester, USA). The haemolymph of the linden bug
was drawn after cutting off the distal part of an
antenna; that of the cotton leaf worm after
entomological pin injury.
Determination of phagocytosis
In our pilot study prior to this, we observed that a
mixture of haemolymph with tested particles
blackens, so that the phagocytosis cannot be
investigated. Evaluating the stability of haemocytes in
Grace's medium we found that the blackening was
eliminated for microscopical examinations but that
the granulocytes decay within a few minutes,
although plasmatocytes were the most resistant
(Buresova and Berger 2002). In the present paper, we
used Ringer solution to prevent both the blackening
and the destruction of cells during the incubation
times used.
A suspension of 2.5 microl HEMA particles (radiation
copolymer of 2-hydroxyethylmethacrylate manufactured by Artim Prague, Czech Republic, diameter
1.2 microm), was incubated in 15 microl of the Ringer solution
and 5 microl of haemolymph for 20 minutes at 37 °C
(Berger 1988). Smears were air-dried and then stained
using the Pappenheim panoptic method. At least 100
haemocytes from each animal were examined (Nikon
Ecclipse 50i, Plan Apo 100x/1.40 oil, immerse oil).
Phagocytic activity is expressed as the ratio
between the number of haemocytes with phagocytised
particles and the number of all evaluated haemocytes.
A phagocytic index is the average of engulfed
particles per one phagocytising haemocyte.
The NBT (Pick et al. 1981) was made using 25 microl
of the Ringer solution containing 0.1% of nitroblue
tetrazolium (NBT, Sigma-Aldrich, Prague, Czech
Republic, Cat. No 68H5075) and 25 microl of fresh
haemolymph at 37 °C for 20 min. Several samples
were used for smears which were immediately fixed
in formaldehyde steam for 10 min and then stained by
nuclear fast red (Merck, Darmstadt, Germany, Cat.
No 8598671). Incubation in a further sample was
stopped by 60 microl of 2M KOH and 70 microl of
dimethylsuphoxide (Rook et al. 1985). Dark nitroblue
formazan was measured in the Sunrise (Tecan Group
Ltd., Mannedorf, Switzerland) reader at 620 nm
against blank samples without a haemolymph.
Statistic analysis
The results are expressed as mean±s.e.m. Data were
compared using the two-sided Mann-Whitney U test
at the significance level 2alpha=0.05.
RESULTS
The phagocytic ability of haemocyte populations was
evaluated by ingestion of HEMA particles and by the
test for the metabolic capacity to kill ingested cells on
the model of the reduction of NBT to formazan. Our
findings demonstrate that haemocytes of both
examined species may have a certain role in defense
responses to foreign microorganisms in both species
examined.
Phagocytic activity and index
Using linden bug haemocytes, HEMA particles were
ingested by prohaemocytes (Fig. 1), granulocytes and
plasmatocytes and no statistically significant
difference was observed between male and female
haemocytes (Table 1). Granulocytes ingest a
significantly lower number of particles than
prohaemocytes and plasmatocytes. No phagocytosis
was observed in the spherulocytes. Comparing data
on the ingestion of yeast cells and HEMA particles we can see that the phagocytic activity for
prohaemocytes and plasmatocytes was significantly
higher using HEMA particles while their ingestion by
granulocytes was lower, but statistically not
significant.
Table 1. Phagocytosis estimated by ingestion in haemocytes.
|
 Linden bug Linden bug
|
 Cotton leaf worm Cotton leaf worm
| |
M |
F |
M |
F | | Phagocytic activity (%) | | Prohaemocytes |
14.9a±2.8b |
12.7±1.9 |
57.7±6.3 |
43.9±4.3+ | | Granulocytes |
3.0±2.0* |
2.4±1.8* |
57.5±3.5 |
46.9±3.3 | | Plasmatocytes |
9.1±0.8 |
10.3±1.7 |
12.7±10.5 |
25.8±12.3 | | Spherulocytes |
0.0±0.0* |
0.0±0.0* |
0.0±0.0* |
0.0±0.0* | | All populations of
haemocytes |
9.0±0.7 |
10.4±1.5 |
57.0±3.7 |
43.5±3.3+ | |
|
|
|
| | Phagocytic index | | All |
3.21±0.35 |
3.26±0.28 |
4.8±0.2 |
3.0±0.3+ | | N |
10 |
10 |
9 |
10 |
a mean, b standard error of mean, * statistically significant versus All, + statistically significant as compared with males

Fig. 1. Ingestion of HEMA particles and by a cotton leaf worm prohaemocyte (left) and granulocyte (middle) and by a
linden bug prohaemocyte (right).
Phagocytosis was expressed in cotton leaf worm
haemocytes at a significantly higher rate compared
with linden bug cells (Table 1, phagocytic activity).
Although the number of phagocyting cotton leaf
worm haemocytes was 4-5 times higher than linden
bug cells, the number of ingested particles per cell,
i.e. phagocytic index, was almost equal. It was
slightly increased in cotton leaf worm males.
NBT test
We observed that prohaemocytes, granulocytes and
plasmatocytes can reduce nitroblue tetrazolium
(Fig. 2) while no spherulocyte with black precipitate
was found. We found the maximum of formazan
concentration following 2 hrs of linden bug
haemocyte incubation, while its peak in cotton leaf
worm haemocytes was reached following 8 hr
incubation. Values from the cotton leaf worm
haemocytes were higher. No significant difference
was found between male and female haemocytes in
formazan production (Table 2).
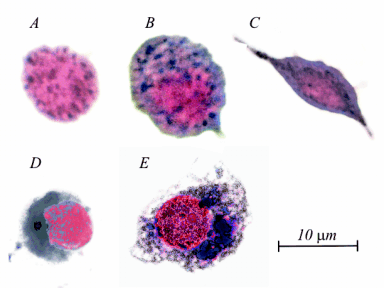
Fig. 2. Formazan (dark precipitates) in prohaemocyte (A), granulocyte (B) and plasmatocyte (C) of linden bug and in
prohaemocyte (D) and granulocyte (E) of cotton leaf worm.
Table 2. Nitroblue tetrazolium reduction in all types of haemocytes in NBT test. Absorbance measured in Elisa reader at
620 nm.
|
 Linden bug Linden bug |
 Cotton leaf worm Cotton leaf worm | | No haemocytes (blank) |
M |
F |
M |
F | | 0.00c±0.00b |
0.52a±0.03b§ |
0.53±0.04§ |
3.21±0.16§ |
3.10±0.35§ |
c mean of ten samples, § statistically significant versus blank samples
Other symbols as in Table 1.
DISCUSSION
The data presented on the phagocytosis and NBT tests
suggest that haemocytes in both species evaluated
may have a role in defence responses to foreign
organisms, although this activity of spherulocytes
seems to be inconsiderable. The phagocytic activity
of prohaemocytes in both insects was an unexpected
finding in contradiction to the data on several other
taxa (cf. Hernandez et al. 1999).
Ingestion tested by HEMA particles seems to be
better estimated than similar evaluations of mammal
phagocytes. We observed lower phagocytic activity in
the linden bug while ingestion in cotton leaf worm
prohaemocytes and granulocytes was close to that in
mammal neutrophils (Berger 1988). The mean
number of ingested particles was lower in the linden
bug haemocytes than in mammalian granulocytes
while the phagocytic index for cotton leaf worm
haemocytes seemed to be similar. Ehlers and
co-workers (1992) also showed that ingestion ability
depends on the character of the particles used, and
that it has a similar influence on methods of
phagocytic ability in mammals (cf. Slapnickova and
Berger 2002). Nevertheles, it seems that HEMA
particles are ingested intensively (Berger 1988,
Buresova and Berger 2002) and their use could better
reveal interspecific variances. The values of
phagocytic activity are lower for linden bug
haemocytes, compared with both mammalian blood
cells (Berger 1988) and the data presented here on the
cotton leaf worm, are close to similar data on other
insect species, Wax Moth, Galleria mellonella
(Wiesner et al. 1996).
Our finding of many positive cells in the NBT test
could suggest that a lower phagocytic activity of
linden bug haemocytes is apparent, maybe as a result
of the faster disintegration of these haemocytes in
vitro; that is, granulocytes wear off in vitro for their
degranulation during incubation for ingestion
examinations, as we have estimated by differential
counts in a previous pilot study (Buresova and Berger
2002) and they could lead to a morphology which is
close to plasmatocytes in panoptically stained smears,
while granulocytes remain present during the NBT
test.
The NBT test reflects the production of
superoxide anions (Liochev et al. 1995). We found
formazan in the prohaemocytes, granulocytes and
plasmatocytes of both linden bug and cotton leaf
worm. Formazan formation was also found in the
haemocytes of several species (Toru 1994, Glupov et
al. 2001). In contrast, Hyrsl and co-workers (2004)
did not find formazan in the isolated larval
haemocytes in silkworm nor did Glupov et al. (2001)
in small tortoiseshell.
Different findings of phagocytic characteristics in
the haemocytes of various insect species could
suggest that the role of phagocytosis in invertebrate
species could be less uniform than that in vertebrate
blood cells. Another explanation for the in-consistencies in the literature on insect phagocytosis
could be the larger diversity in metabolic pathways
during phagocytic processes.
We found that the most important characteristics
of phagocytosis in both linden bug and cotton leaf
worm haemocytes can be examined by methods
commonly used in both human and veterinary
haematology. We suppose that there could be
alternative biomodels applicable to haematotoxi-cological express assays - so-called screening; for
example, cotton leaf worm larvae had characteristics
close to mammals. The insect species evaluated
would be alternative biomodels without juridical
limitations concerning animal welfare and which
would moreover be cheaper (Berger 2009a).
ACKNOWLEDGEMENT
Spodoptera larvae material was kindly provided by
Prof Gelbic. This work was supported in part by the
Ministry of Education CR, grant no. 2295/09.
REFERENCES
Berger J. HEMA-particles phagocytosis of rat neutrophils. Folia
Haematol. 115: 833-836, 1988.
Berger J. Preclinical testing on insects predicts human haematotoxic
potentials. Lab Anim. 43: 328-332.2009a.
[CrossRef]
[PubMed]
Berger J. New haemolytic potential assay on an alternative insect
model. Basic Clin Pharmacol Toxicol. 105: 315-318, 2009b.
[CrossRef]
Berger J. Alternative haematotoxicological testing. J Appl Biomed.
8: 19-22, 2010.
[JAB]
[CrossRef]
Berger J, Slavickova K. Morphological characterization of hemocyes in
the adult linden bug, Pyrrhocoris apterus (L.) (Heteroptera). Zool
Stud. 47: 466-472, 2008.
Buresova V, Berger J. Phagocytosis of Pyrrhocoris apterus haemocytes.
In Berger J (ed.), Cells IV. Kopp Publ., Ceske Budejovice 2002, p. 178.
Ehlers D, Zosel B, Werner M, Kauschke E, Ehlers ML. Comparison of
in vivo and in vitro phagocytosis in Galleria mellonella L. Parasitol
Res. 78: 354-359, 1992.
[CrossRef]
Gelbic I, Strbackova J, Berger J. Influence of metyrapone on the
morphology of hemocytes of the Egyptian cotton leafworm Spodoptera
littoralis (Boisd.). Zool Stud 45: 371-377, 2006.
Glupov VV, Khvoshevskaya MF, Lozinskaya YL, Dubovski IM, Martemyanov
VV, Sokolova JY. Application of the nitroblue tetrazolium-reduction
method for studies on the production of reactive oxygen species in
insect haemocytes. Cytobios. 106(Suppl. 2): 165-168, 2001.
[PubMed]
Hernandez S, Lanz H, Rodriguez MH, Torres JA, Martinez-Palomo A,
Tsutsumi V. Morphological and cytochemical characterization of female
Anopheles albimanus (Diptera: Culicidae) hemocytes. J Med Entomol.
36: 426-434, 1999.
[PubMed]
Hyrsl P, Ciz M, Kubala L, Lojek A. Silkworm (Bombyx mori) hemocytes
do not produce reactive oxygen metabolites as a part of defence
mechanisms. Folia Microbiol. 49: 315-319, 2004.
[CrossRef]
Johnston RB, Jr., Baehner RL. Chronic granulomatcus disease:
correlation between pathogenesis and clinical findings. Pediatrics.
48: 730-739, 1971.
[PubMed]
Liochev SI, BatinicHaberle I, Fridovich I. The effect of detergents
on the reduction of tetrazolium salts. Arch Bioch Biophys. 324: 48-52,
1995.
[CrossRef]
[PubMed]
Marmaras VJ, Lampropoulou M. Regulators and signalling in insect
haemocyte immunity. Cell Signal. 21: 186-195, 2009.
[CrossRef]
[PubMed]
Pick E, Charon J, Mizel D. A rapid densitometric microassay for
nitroblue tetrazolium reduction and application of the microassay to
macrophages. J Reticuloendothel Soc. 30: 581-593, 1981.
[PubMed]
Picmonova V, Berger J. Genistein effects on haematoimmune cells in a
newly developed alternative toxicological model. Exp Toxicol Pathol.
2012 (in press), doi: 10.1016/j.etp.2010.10.006.
[CrossRef]
[PubMed]
Pohanka M, Bandouchova H, Vlckova K, Zdarova Karasova J, Kuca K,
Damkova V, Peckova L, Vitula F, Pikula J. Square wave voltammetry on
screen printed electrodes: comparison to ferric reducing antioxidant
power in plasma from model laboratory animal (Grey Partridge) and
comparison to standard antioxidants. J Appl Biomed. 9: 103-109, 2011.
[JAB]
[CrossRef]
Rook GAW, Steele J, Umar S, Dockrell HM. A simple method for the
solubilisation of reduced NBT, and its use as a colrimetric assay for
activation of human macrophages by c-interferon. J Immunol Meth.
82: 161-167, 1985.
[CrossRef]
Sanchez Y, Amran D, de Blas E, Aller P. Arsenic trioxide as an anti-
tumour agent: mechanisms of action and strategies of sensitization. J
Appl Biomed. 8: 199-208, 2010.
[JAB]
[CrossRef]
Slapnickova M, Berger J. Rat neutrophil phagocytosis following feed
restriction. Comp Clin Pathol. 11: 172-177, 2002.
[CrossRef]
Stanley DW, Miller JS. Eicosanoid actions in insect cellular immune
functions. Entomol Exper Appl. 119: 1-13, 2006.
[CrossRef]
Steevels TAM, Meyaard L. Immune inhibitory receptors: Essential
regulators of phagocyte function. Eur J Immunol. 41: 575-587, 2011.
[CrossRef]
[PubMed]
Strand MR. The insect cellular immune response. Insect Sci. 15: 1-14,
2008.
[CrossRef]
Toru A. Superoxide generation in vitro in lepidopteran larval
haemolymph. J Insect Physiol. 40: 165-171, 1994.
[CrossRef]
Vetvicka V, Fornusek L, Kopecek J, Kaminkova J, Kasparek L, Vranova
M. Phagocytosis of human-blood leukocytes - a simple
micromethod. Immunol Lett. 5: 97-100, 1982.
[CrossRef]
Wang CJK, Schwarz J. Phagocytosis of yeast cells in vitro. Am J
Pathol. 35: 901-907, 1959.
[PubMed]
Wiesner A, Wittwer D, Gotz P. A small phagocytosis stimulating factor
is released by and acts on phagocytosing Galleria mellonella
haemocytes in vitro. J Insect Physiol. 42: 829-835, 1996.
[CrossRef]
Yutin N, Wolf MY, Wolf YI, Koonin EV. The origins of phagocytosis and
eukaryogenesis. Biol Direct. 4: Art No 9, 2009.
|
BACK
|



