Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 1, p 41-50
DOI 10.2478/v10136-011-0009-0
Jerte Valley cherry-based product modulates serum inflammatory markers in rats and ringdoves
Jonathan Delgado, Maria del Pilar Terron, Maria Garrido, Carmen Barriga, Javier Espino, Sergio Damian Paredes, Ana Beatriz Rodriguez
Address: Ana Beatriz Rodriguez, Department of Physiology, Neuroimmunophysiology and Chrononutrition Research Group, Faculty of Science, University
of Extremadura Avda. de Elvas s/n, 06006, Badajoz, Spain
moratino@unex.es
Received 4th May 2011.
Revised 27th May 2011.
Published online 15th July 2011.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
References
SUMMARY
Ageing is commonly accompanied by a chronic subclinical inflammatory status that coexists with immune dysfunction. Consumption of foods rich in
antioxidants is associated with a lower incidence of chronic diseases. The aim of this study was to evaluate the effect of the consumption of a
Jerte Valley cherry-based beverage on the inflammatory load in two different animal species: rats and ringdoves (Streptopelia risoria); each
divided into two age groups. To this purpose, circulating levels of both pro-inflammatory (IL-1beta and TNF-alpha) and anti-inflammatory (IL-4 and
IL-2) cytokines were measured before and after a 10-day treatment with the Jerte Valley cherry-based beverage. Our results suggest that the 10-day
treatment with the Jerte Valley cherry-based beverage modulated the balance of pro- and anti-inflammatory cytokines in both rats and ringdoves by
down-regulating the levels of pro-inflammatory (IL-1beta and TNF-alpha) cytokines and up-regulating the levels of anti-inflammatory (IL-4 and IL-2)
cytokines. Moreover, old animals showed imbalanced levels of inflammatory markers towards a pro-inflammatory status, thereby underlining the fact
that ageing is usually accompanied by systemic inflammation and inflammation-related chronic diseases. In conclusion, since an increased dietary
intake of vegetable-derived bioactive compounds may retard age-related immune dysfunctions and prolong life-span, supplementing diets with the
cherry-based beverage may reduce the inflammatory load by modulating the serum concentrations of some markers of inflammation.
KEY WORDS
sweet cherry; inflammation; cytokine; melatonin; rat; ringdove
INTRODUCTION
Both chronic systemic inflammation and low grade
inflammation may contribute to the reduction of the
human life span (Finch and Crimmins 2004).
Although there is limited information on markers of
systemic inflammation in healthy adults, circulating
cytokines may act as biomarkers for disease
progression (Emery et al. 2008). Thus, it has been
reported that there is an association between chronic
inflammation and many of the prevalent diseases
found in the developed world, including obesity
(Engstrom et al. 2004), diabetes (Hanley et al. 2004)
and cancer (Hofseth and Ying 2006). Moreover, the
secretion by lymphocytes of pro-inflammatory
cytokines, such as interleukin-1 (IL-1) and tumour
necrosis factor-alpha (TNF-alpha) is responsible for initiating
inflammation in the pathogenesis of chronic diseases,
such as rheumatoid arthritis (Horwood 2008).
The ability of fruits and vegetables to protect
against diseases prevalent in the developed world is
well documented (Heber 2004). Although the
mechanisms of protection are not fully understood, it
is known that there are hundreds of potentially
beneficial ingredients in plant foods, and there is
relatively consistent evidence, in both humans and
animals (Heber 2004, Mayorga et al. 2004), that their
antioxidant and anti-inflammatory properties play a
role in this protection. Cherries are an important
source of phytochemicals and reportedly have
important health-promoting qualities, including
anti-inflammatory effects (McCune et al. 2011). It has
been demonstrated that they inhibit the
cyclooxygenase (COX) enzymes responsible for the
inflammatory response (Seeram et al. 2001), and a
pilot study investigating the effects of sweet cherry
consumption on inflammatory markers in humans has
revealed that cherry consumption can reduce serum
C-reactive protein (hsCRP) levels (Kelley et al.
2006).
Although the anti-inflammatory effects of cherries
have been attributed to anthocyanins, it has been
proposed that the beneficial properties of fruits and
vegetables come from the additive and synergistic
effects of their phytonutrients and that isolated dietary
supplements do not exhibit these same benefits
(Milde et al. 2007). In this regard, sweet-cherries
from Jerte Valley (Extremadura, Spain) contain not
only high concentrations of anthocyanin pigments and
other phenolic compounds (Gonzalez-Gomez et al.
2009), but also substantial amounts of melatonin,
serotonin (Gonzalez-Gomez et al. 2010) and
tryptophan (Cubero et al. 2010), as recently reported
in seven different cultivars of these fruits: Bourlat,
Navalinda, Van, Ambrunes, Pico Limon, Pico Negro,
and Pico Colorado.
The amino acid tryptophan, the neurotransmitter
serotonin, and the indole melatonin are present in
various fruits and vegetables (Paredes et al. 2009a).
These bioactive compounds participate in the
physiological regulation of sleep as well as in the
improvement of antioxidant defences (Paredes et al.
2007a, b, c). Moreover, melatonin possesses both
immunomodulatory (Carrillo-Vico et al. 2005a) and
anti-inflammatory (Carrillo-Vico et al. 2005b)
properties, and is also a potent free radical scavenger
(Tan et al. 1993). In this respect, it has been reported
that both a Jerte Valley cherry-enriched diet (Garrido
et al. 2010) and the intake of a Jerte Valley
cherry-based nutraceutical product (Garrido et al.
2009) exhibit sleep-promoting actions, and increase
urinary 6-sulfatoxymelatonin (aMT6-s), a metabolite
that is considered to reflect the nocturnal melatonin
concentration, as well as antioxidant status in young,
middle-aged, and elderly subjects. Taking into
account the potential health-promoting actions of
Jerte Valley sweet-cherries, the purpose of this work
was to evaluate the effect of the consumption of a
Jerte Valley cherry-based product on inflammatory
load in both rats (a nocturnal animal) and ringdoves
(a diurnal animal; Streptopelia risoria) from two
different age groups: young and old. To this end,
circulating levels of both pro-inflammatory (IL-1
and TNF-alpha) and anti-inflammatory (IL-4 and IL-2)
cytokines were measured before and after a 10-day
treatment with the Jerte Valley cherry-based
beverage.
MATERIAL AND METHODS
Animals
Male Wistar rats of 6-7 months of age (young) and
18-20 months of age (old, given an average life span
of 22-24 months) were used in the study (n=8 per age
group). Rats were individually housed under
controlled environmental conditions (20 °C; 70%
humidity), maintained under a 12/12 h light/dark
photoperiod (darkness from 19:00 to 07:00 h) and fed
ad libitum (food and water). All handling during
lights-off was done under dim red light (<2 lux).
Similarly, both male and female ringdoves
(Streptopelia risoria) of 4-5 years of age (young) and
12-14 years of age (old, given an average life span of
15 years) were used in the study (n=8 per age group)
and individually housed under the same conditions as
described above.
The study was approved by the Ethical Committee
of the University of Extremadura (Badajoz, Spain) in
accordance with the National Institute of Health
Guide for the Care and Use of Laboratory Animals,
and the European Community's Council Directives
(86/609/EEC).
Animal treatment
Both young and old animals (rats and birds) were
watered with a Jerte Valley cherry-based beverage
(Spanish patent no. ES 2342141 B1) for a 10-day
period. The beverage was made of 27.85 g powdered
freeze-dried product mix diluted in 250 ml of water.
This product mix consisted of 18.85 g pitted freezedried cherries (equivalent to 141 g fresh cherries) in
equal parts of 4 Jerte Valley cherry cultivars (Bourlat,
Navalinda, Pico Negro, and Pico Colorado), plus
7.5& g maltodextrin, and 1.5 g ascorbic acid.
The cherry-based beverage was freshly prepared
every day. Feeding bottles containing the cherry
based beverage were wrapped in tinfoil to avoid
oxidization and/or destruction of light sensitive
compounds, e.g. melatonin. Basal parameters were
obtained from animals that had not been watered with
the cherry-based beverage, but with drinking water.
Serum collection
Blood samples were drawn from all rats (n=16) and
birds (n=16) at 08:00 h, 18:00 h, and the time
corresponding to each group's acrophase of the
melatonin rhythm, allowing at least 1 week between
consecutive samplings. Based on previous research,
the acrophases of the melatonin rhythm (times at
which the variables reach their maximums) in basal
conditions, were established at 02:00 h and 01:00 h in
young and old ringdoves, respectively (Paredes et al.
2006), and at 02:00 h in both groups of rats (Sanchez
et al. 2008). The collections (1 ml) were made with a
25-gauge needle and a syringe, taking blood from the
lateral tail vein (rats) or brachial vein (birds) and then
transferring it unheparinized to a pre-prepared tube
containing serum-separating gel. The samples were
centrifuged at room temperature for 30 min at 300xg.
The serum was then aliquotted into Eppendorf vials
and kept frozen at -30 °C until assay. Nocturnal
collections were performed under dim red light,
which the animals perceive as darkness. The
extractions were performed before initiating the
treatment (basal values) and at the end of the
treatment.
Cytokines determination in serum
Serum cytokines (IL-1beta, IL-4, IL-2 and TNF-alpha) levels
were measured using a commercial enzyme-linked
immunosorbent assay (ELISA), following the
manufacturer's instructions. All ELISA kits were
purchased from DRG (Marburg, Germany), except
for a rat IL-4 ELISA kit that was acquired from
Invitrogen (Barcelona, Spain). Determinations were
made in duplicate, and cytokine results are expressed
in picograms per millilitre. As aforementioned,
cytokine basal levels were blood values from animals
studied before treatment.
Statistical analysis
Data are expressed as mean ± S.E.M. of the numbers
of determinations carried out in duplicate. To
compare the different treatments, statistical
significance was calculated by one-way analysis of
variance followed by a post hoc Tukey test. The
significance level was set at alpha=0.05.
RESULTS
Several pro- and anti-inflammatory cytokines were
analysed at three different time points. Thus, serum
concentrations of pro-inflammatory IL-1beta in treated
young rats (Fig. 1A) were substantially lowered at all
hours compared to young rats in basal conditions,
whereas, in old rats administered with the
cherry-based beverage, serum IL-1beta levels only
diminished at dawn (08:00 h) and in the acrophase of
the melatonin rhythm (02:00 h). Moreover, old rats
showed decreased IL-1beta levels after treatment at
dawn (08:00 h) compared to young rats (Fig. 1A).
Similar results were obtained in ringdoves. In fact,
after the 10-day treatment with the cherry-based
beverage, serum IL-1beta levels significantly decreased
both at dawn and at 18:00 h in young ringdoves, as
well as at 18:00 h and at 01:00 h (acrophase of the
melatonin rhythm) in old ringdoves (Fig. 1B).
However, in this case, old birds seemed to show
substantially higher IL-1beta basal levels at all hours
than young birds (Fig. 1B).
Serum levels of TNF-alpha were also analysed as
pro-inflammatory markers. In this regard, the 10-day
treatment with the cherry-based beverage only
modified the serum levels of TNF-alpha at dawn (08:00 h)
in old rats, since they dropped remarkably after the
treatment (Fig. 2A). Old rats showed higher TNF-alpha
basal levels than young rats (Fig. 2A), these
differences being statistically significant at 18:00 h
and 02:00 h. Likewise, the consumption of the Jerte
Valley cherry-based beverage caused a significant
decrease in TNF-alpha serum levels at dawn (08:00 h) in
young ringdoves, as well as at 18:00 h and at 01:00 h
(acrophase of the melatonin rhythm) in old ringdoves
(Fig. 2B). Like old rats, old ringdoves exhibited
greater basal levels of TNF-alpha at the different hours
studied than young ringdoves (Fig. 2B).
Serum levels of IL-4 and IL-2 were subsequently
measured as anti-inflammatory markers. Interestingly,
the 10-day treatment with the Jerte Valley
cherry-based beverage led to increased IL-4
concentrations at all hours tested in both young and
old rats (Fig. 3A). Moreover, it is worth noting that
old rats presented lower IL-4 basal levels than young
rats at all hours (Fig. 3A). Similarly, the consumption
of the cherry-based beverage largely enhanced the
serum levels of IL-4 at all hours tested in both young and old ringdoves (Fig. 3B). Like the old rats, old
ringdoves showed lower basal levels of IL-4 at dawn
and at the time corresponding to the old ringdoves'
acrophase of the melatonin rhythm (Fig. 3B).
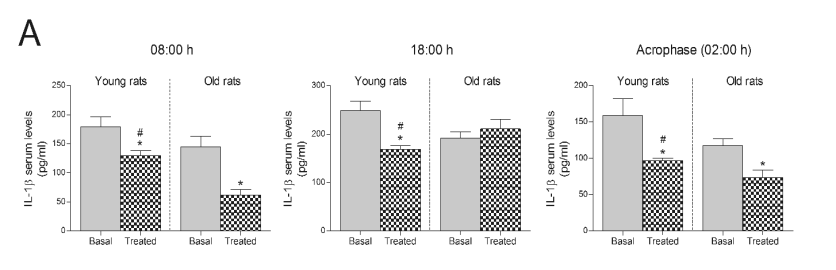
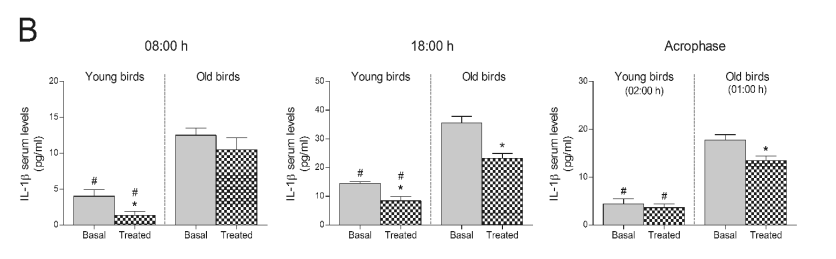
Fig. 1. Effect of consuming a Jerte Valley cherry-based beverage on circulating concentrations of IL-1beta in rats and ringdoves. Serum
IL-1beta levels in rats (A) and ringdoves (B) before (basal)
and after (treated) the consumption of the cherry-based beverage. Samples were analysed at different times, as indicated in Material and methods.
Values are expressed as pg/ml, n=8.
* statistically significant regarding basal values, # statistically significant regarding its corresponding value in old animals
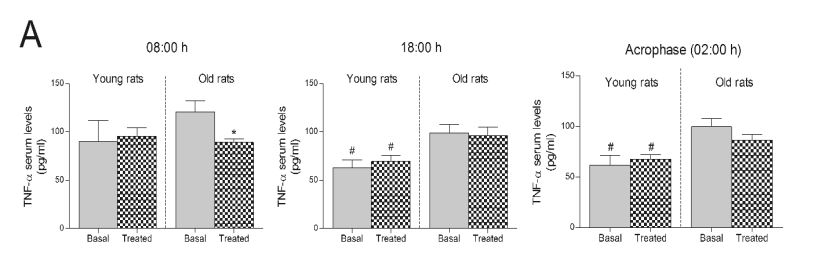
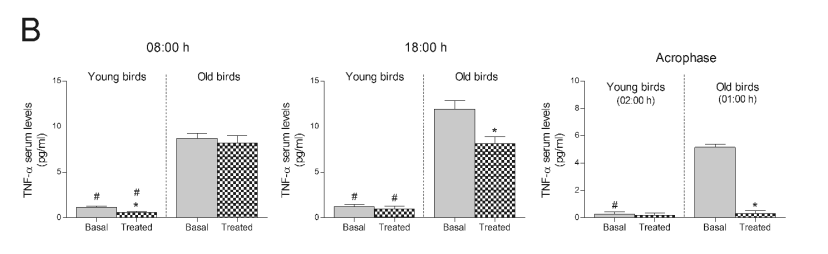
Fig. 2. TNF-alpha serum levels after the consumption of a Jerte Valley cherry-based beverage in rats and ringdoves. Serum TNF-alpha levels
in rats (A) and ringdoves (B) before (basal) and after
(treated) the consumption of the cherry-based beverage. Samples were analysed at different times, as indicated in Material and methods. Values are
expressed as pg/ml, n=8.
* statistically significant regarding basal values, # statistically significant regarding its corresponding value in old animals
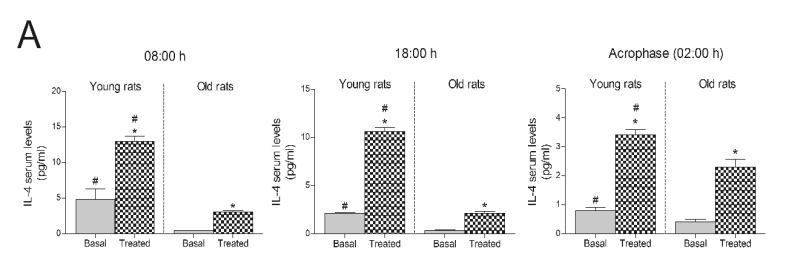
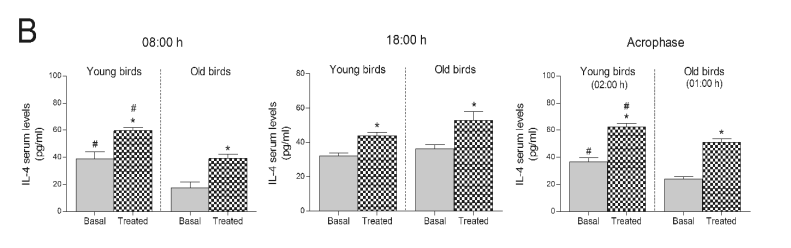
Fig. 3. Serum IL-4 concentrations in rats and ringdoves that consumed a Jerte Valley cherry-based beverage. Serum IL-4 levels in rats
(A) and ringdoves (B) before (basal) and after (treated) the consumption of the cherry-based beverage. Samples were analysed at
different times, as indicated in Material and methods. Values are expressed as pg/ml, n=8.
* statistically significant regarding basal values, # statistically significant regarding its corresponding value in old animals
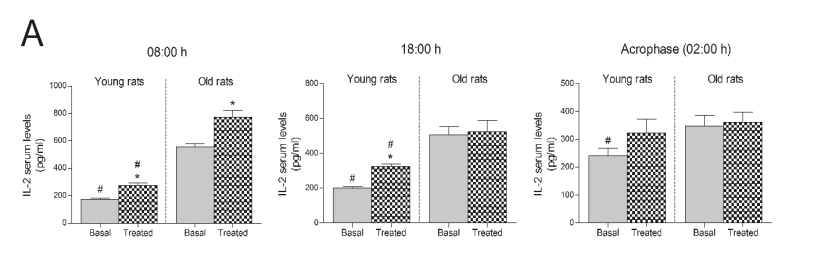
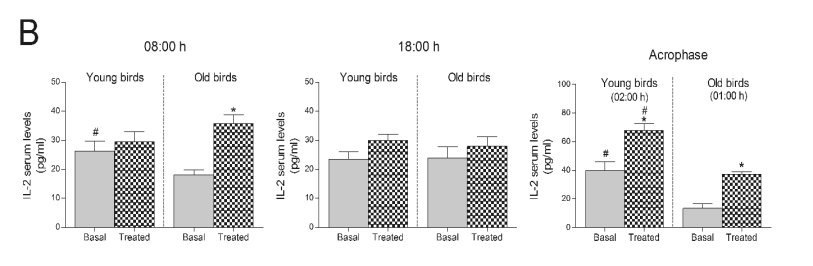
Fig. 4. Concentrations of IL-2 in rats and ringdoves supplemented with a Jerte Valley cherry-based beverage. Serum IL-2 levels in rats
(A) and ringdoves (B) before (basal) and after (treated)
the consumption of the cherry-based beverage. Samples were analyzed at different times, as indicated in Material and methods. Values are expressed
as pg/ml, n=8.
* statistically significant regarding basal values, # statistically significant regarding its corresponding value in old animals
Finally, serum concentrations of anti-inflammatory
IL-2 in treated young rats were clearly raised at dawn
(08:00 h) and at 18:00 h compared to young rats in
basal conditions, while, in the treated old rats, serum
IL-2 levels were only augmented at dawn (Fig. 4A).
In this case, old rats exhibited higher IL-2 basal levels
at all hours tested than young rats (Fig. 4A).
Additionally, the 10-day treatment with the Jerte
Valley cherry-based beverage led to increased IL-2
concentrations at dawn (in old ringdoves) and at the
time corresponding to each group's acrophase of the
melatonin rhythm, i.e. 02:00 h in young ringdoves and
01:00 h in old ringdoves (Fig. 4B). Like basal IL-4,
basal levels of IL-2 were much lower in old ringdoves
both at dawn and the time corresponding to their
acrophase of the melatonin rhythm (Fig. 4B).
DISCUSSION
Consumption of foods rich in antioxidants is
associated with a lower incidence of chronic diseases
such as cardiovascular disease and cancer (Galassetti
and Pontello 2006, Gonzalez 2006). In this study, we
have shown that a 10-day treatment with a Jerte
Valley cherry-based beverage modulates the balance
of pro- and anti-inflammatory cytokines in both rats
and ringdoves by down-regulating the levels of
pro-inflammatory (IL-1beta and TNF-alpha) cytokines and
up-regulating the levels of anti-inflammatory (IL-4
and IL-2) cytokines. Our results are consistent with
those reported with phenolic compounds in animal
models (Tall et al. 2004), preliminary human studies
with cherries (Jacob et al. 2003), and in vitro studies
with phenolic extracts from cherries (Wang et al.
1999). Moreover, the anti-inflammatory effects of
cherries have previously been investigated in animal
models of arthritis. Thus, He et al. (2006), using male
Sprague Dawley mice, showed that animals fed at the
highest dose of total cherry anthocyanins (40 mg/kg)
exhibited significantly lower TNF-alpha and prostaglandin
E2 (PGE2) levels, thereby providing preliminary
evidence of the potential role of cherries in reducing
the inflammatory response in those with
inflammation-related chronic illness.
As cytokine serum levels may vary throughout the
day (Paredes et al. 2009b), it is important to note that
the time of sample collection may affect the results
obtained so that inaccurate conclusions may be drawn.
Actually, our findings show that the cherry-based
beverage was able to moderate the inflammatory load
of our animal subjects depending on the time at which
serum samples were taken. For instance, no changes
were observed after the 10-day treatment with the
cherry-based beverage in IL-1beta and TNF-alpha serum
levels of old birds at dawn (08:00 h), while a
reduction was showed in samples taken at 18:00 h or
01:00 h.
A common finding in the elderly population is a
chronic subclinical inflammatory status that coexists
with immune dysfunction, and these interconnected
processes are of sufficient magnitude to impact health
and survival time (Han and Ulevitch 2005). For
example, the onset and course of age-associated
diseases, such as cardiovascular disease, osteoporosis
and arthritis, are influenced by the level of
pro-inflammatory cytokines (Gouin et al. 2008).
Accordingly, old animals used in this study showed
imbalanced levels of inflammatory markers towards a
pro-inflammatory status, thereby underlining the fact
that ageing is usually accompanied by systemic
inflammation and inflammation-related chronic
illnesses. Therefore, it is worth noting the possibility
that nutritional interventions, like the consumption of
the cherry-based beverage, could prevent or delay the
functional deterioration of the immune system that
accompanies ageing and even, perhaps, return it to
that of the "younger" situation, as previously
suggested (Dorshkind et al. 2009). In this respect, it
has been previously reported that food restriction
delays the loss of several cellular immune functions,
retards the onset of many diseases during ageing and,
consequently, extends significantly both rat and
human life-span (Byun et al. 1995, Berger et al.
2005).
Melatonin is known to exhibit immunomodulatory
actions that are mainly mediated through the
modulation of cytokine production (Carrillo-Vico et
al. 2003a) via binding to specific receptors expressed
by different immune cells (Carrillo-Vico et al. 2003b).
Since Jerte Valley sweet-cherries contain substantial
amounts of melatonin (Gonzalez-Gomez et al. 2010),
it is reasonable to assume that the anti-inflammatory
properties showed by the Jerte Valley cherry-based
beverage in this study may be attributed to melatonin.
Nevertheless, as it has been proposed that the
beneficial properties of fruits and vegetables come
from the additive and synergistic effects of their
phytonutrients (Milde et al. 2007), the involvement of
other antioxidants, such as phenolic acids,
anthocyanins and carotenoids, cannot be ruled out.
Increased dietary intake of vegetable-derived
bioactive compounds may retard age-related
decrements in immune function and prolong life-span.
Therefore, the anti-inflammatory effects of the
cherry-based beverage may be of clinical significance
and should be investigated in further studies. In fact,
the close relationship between chronic inflammation
and poor human health suggests that the Jerte Valley
cherry-based beverage is likely to be a beneficial
addition to the human healthy diet.
ACKNOWLEDGEMENTS
This research was supported by UEx grant (Plan de
Iniciacion a la Investigacion, Accion VII - 18L202).
M. Garrido is beneficiary of a grant from UEx (Plan
de Iniciacion a la Investigacion, Accion II - no.
1059). J. Espino is beneficiary of a grant from
Ministerio de Educacion (AP2009-0753). Sergio D.
Paredes is beneficiary of a grant from Consejeria de
Economia, Comercio e Innovacion - Fondo Social
Europeo (Junta de Extremadura, REI09009).
REFERENCES
Berger J, Machackova M, Berger Z. Effects of feed restriction on the nucleolar structure and function in lymphocytes. Basic Clin Pharmacol Toxicol.
97: 236-237, 2005.
[CrossRef]
[PubMed]
Byun DS, Venkatraman JT, Yu BP, Fernandes G. Modulation of antioxidant activities and immune responses by food restriction in ageing Fisher-344 rats.
Ageing Clin Exp Res. 7: 40-48, 1995.
[PubMed]
Carrillo-Vico A, Garcia-Maurino S, Calvo JR, Guerrero JM. Melatonin counteracts the inhibitory effect of PGE2 on IL-2 production in human lymphocytes
via its mt1 membrane receptor. FASEB J. 17: 755-757, 2003a.
[CrossRef]
[PubMed]
Carrillo-Vico A, Garcia-Perganeda A, Naji L, Calvo JR, Romero MP, Guerrero JM. Expression of membrane and nuclear melatonin receptor mRNA and protein
in the mouse immune system. Cell Mol Life Sci. 60: 2272-2278, 2003b.
[CrossRef]
[PubMed]
Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 27: 189-200,
2005a.
[CrossRef]
[PubMed]
Carrillo-Vico A, Lardone PJ, Naji L, Fernandez-Santos JM, Martin-Lacave I, Guerrero JM, Calvo JR. Beneficial pleiotropic actions of melatonin in an
experimental model of septic shock in mice: regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and
anti-apoptotic effects. J Pineal Res. 39: 400-408, 2005b.
[CrossRef]
[PubMed]
Cubero J, Toribio F, Garrido M, Hernandez MT, Maynar J, Barriga C, Rodriguez AB. Assays of the amino acid tryptophan in cherries by
HPLC-Fluorescence. Food Anal Methods. 3: 36-39, 2010.
[CrossRef]
Dorshkind K, Montecino-Rodriguez E, Signer RAJ. The immune system: is it ever too old to be young again? Nat Rev Immunol. 9: 57-62, 2009.
[CrossRef]
Emery P, McInnes IB, van Vollenhoven R, Kraan MC. Clinical identification and treatment of a rapidly progressing disease state in patients with
rheumatoid arthritis. Rheumatology. 47: 392-398, 2008.
[CrossRef]
[PubMed]
Engstrom G, Hedblad B, Stavenow L, Jonsson S, Lind P, Janzon L, Lindgarde F. Incidence of obesity-associated cardiovascular disease is related to
inflammation-sensitive plasma proteins: a population-based cohort study. Arterioscler Thromb Vasc Biol. 24: 1498-1502, 2004.
[CrossRef]
Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 305: 1736-1739, 2004.
[CrossRef]
Galassetti P, Pontello A. Dietary effects on oxidation of low-density lipoprotein and atherogenesis. Curr Atheroscler Rep. 8: 523-529, 2006.
[CrossRef]
[PubMed]
)
Garrido M, Espino J, Gonzalez-Gomez D, Lozano M, Cubero J, Toribio-Delgado AF, Maynar-Marino JI, Terron MP, Munoz JL, Pariente JA, Barriga C, Paredes
SD, Rodriguez AB. A nutraceutical product based on Jerte Valley cherries improves sleep and augments the antioxidant status in humans. e-SPEN J. 4:
321-323, 2009.
[CrossRef]
Garrido M, Paredes SD, Cubero J, Lozano M, Toribio-Delgado AF, Munoz JL, Reiter RJ, Barriga C, Rodriguez AB. Jerte Valley cherry-enriched diets
improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidantcapacity in the urine of middle-aged and elderly humans. J Gerontol A
Biol Sci Med Sci. 65: 909-914, 2010.
[CrossRef]
[PubMed]
Gonzalez CA. Nutrition and cancer: the current epidemiological evidence. Br J Nutr. 96: 42-45, 2006.
[PubMed]
Gonzalez-Gomez D, Lozano M, Fernandez-Leon MF, Ayuso MC, Bernalte MJ, Rodriguez AB. Detection and quantification of melatonin and serotonin in eight
Sweet Cherry cultivars (Prunus avium L.). Eur Food Res Technol. 229: 223-229, 2009.
[CrossRef]
Gonzalez-Gomez D, Lozano M, Fernandez-Leon MF, Bernalte MJ, Ayuso MC, Rodriguez AB. Sweet cherry phytochemicals: Identification and characterization
by HPLC-DAD/ESI-MS in sweet-cherry cultivars grown in Valle del Jerte (Spain). J Food Compost Anal. 23: 533-539, 2010.
[CrossRef]
Gouin JP, Hantsoo L, Kiecolt-Glaser JK. Immune dysregulation and chronic stress among older adults: a review. Neuroimmunomodulation. 15: 251-259,
2008.
[CrossRef]
Han J, Ulevitch RJ. Limiting inflammatory responses during activation of innate immunity. Nat Immunol. 6: 1198-1205, 2005.
[CrossRef]
[PubMed]
Hanley AJ, Festa A, D'Agostino RB, Jr., Wagenknecht LE, Savage PJ, Tracy RP, Saad MF, Haffner SM. Metabolic and inflammation variable clusters and
prediction of type 2 diabetes: factor analysis using directly measured insulin sensitivity. Diabetes. 53: 1773-1781, 2004.
[PubMed]
He YH, Zhou J, Wang YS, Xiao C, Tong Y, Tang JC, Chan AS, Lu AP. Anti-inflammatory and anti-oxidative effects of cherries on Freund's
adjuvant-induced arthritis in rats. Scand J Rheumatol. 35: 356-358, 2006.
[CrossRef]
[PubMed]
Heber D. Vegetables, fruits and phytoestrogens in the prevention of diseases. J Postgrad Med. 50: 145-149, 2004.
[PubMed]
Hofseth LJ, Ying L. Identifying and defusing weapons of mass inflammation in carcinogenesis. Biochim Biophys Acta. 1765: 74-84, 2006.
[CrossRef]
[PubMed]
Horwood N. Lymphocyte-derived cytokines in inflammatory arthritis. Autoimmunity. 41: 230-238, 2008.
[CrossRef]
[PubMed]
Jacob RA, Spinozzi GM, Simon VA, Kelley DS, Prior RL, Hess-Pierce B, Kader AA. Consumption of cherries lowers plasma urate in healthy women. J Nutr.
133: 1826-1829, 2003.
[PubMed]
Kelley DS, Rasooly R, Jacob RA, Kader AA, Mackey BE. Consumption of bing sweet cherries lowers circulation concentrations of inflammation makers in
healthy men and women. J Nutr. 136: 981-986, 2006.
Mayorga M, Iborra A, Estany S, Martinez P. Protective effect of vitamin E in an animal model of LPS-induced inflammation. Am J Reprod Immunol. 52:
356-361, 2004.
[CrossRef]
[PubMed]
McCune LM, Kubota C, Stendell-Hollis NR, Thomson CA. Cherries and health: a review. Crit Rev Food Sci Nutr. 51: 1-12, 2011.
[CrossRef]
[PubMed]
Milde J, Elstner EF, Grassmann J. Synergistic effects of phenolics and carotenoids on human low-density lipoprotein oxidation. Mol Nutr Food Res. 51:
956-961, 2007.
[CrossRef]
[PubMed]
Paredes SD, Terron MP, Valero V, Cubero J, Barriga C, Reiter RJ, Rodriguez AB. Comparative study of the activity/rest rhythms in young and old
ringdove (Streptopelia risoria): Correlation with the serum levels of melatonin and serotonin. Chronobiol Int. 23: 779-793, 2006.
[CrossRef]
[PubMed]
Paredes SD, Terron MP, Cubero J, Valero V, Barriga C, Reiter RJ, Rodriguez AB. Tryptophan increases nocturnal rest and affects melatonin and
serotonin serum levels in old ringdove. Physiol Behav. 90: 576-582, 2007a.
[CrossRef]
[PubMed]
Paredes SD, Terron MP, Marchena AM, Barriga C, Pariente JA, Reiter RJ, Rodriguez AB. Tryptophan modulates cell viability, phagocytosis and oxidative
metabolism in old ringdoves. Basic Clin Pharmacol Toxicol. 101: 56-62, 2007b.
[CrossRef]
[PubMed]
Paredes SD, Terron MP, Marchena AM, Barriga C, Pariente JA, Reiter RJ, Rodriguez AB. Effect of exogenous melatonin on viability, ingestion capacity,
and free-radical scavenging in heterophils from young and old ringdoves (Streptopelia risoria). Mol Cell Biochem. 304: 305-314, 2007c.
[CrossRef]
[PubMed]
Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ. Phytomelatonin: a review. J Exp Bot. 60: 57-59, 2009a.
[CrossRef]
[PubMed]
Paredes SD, Marchena AM, Bejarano I, Espino J, Barriga C, Rial RV, Reiter RJ, Rodriguez AB. Melatonin and tryptophan affect the activity-rest rhythm,
core and peripheral temperatures, and interleukin levels in the ringdove: Changes with age. J Gerontol A Biol Sci Med Sci. 61: 340-350, 2009b.
[CrossRef]
[PubMed]
Sanchez S, Sanchez CL, Paredes SD, Rodriguez AB, Barrgia C. The effect of tryptophan administration on the circadian rhythms of melatonin in plasma
and the pineal gland of rats. J Appl Biomed. 6: 177-186, 2008.
[JAB]
Seeram NP, Momin RA, Nair MG, Bourquin LD. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine. 8:
362-369, 2001.
[PubMed]
Tall JM, Seeram NP, Zhao C, Nair MG, Meyer RA, Raja SN. Tart cherry anthocyanins suppress inflammation-induced pain behavior in rat. Behav Brain Res.
153: 181-188, 2004.
[CrossRef]
[PubMed]
Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocrinol J. 1: 57-60,
1993.
Wang H, Nair MG, Strasburg GM, Chang YC, Booren AM, Gray JI, DeWitt DL. Antioxidant and anti-inflammatory activities of anthocyanins and their
aglycon, cyanidin, from tart cherries. J Nat Prod. 62: 294-296, 1999.
[CrossRef]
[PubMed]
|
BACK
|









