Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 1, p 9-17
DOI 10.2478/v10136-011-0005-4
Influence of circulating fibrocytes on the growth, proliferation and migration of keratinocytes and fibroblasts
Peng Liu, Gang Hu, Jie Feng, Yan Jin
Address: Gang Hu, Department of Dermatology, Second Hospital of Xi’an Jiaotong University, Xi’an, 710004, China
gang1008@yeah.net
Received 3rd March 2011.
Revised 12th October 2011.
Published online 12th October 2011.
Full text article (pdf)
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
Acknowledgements
Conflict of interests
References
SUMMARY
Circulating fibrocytes (CFs) exhibit an extraordinary degree of plasticity and growth factor repertoire, and because of this they have been investigated for their role in the repair and regeneration of damaged tissues, but yet not adequately for their role in wound healing. In the present study, CFs were co-cultured with keratinocytes (KCs) or fibroblasts (Fbs) and the influences of CFs on the growth, proliferation and migration of KCs and Fbs were investigated. Our results showed that the CFs in the co-culture system could inhibit the growth, proliferation and migration of KCs, while CFs promoted the growth and proliferation of Fbs. Our study demonstrates that CFs can regulate the functions of Fbs, which may be a possible cause of fibrosis.
KEY WORDS
circulating fibrocytes; cell interaction; keratinocytes; fibroblasts; wound healing
Abbreviations: CFs: circulating fibrocytes; Fbs: fibroblasts; KCs: keratinocytes
INTRODUCTION
Circulating fibrocytes (CFs) are bone marrow-derived
mesenchymal progenitors (Mori et al. 2005, Kisseleva
et al. 2006) that can co-express haematopoietic stem
cell antigens and markers of the monocyte lineage
and fibroblasts (Bucala et al. 1994, Yang et al. 2002,
Schmidt et al. 2003, Mori et al. 2005). Studies have
shown that CFs are involved in several aspects of
wound healing, including inflammation, the
production of an extracellular matrix, and
angiogenesis (Bucala et al. 1994, Chesney et al. 1997,
1998, Hartlapp et al. 2001). Previous investigations
have also provided evidence that CFs participate in
fibrotic disorders (Chesney and Bucala 2000, Cowper
2003, Moore et al. 2005, Kisseleva and Brenner
2008). It has been confirmed that the capacity of
fibrocytes from healthy subjects or from burn patients
to produce collagens is inferior to that of dermal
fibroblasts, and that CFs can regulate the activities of
local fibroblasts through secreting the transforming
growth factor beta 1 (TGF-beta1) or the connective
tissue growth factor (CTGF) in the healing of burn
wounds (Wang et al. 2007). Therefore, CFs may play
an indirect, regulatory role in wound healing by
affecting the functions of dermal fibroblasts.
Nevertheless, the increase of CFs in the
hypertrophic scar (Yang et al. 2002, 2005) indicates
that CFs contribute to wound repair by regulating not
only the fibroblasts' activities, but their differentiation
and proliferation. In the proliferative phase, the
migration and proliferation of keratinocytes (KCs),
fibroblasts (Fbs) and endothelial cells can lead to
re-epithelialization and tissue granulation. At the
same time, CFs can interact with local KCs and Fbs.
We therefore postulate that CFs can impact the
proliferation and migration of local cells through
direct cell-cell interaction except for the paracrine.
In the present study, the CFs were isolated and
co-cultured with KCs or dermal Fbs, and the
influences of CFs on the growth, proliferation,
migration and cell cycle of KCs and Fbs were
investigated. Our results showed that CFs in the
co-culture system compromised the functions of KCs
but promoted those of Fbs. Therefore, we conclude
that CFs play an important role in fibrosis during
wound healing.
MATERIALS AND METHODS
Cell culture
This study was approved by the Ethical Committee of
the Fourth Military Medical University. CFs were
isolated by the leukopheresis of healthy human
donors. Briefly, the sample was diluted with
phosphate-buffered saline (PBS) at the volume ratio
of 1:1 in heparinized tubes. Then, 7 ml of this
solution was transferred into a tube containing 7 ml of
Percoll-Paque (1.073 g/ml; GE Healthcare, UK), and
the nucleated cells were isolated by density gradient
centrifugation at 2,100 rpm for 20 min. The nucleated
cells were seeded in T25 plastic dishes at a density of
1x105 cells/cm3 and followed by incubation in a
low-glucose Dulbecco's modified Eagle's medium
(DMEM) containing 7% foetal calf serum (FCS;
Gibco, USA), 2 mM-glutamine, 100 U/ml penicillin,
100 mg/ml of streptomycin at 37 °C in a humidified
atmosphere with 5% CO2. Three days later,
non-adherent cells were removed by refreshing the
medium every 3 days. After 10-14 days of culture,
the proportion of Fbs with spindle-shaped
morphology was analysed (greater than 75%). When
the cells reached 100% confluence, the adherent cells
were harvested by incubation in 0.05% (w/v)
EDTA/PBS for 5 min at room temperature, and then
were gently aspirated and collected for the following
experiments.
Proliferation of CFs
At same condition of cell culture, CFs were inoculated into 24 well plates at 4x103/cm3, and every eight wells were regarded as a individual induction group. One of these groups was treated with 50 ng/ml macrophage colony-stimulating growth factor (M-CSF, Sigma, USA) and 50 ng/ml recombinant human thrombopoietin (TPO, Sigma, USA) for another. An individual group was leaved untreated. Cell counting for each well of different groups was performed by inspection under a light microscopy every 24 h, lasted for 8 days, and cell growth curves were drawn. The results are the mean of cell counts from individuals.
Phenotype of CFs
Because of the difficulties in passaging, CFs in the early and late phase (3 and 7 days of primary culture) were stained with the following mouse anti-human antibodies (mAbs) conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE) or peridinin-chlorophyll-protein complex (PerCP): CD11b, CD14, CD34, CD44, CD45 and CD90. Cells in the negative controls were incubated with isotype-matched mouse anti-human mAb to an irrelevant antigen. The cells were analysed in an FACS Calibur flow cytometer (BD Biosciences, USA) with CellQuest software.
Osteogenic and adipogenic induction
CFs were seeded in a 6-well plate at a density of 5x105 cells/well in basic medium for 24 h to allow adherence. Then, the medium was replaced with osteogenic induction medium containing DMEM, 10% FCS, 0.1 microM dexamethasone, 10 mM beta-glycerolphosphate and 50 mg/l ascorbate-2-phosphate, and adipogenic induction medium consisting DMEM, 10% FCS, 0.25 microM dexamethason, 100 microM indomethacin, 0.5 mM 3-isobutyl methylxanthine and 10 mg/l insulin. Cells were maintained for 2 weeks and media were replaced twice weekly. The mineralized nodules were stained with Alizarin Red S and neutral lipid vacuoles with Oil-red O (Sigma, USA).
Analysis of proliferation, migration and cell cycle
A CFs feeder layer was obtained after 10 to 14 days
of culture in a 24-well plate and then irradiated at
1400 cGy. The prepared feeder layer was maintained
in a humidified atmosphere with 5% CO2 at 37 °C and
used for the subsequent experiments within 5 days
after irradiation. The irradiation of CFs layers at 1400
cGy prevents the overgrowth of the feeder while
maintaining optimal viability and growth factor
production. Four groups were included: 1) KCs alone;
2) KCs+CFs; 3) Fbs alone; 4) Fbs+CFs.
To determine the effects of CFs on the proliferation of KCs and Fbs, KCs and Fbs were seeded on the CFs feeder layer (2x105 cells/well) for 24 h in a conditioned medium (DMEM containing 10% fetal bovine serum, Fbs or serum-free medium for the culture of human keratinocytes, K-SFM). When the cell confluence reached 80%, cells were trypsinized for detection of proliferation by MTT assay which was performed from day 1 to day 7. The culture medium was then removed, cells were washed with PBS and a serum-free DMEM was added. About 100 microl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl- tetrazolium bromide (MTT) solution (5 microg/ml) were added to each well followed by incubation for 2 h in a CO2 incubator at 37 °C. Then, 1 ml of 20% SDS/DMF solution was added to each well followed by shaking for 15 min on an orbital shaker. These cells were transferred to cuvettes and the optical density was measured at 570 nm. For the BrdU assay, 50 microM 5-bromo-2'-deoxyuridine, BrdU (Sigma, USA) was added.
The migration of co-cultured KCs and Fbs was
determined by scratching assay. A representative area
of KCs and Fbs at 12 h after scratching was
presented. KCs, Fbs and co-cultured KCs+CFs and
Fbs+CFs were trypsinized and then fixed in 70%
alcohol followed by analysis of cell cycle by flow
cytometry (Beckman Coulter, USA).
Statistical analysis
Statistical analysis was carried out with SPSS version
13.0 statistic software package (Cary, USA). We used
two sided t-test at the significance level 2alpha=0.05.
RESULTS
CFs has a spindle-shaped morphology, grows in the
presence of anti-macrophage colony stimulating
factor (M-CSF) and can transform into
macrophage-like cells
The adherent cells exhibited a spindle-shaped
morphology, and some transformed into
macrophage-like cells during the culture (Fig. 1a, b).
In the presence of M-CSF and TPO (50 ng/ml), CFs
could maintain the spindle-shaped morphology and
proliferate for a long time (Fig. 1c). CFs were
difficult to proliferate and passage under normal
conditions, and macrophage-like cells could spread in
the disk for more than one month supporting the
absence of M-CSF (Fig. 1d). The cell count was
significantly increased after 6 days of incubation with
M-CSF and TPO at a concentration of 50 ng/ml
(Fig. 1e).
Phenotypes of CFs
The CFs displayed CD11b/CD14low/CD34/
CD45high/CD44high/CD90 (Fig. 2a), and showed
enhanced expressions of CD34 (from 2.99% to
21.06%) and CD45 (from 96.8% to 98.92%) in the
early and late phase of primary culture (Fig. 2b). The
expression of isotype-control was about 1%. These
results indicate the maturation of CFs, and the high
expression of CD45 confirms its haematopoietic
origin.
Multipotency of CFs
Maintained in the specific induction medium, the CFs
possessed osteogenic and adipogenic potentials and
were positive for Alizarin Red S and Oil red-O
(Fig. 3a, b).
CFs inhibit the proliferation and migration of KCs in vitro
In the scratching assay, cells migrated toward the
center of the scratch and only a small amount of cell
were confluent after 12 h of co-culture of CFs and
KCs, which was much less than that after culture of
the KCs alone (Fig. 4a). By MTT assay, the growth
and proliferation of KCs were significantly inhibited
in the co-culture system of CFs and KCs when
compared with those after culture of KCs alone
(statistically significant) (Fig. 4b). A smilar result was
achieved by BrdU assay (statistically significant)
(Fig. 4c). Moreover, the proportion of cells in the
G2+S phase and S phase was 13.4 and 13.4
respectively in the co-culture system of CFs and KCs,
and 20.6 and 20 respectively in the culture of KCs
alone (Fig. 4d), which indicates the decrease of DNA
synthesis. These results suggest that CFs co-cultured
with KCs can suppress the growth, proliferation and
migration of KCs.
CFs promote the proliferation and migration of Fbs in vitro
In contrast to the effects of CFs on KCs in the
scratching assay, cells migrated toward the center of
the scratch and a large number of cells were confluent
after 12 h of co-culture of the CFs and Fbs, which
was much more than that in the culture of Fbs alone
(Fig. 5a). The MTT assay showed that the growth and
proliferation of KCs were significantly promoted after
co-culture with CFs when compared with those in the
culture of Fbs alone (statistically significant)
(Fig. 5b). A similar result was achieved by BrdU
assay (Fig. 5c). Moreover, the proportion of cells in
the G2+S phase and S phase was 51 and 39,
respectively in the co-culture system of CFs and Fbs,
and 31 and 20.5 in culture of Fbs alone (Fig. 5d).
These findings suggest that CFs co-cultured with Fbs
can promote the growth, proliferation and migration
of Fbs.

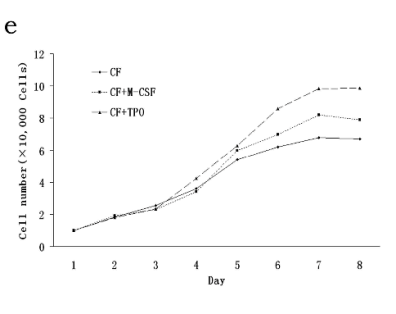
Fig. 1. CFs exhibits a spindle-shaped morphology, grows in the presence of M-CSF and transforms into macrophage-like cells. Adherent cells exhibit a spindle-shaped morphology, and some transformed to macrophage-like cells during the culture
(a, b). In the presence of M-CSF (50 ng/ml), CFs could maintain the spindle-shaped morphology and proliferate for a long time
(c). CFs were difficult to proliferate and passage under normal condition, and macrophage-like cells could spread in the disk for
more than one month supporting the absence of M-CSF (d). The cell count was significantly increased after 6 days of incubation
with M-CSF and TPO at a concentration of 50 ng/ml (e).
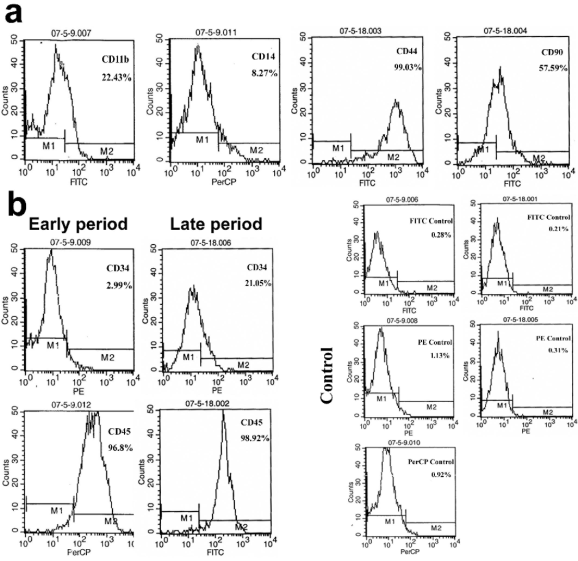
Fig. 2. CFs displayed CD11b/CD14low/CD34/CD45high/CD44high/CD90 (a), and showed an enhanced expression of CD34
(from 2.99% to 21.06%) and CD45 (from 96.8% to 98.92%) in the early and late phase of primary culture (b). The expression
of isotype-control was about 1%.
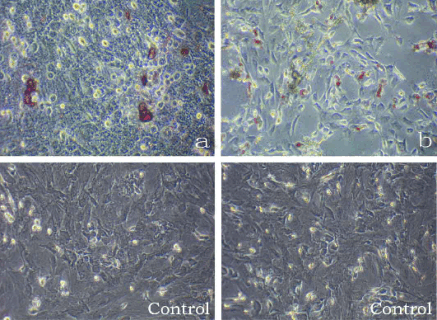
Fig. 3. CFs maintained in the specific induction media, CFs possessed osteogenic and adipogenic potentials and were positive
for Alizarin Red S and Oil red-O stainings, respectively (a, b).
DISCUSSION
The present study aimed to determine the influences
of CFs on the cell functions of KCs and Fbs in the
co-culture system. Our results demonstrated that CFs
could promote the growth, proliferation and migration
of Fbs in vitro in the co-culture system. In humans, CFs can be found in tumours, skin wounds,
hypertrophic scars, bronchial asthma, pulmonary
fibrosis, and nephrogenic fibrosing dermopathy
(Schmidt et al. 2003, Quan et al. 2004, Mori et al.
2005, Yang et al. 2005, Mehrad et al. 2007). In
animal models, CFs are associated with experimental
fibrosis of lung, kidney and liver, carotid artery
intimal hyperplasia, chronic granulomatous disease,
and skin wounding (Direkze et al. 2003, Epperly et al.
2003, Hashimoto et al. 2004, Phillips et al. 2004,
Quan et al. 2004, Kisseleva et al. 2006, Moore et al.
2006, Sakai et al. 2006, Varcoe et al. 2006).
Therefore, CFs may play an important role in fibrosis
in numerous forms.
Although the specific impact of CFs on healing is
unclear, there are at least two ways in which CFs
exert their effects. One is that CFs can differentiate
into fibroblasts secreting an essential extracellular matrix at the injury site. Fibrocytes cultured ex vivo
can express numerous extracellular matrix molecules,
including vimentin, fibronectin, collagen I and
collagen III, and fibrocytes in the wounds has the
ability to express collagen, suggesting their roles in
wound repair (Bucala et al. 1994, Chesney et al.
1998, Yang et al. 2005). The other is that CFs can
regulate the biological behavior of fibroblasts via
paracrine. Wang et al. (2007) demonstrated that CFs
could enhance the activities of local fibroblasts
through producing growth factors. In both ways, CFs
may result in severe fibrosis, which has been
demonstrated by the findings that CFs have been
found in the areas with connective tissue matrix
deposition in fibrotic liver, lung and kidney (Chesney
et al. 1998, Chesney and Bucala 2000, Hashimoto et
al. 2004, Phillips et al. 2004, Kisseleva et al. 2006,
Sakai et al. 2006, Bellini and Mattoli 2007, Mehrad et
al. 2007).
In addition, our results also revealed that CFs
could inhibit the growth, proliferation and migration
of KCs. Few studies report the influence of CFs on
KCs and in vivo transplantation of CFs or KCs
showed enhanced re-epithelialization, which implies
the mechanism of epithelial regeneration is more
complex than that of fibrosis. It is well known that the
migration and proliferation of epithelial cells can be promoted by the surrounding cells at the wound
margin and/or by the increase of secreted growth
factors and their receptors on the epithelial cells.
Future studies are required to explain this
discrepancy.
In conclusion, CFs can improve the cell functions
of Fbs, which may be an important cause of fibrosis.
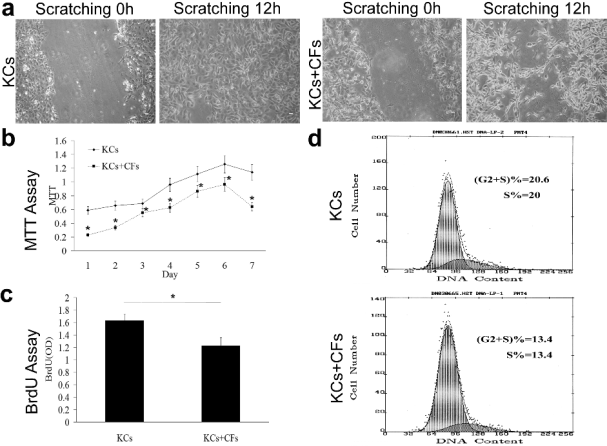
Fig. 4. CFs inhibit the proliferation and migration of KCs in vitro. In the scratching assay, cells migrated toward the center
of the scratch and only a small amount of cells were confluent after 12 h of co-culture of CFs and KCs, which was much less than
that in the culture of KCs alone (a). In the MTT assay, the growth and proliferation of KCs were markedly inhibited in the
co-culture system of CFs and KCs when compared with those in the culture of KCs alone (statistically significant) (b). A similar
result was obtained by BrdU assay (statistically significant) (c). Moreover, the proportion of cells in the G2+S phase and S phase
was 13.4 and 13.4 respectively in the co-culture system of CFs and KCs, and 20.6 and 20 respectivel in the culture of KCs alone
(d), which indicates the decrease of DNA synthesis.
ACKNOWLEDGEMENTS
The study was supported by the National High
Technology Research and Development Program of
China (863 Project) (No: 2006AA02A119) and
Fundamental Research Funds for the Central.
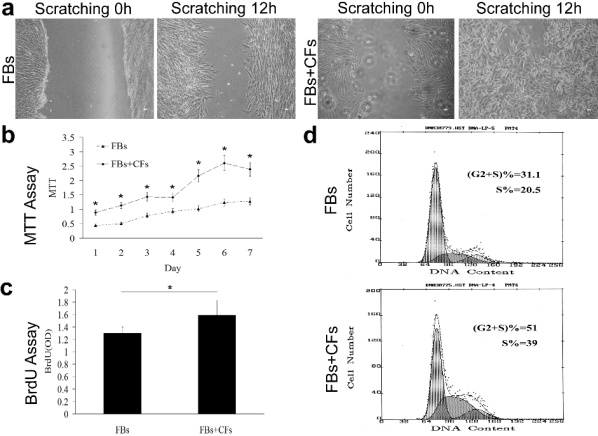
Fig. 5. CFs promotes the proliferation and migration of Fbs in vitro. In contrast to the effects of CFs on KCs in the scratching
assay, cells migrated toward the center of the scratch and a large amount of cells were confluent after 12 h of co-culture of CFs
and Fbs, which was much more than that in the culture of Fbs alone (a). An MTT assay showed the growth and proliferation of
Fbs were significantly increased in the co-culture system of CFs and KCs when compared with those in the culture of Fbs alone
(statistically significant) (b). A similar result was obtained by a BrdU assay (statistically significant) (c). Moreover, the proportion of cells in the G2+S phase and S phase was 51 and 39 respectively in the co-culture system of CFs and Fbs, and 31 and 20.5 in
culture of Fbs alone (d).
CONFLICT OF INTERESTS
The authors declare no conflict of interests in this
manuscript.
REFERENCES
Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 87:
858-870, 2007.
[CrossRef]
Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med.
1: 71-81, 1994.
Chesney J, Bucala R. Peripheral blood fibrocytes: mesenchymal precursor cells and the pathogenesis of fibrosis. Curr Rheumatol Rep. 2: 501-505,
2000.
Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in
situ. Proc Natl Acad Sci USA. 94: 6307-6312, 1997.
Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood
fibrocytes. J Immunol. 160: 419-425, 1998.
Cowper SE. Nephrogenic fibrosing dermopathy: the first 6 years. Curr Opin Rheumatol. 15: 785-790, 2003.
Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by
bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 21: 514-520, 2003.
[CrossRef]
Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol. 29:
213-224, 2003.
[CrossRef]
Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and
promote angiogenesis in vivo. FASEB J. 15: 2215-2224, 2001.
[CrossRef]
Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 113: 243-252, 2004.
[CrossRef]
Kisseleva T, Brenner DA. Fibrogenesis of parenchymal organs. Proc Am Thorac Soc. 5: 338-342, 2008.
[CrossRef]
Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. Bone marrow-derived fibrocytes participate in
pathogenesis of liver fibrosis. J Hepatol. 45: 429-438, 2006.
[CrossRef]
Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung
disease. Biochem Biophys Res Commun. 353: 104-108, 2007.
[CrossRef]
Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar
space after fibrotic injury. Am J Pathol. 166: 675-684, 2005.
[CrossRef]
Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell
Mol Biol. 35: 175-181, 2006.
[CrossRef]
Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone
marrow. Exp Cell Res. 304: 81-90, 2005.
[CrossRef]
Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in
response to CXCL12 and mediate fibrosis. J Clin Invest. 114: 438-446, 2004.
[CrossRef]
Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell
Biol. 36: 598-606, 2004.
[CrossRef]
Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine, SLC/CCL21/CCR7 signaling regulates
fibrocytes in renal fibrosis. Proc Natl Acad Sci USA. 103: 14098-14103, 2006.
[CrossRef]
Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J
Immunol. 171: 380-389, 2003.
Varcoe RL, Mikhail M, Guiffre AK, Pennings G, Vicaretti M, Hawthorne WJ, Fletcher JP, Medbury HJ. The role of the fibrocyte in intimal hyperplasia. J
Thromb Haemost. 4: 1125-1133, 2006.
[CrossRef]
Wang JF, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair
Regen. 15: 113-121, 2007.
[CrossRef]
Yang L, Scott PG, Giuffre J, Shankowsky HA, Ghahary A, Tredget EE. Peripheral blood fibrocytes from burn patients: identification and quantification
of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 82: 1183-1192, 2002.
Yang L, Scott PG, Dodd C, Medina A, Jiao H, Shankowsky HA, Ghahary A, Tredget EE. Identification of fibrocytes in postburn hypertrophic scar. Wound
Repair Regen. 13: 398-404, 2005.
[CrossRef]
|
BACK
|







