Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 1, p 51-61
DOI 10.2478/v10136-011-0018-z
Soman and VX: different effect on cellular signalling
Jaroslav Pejchal, Jan Osterreicher, Jiri Kassa, Ales Tichy, Zuzana Sinkorova, Lenka Zarybnicka, Klara Kubelkova, Kamil Kuca
Address: Kamil Kuca, Center of Advanced Studies, Faculty of Military Health Sciences, University of Defence, Trebesska 1575, 500 01 Hradec Kralove,
Czech Republic
kucakam@pmfhk.cz
Received 16th May 2011.
Revised 26th July 2011.
Published online 10th November 2011.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
Acknowledgements
References
SUMMARY
The purpose of our study was to examine the early expression of p21 and activated transcription factors ATF-2, CREB, Elk-1, p53 after soman and VX
poisoning, to throw light on the pathogenetic mechanism of nerve agent-induced non-specific effects. Male Wistar rats were i.m. poisoned by soman
(60 microg.kg-1 - 70% LD50) or VX (8 microg.kg-1 - 70% LD50). Samples were taken 4, 24, and 72 hours
after poisoning, immunohistochemically stained and phospho-ATF-2Thr-69/71, phospho-CREBSer-133,
phospho-Elk-1Ser-383, phospho-p53Ser-15, and protein p21 expressions were measured using computer Image analysis in apical
and cryptal enterocytes of the colon transversum. After soman poisoning, we observed an increased phospho-CREB in cryptal enterocytes 4, 24, and 72
h after poisoning, while apical enterocytes expressed increased phospho-CREB only 72 h after intoxication. Phospho-Elk-1 significantly dropped 4
and 24 h after soman poisoning in the cryptal compartment. Activation of ATF-2 and p53 and expression of p21 were not changed 4, 24, and 72 h after
soman poisoning. VX poisoning did not change any of measured parameters. Soman and VX showed a different effect on cellular signalling. Soman seems
to cause additional effects, which are not related to the basic mechanism of nerve agent-induced toxicity and which temporarily suppress promitotic
pathways of proliferating cells and persist in cells during the differentiation process.
KEY WORDS
soman; VX; ATF-2; CREB, Elk-1; p21; p53; enterocyte; image analysis
INTRODUCTION
Nerve agents are highly toxic organophosphates
representing potential threats to both the military and
the civilian population. The basic mechanism of their
toxicity is well known and lies in irreversible binding
to and inactivation of acetylcholinesterase (AChE, EC
3.1.1.7), which is associated with the accumulation of
acetylcholine at the synapses and overstimulation of
the cholinergic nervous system (Marrs 1993). By
contrast, less is known about nerve agent-induced
non-specific effects including the influence on
non-cholinergic neurotransmitter levels and especially
oxidative stress interfering with cellular DNA
metabolism and resulting in organophosphate
genotoxicity and mutagenicity (Kassa et al. 2000,
Klaidman et al. 2003, Bajgar 2004). Oxidative stress
and long-term alteration of DNA are considered to
contribute to the long-term toxic effects of nerve
agents (Pazdernik et al. 2001, Klaidman et al. 2003).
Therefore, finding the mechanisms of nerve
agent-induced non-specific effects might contribute to
early diagnosis and complex treatment of nerve agent
poisoning.
Oxidative stress causes generalized damage to all
molecular components (DNA, proteins, lipids) and
even gives rise to the most deleterious form of
cellular lesion, called double strand breaks (DSB)
(Steinboeck et al. 2010). Eukaryotic cells respond to
oxidative stress and/or DNA damage by activating
multiple signal transduction pathways to maximize
cellular survival while minimizing the chance of
carcinogenesis (Takekawa et al. 2000). The most
important signalling pathways related to oxidative
stress and/or DNA damage are pathways related to
protein p53 and mitogen-activated protein kinase
(MAPK) pathways (Giaccia et al. 1998, Kyriakis and
Avruch 2001).
The protein p53 is a transcription factor whose
function is regulated through phosphorylation at
multiple sites by different kinases (Kyriakis and
Avruch 2001). The presence of DSB induces
phosphorylation of p53 at serine 15 by
DNA-dependent protein kinase (DNA-PK) and ataxia
telangiectasia mutated kinase (ATM). Both kinases
are at the top of the DNA damage signalling network
and play a key role in the response of p53 to DNA
damage (Lakin and Johnson 1999). Phosphorylation
of p53 at serine 15 modulates its stability and
sequence-specific DNA binding activity leading to
transcription of several p53 targets, including the
cyclin-dependent kinase inhibitor p21 (el-Deiry et al.
1993, Wittlinger et al. 2007). Protein p21 blocks the
cell cycle which allows the cells to assess the extent
of DNA damage and initiate repair or to trigger an
apoptotic response (Jung et al. 2010).
In contrast to DNA damage specific phospho-rylation of p53 at serine 15, MAPK signalling
pathways are activated in response to many different
stimuli such as gamma-radiation, UV radiation, DNA
damaging reagents, osmotic shock, and oxidant
stressors (Johnson and Lapadat 2002). So far, three
main groups of MAPK cascades have been identified:
ERK (extracellular signal-regulated kinase) pathway,
preferably activated by mitogenic stimuli, p38 and
JNK (c-Jun N-terminal kinase) pathways regulated by
environmental stressors. These cascades modulate the
function of a very wide range of transcription factors,
including Elk-1, ATF-2, CREB, through which
MAPK pathways adjust cellular transcription
phenotype to the new conditions - to the presence of
damage and/or stress stimulation (Kyriakis and
Avruch 2001).
To evaluate the effects of nerve agents on cellular
signalling, we investigated the impact of soman and
the VX agent on phosphorylation of p53 at serine 15,
the expression of protein p21 and activation of
MAPK-related transcription factors Elk-1, ATF-2,
and CREB in vivo. Because of the different
penetration of both nerve agents through the
blood-brain barrier (Bajgar et al. 2010), a model of rat
colon enterocytes was chosen for the study.
MATERIAL AND METHODS
Animals
Male Wistar rats aged 12-16 weeks and weighing
250-300 g (Navel, Konarovice, Czech Republic)
were kept in an air-conditioned room (22±2 °C and
50±10% relative humidity, with lights from 7.00 to
19.00 hours) and allowed access to standard food and
tap water ad libitum. Before the start of the
experiment (soman, VX, or saline administration),
animals spent 15 days of acclimatization in the
laboratory vivarium. Handling of the experimental
animals was done under the supervision of the Ethics
Committee of the Faculty of Military Health Sciences
in Hradec Kralove (Czech Republic).
Chemicals
Soman (GD; pinacolyl methylphosphonofluoridate)
and the VX agent [O-ethyl S-(2-isopropylaminoethyl) methyl phosphonothioate] were obtained
from the Military Technical Institute in Brno (Czech
Republic). Their purity (97-98%) was assayed by
acidimetric titration. All other drugs and chemicals of
analytical grade were obtained commercially and
used without further purification. Soman, the VX
agent and saline were administered intramuscularly
(i.m.) at a volume of 1 ml/kg of body weight (b.w.).
Procedure
Soman experiment
Saline was administered i. m. to twenty-four control
rats divided into three groups and all of them were
killed by cervical dislocation 4, 24 and 72 hours after
saline administration, respectively. Soman was
administered i. m. at a dose of 60 microg/kg (70% LD50),
to twenty-four experimental rats who were then
divided into three groups, all of which were killed by
cervical dislocation 4, 24 and 72 hours after the
poisoning, respectively.
VX experiment
Saline was administered i.m. to twenty-four control
rats who were then divided into three groups all of
which were killed by cervical dislocation 4, 24 and
72 hours after saline administration, respectively. The
VX agent was administered i. m. to thirty
experimental rats at a dose 8 microg/kg (70% LD50); they
were divided into three groups, all of which were
killed by cervical dislocation 4, 24 and 72 hours after
VX poisoning, respectively.
Histological examination
The central part of the colon transversum was
removed from the rats and carefully fixed with a 10%
neutral buffered formalin (Chemapol, Prague, Czech
Republic). Samples were subsequently embedded into
paraffin (Paramix, Holice, Czech Republic), 5 m
thick tissue sections were cut and immuno-histochemical detection of ATF-2 phosphorylation at
threonine 69 and 71, CREB phosphorylation at serine
133, Elk-1 phosphorylation at serine 383, p53
phosphorylation at serine 15, and protein p21 was
performed with a standard peroxidase technique.
After blocking of the endogenous peroxidase activity
for 20 min [1.8 ml of 30% hydrogen peroxide
(Vitrum, Prague, Czech Republic) in 100 ml methanol
(Kulich, Hradec Kralove Czech Republic)], the tissue
sections were incubated for 1 hour with antibodies:
rabbit polyclonal anti-phospho-ATF-2Thr-69/71 diluted
1:50; rabbit monoclonal anti-phospho-CREBSer-133
diluted 1:100; mouse monoclonal anti-Elk-1Ser-383
diluted 1:100 (all from Biotech, Prague, Czech
Republic); rabbit polyclonal-anti-p53Ser-15 diluted
1:100 (Merck, Ricany, Czech Republic), and mouse
monoclonal anti-p21 diluted 1:50 (ITA-interact,
Prague, Czech Republic) in phosphate buffered saline
(PBS, Sigma-Aldrich, Prague, Czech Republic) pH
7.2 and were then washed three times in PBS. All
slides were then incubated for 20 min with secondary
antibodies. A ready-to-use biotinylated anti-rabbit
secondary antibody (DakoCytomation, Prague, Czech
Republic) was used for the slides previously
incubated with rabbit primary antibodies and
biotin-SP-conjugated AffiniPure donkey anti-mouse
secondary antibody diluted 1:500 (Spinchem, Plzen,
Czech Republic) was used for slides previously
incubated with mouse primary antibodies. The excess
of secondary antibodies was then washed off with
PBS. Subsequently, all slides were incubated with
streptavidin horseradish peroxidase (Dako
Cytomation, Prague, Czech Republic) under the same
conditions as the secondary antibody, washed with
PBS and finally, 0.05% 3,3´-diaminobenzidi-netetrahydrochloride-chromogen solution (Sigma-
Aldrich, Prague, Czech Republic) in PBS containing
0.02% hydrogen peroxide, was added for 10 min to
visualize the antigen-antibody complex in situ.
Image analysis
Stained samples were evaluated using the BX-51
microscope (Olympus, Prague, Czech Republic) and
computer image analysis ImagePro 5.1. (Media
Cybernetics, Bethesda, USA). Ten microscopic fields
at a 400fold original magnification were randomly
selected from each rat sample. Image analysis was
performed separately in two compartments - in apical
enterocytes and in enterocytes of lateral sides of
crypts in the area of 2250 microm2 representing 30-40
cells per field and compartment. The immuno-reactive
structures of inverted RGB scale were detected in the
range: red 56-255, green 76-255, and blue 94-255,
where 0 is white and 255 is the colour black.
Subsequently, integral optical density (IOD) of
viewing fields was measured. The IOD parameter
reflects the intensity of positivity within the detected
area. The scale represents levels from 0 to 2 x 105 for
the detected area.
Histopathological evaluation of staining intensity
The samples were examined in a "blind" manner by
two independent observers. The level of staining was
estimated semiquantitatively by using the
immunoreactive score (IRS) (Remmele and Stegner
1987). The IRS is calculated by multiplying the
staining intensity (graded between 0 and 3) and the
percentage of positive cells (graded between 0 and 4:
0, negative; 1, 1-10%; 2, 11-50%; 3, 51-80%; 4,
81-100%). It allows a maximum value of 12. On the
basis of IRS, the staining pattern was defined as:
negative (IRS: 0), weak (IRS: 1-4), moderate (IRS:
5-8), and strong (IRS: 9-12).
Statistical analysis
The Mann-Whitney test was used for the statistical
analysis giving mean ± 2 x S.E.M. (Standard error of
mean). The differences were considered significant
when p0.05.
RESULTS
Mortality and symptoms of nerve agent poisoning
After soman poisoning, 5 of 24 animals died prior to
the time of sample collection (Table 1). This result is
comparable to VX poisoning, in which 6 of 30
animals died prior to the time of sample collection
(Table 2). Symptoms exhibited with soman and VX
poisoning (salivation, chewing, generalised
convulsions and/or convulsions in the forelimb or
hindlimb) were present in all animals early after
intoxication and disappeared within 24 hours after
soman as well as VX poisoning.
Table 1. Survival of rats following exposure to soman.
| Groups (time of sample collection) |
Survival in the time of sample collection
(survived/total) |
Time of death (min) | | Saline (4 hours) |
8/8 |
| | Saline (24 hours) |
8/8 |
| | Saline (72 hours) |
8/8 |
| | Soman (4 hours) |
6/8 |
152, 180 | | Soman (24 hours) |
6/8 |
200, overnight | | Soman (72 hours) |
7/8 |
181 |
Table 2. Survival of rats following exposure to VX.
| Groups (time of sample collection) |
Survival in the time of sample collection
(survived/total) |
Time of death (min) | | Saline (4 hours) |
8/8 |
| | Saline (24 hours) |
8/8 |
| | Saline (72 hours) |
8/8 |
| | Soman (4 hours) |
8/10 |
110, 130 | | Soman (24 hours) |
8/10 |
156, overnight | | Soman (72 hours) |
8/10 |
62, overnight |
Effect of nerve agents on activation of p53, Elk-1,
CREB, ATF-2 and and expression of protein p21
Phospho-p53Ser-15
In comparision with the control animals, we did not
find any significant changes in phospho-p53Ser-15
levels 4, 24, and 72 hours after soman or VX
poisoning (Table 3 and 8).
Expression of p21
Similarly to phospho-p53Ser-15, p21 expression was not
found to be significantly changed 4, 24, and 72 hours
after soman or VX intoxication (Table 4 and 8).
Phospho-Elk-1Ser-383
In comparison with the control animals,
phospho-Elk-1Ser-383 was significantly decreased in
cryptal enterocytes 4 and 24 hours after soman
intoxication. The IOD values decreased 2.7- and
1.6-fold, respectively. A significant change of Elk-1
activation was also measured oculometrically in
crypts 4 hours after soman poisoning. On the other
hand, VX poisoning did not change activation of
Elk-1 compared to control values (Table 5 and 8).
Phospho-CREBSer-133
The level of phospho-CREBSer-133 in apical
enterocytes was significantly increased 72 hours after
soman administration (IOD increased 3.1-fold), while
cryptal phospho-CREB levels were found to be
significantly higher 4, 24, and 72 hours after soman
intoxication with IOD values being 3.3-, 3.2-, and
1.7-fold increased, respectively. Significantly higher
phospho-CREBSer-133 was measured oculometrically in
apical enterocytes 72 hours after soman poisoning.
VX poisoning did not change the activation of CREB
in both compartments compared to control values
(Table 6 and 8).
Phospho-ATF-2Thr-69/71
No significantly different changes of phospho-ATF-2
levels were measured in rat colon enterocytes 4, 24,
and 72 hours after soman or VX intoxication (Table 7
and 8).
Table 3. Average IOD values of phospho-p53Ser-15 per microscopic field ± 2 x S.E.M.
|
Integral optical density
(IOD) | | time (h) |
4 |
24 |
72 | |
apical enterocytes | | control |
400±100 |
400±100 |
400±100 | | SOMAN |
500±100 |
700±300 |
500±100 | | control |
1900±900 |
3100±1400 |
3800±1500 | | VX |
1200±600 |
3200±900 |
2800±1200 | |
cryptal enterocytes | | control |
0±0 |
100±100 |
0±0 | | SOMAN |
0±0 |
100±0 |
0±0 | | control |
100±100 |
200±100 |
100±100 | | VX |
0±0 |
100±100 |
100±100 |
Note: Although the same primary antibodies (same supplier, same catalogue number) were used for soman and VX experiments,
their batches differed. Therefore, significant colour differences between soman and VX control groups can be found. See also
Table 6 and 7.
Table 4. Average IOD values of protein p21 expression per microscopic field ± 2 x S.E.M.
|
Integral optical density
(IOD) | | time (h) |
4 |
24 |
72 | |
apical enterocytes | | control |
900±300 |
2000±600 |
1700±1200 | | SOMAN |
1000±300 |
2500±1300 |
1500±900 | | control |
1400±500 |
1500±100 |
1300±700 | | VX |
1100±500 |
1400±400 |
1000±500 | |
cryptal enterocytes | | control |
100±0 |
100±0 |
100±100 | | SOMAN |
0±0 |
200±100 |
0±0 | | control |
100±100 |
100±100 |
100±100 | | VX |
100±100 |
100±100 |
100±100 |
Table 5. Average IOD values of phospho-Elk-1Ser-383 per microscopic field ± 2 x S.E.M.
|
Integral optical density
(IOD) | | time (h) |
4 |
24 |
72 | |
apical enterocytes | | control |
39500±6000 |
32400±6500 |
28600±3700 | | SOMAN |
31600±3900 |
31300±7200 |
36800±6300 | | control |
35600±5100 |
32600±3400 |
31700±4700 | | VX |
34600±4300 |
35500±3500 |
37000±5000 | |
cryptal enterocytes | | control |
36900±6400 |
28800±6100 |
30000±3700 | | SOMAN |
13600±4200* |
17500±5500* |
22700±4200 | | control |
32500±5800 |
31000±6000 |
25800±4500 | | VX |
30600±5100 |
26700±3600 |
24600±3800 |
* statistically significant as compared with control
Table 6. Average IOD values of phospho-CREBSer-133 per microscopic field ± 2 x S.E.M.
|
Integral optical density
(IOD) | | time (h) |
4 |
24 |
72 | |
apical enterocytes | | control |
700±100 |
1200±300 |
1400±700 | | SOMAN |
900±200 |
2000±900 |
4300±2300* | | control |
2400±600 |
2500±900 |
3500±1100 | | VX |
2900±800 |
3700±800 |
4500±2200 | |
cryptal enterocytes | | control |
300±100 |
500±100 |
1300±600 | | SOMAN |
1000±200* |
1600±400* |
2200±700* | | control |
1200±200 |
1100±300 |
1200±300 | | VX |
1200±400 |
1600±500 |
900±200 |
symbols as in Table 5
Table 7. Average IOD values of phospho-ATF-2Thr-69/71 per microscopic field ± 2 x S.E.M.
|
Integral optical density
(IOD) | | time (h) |
4 |
24 |
72 | |
apical enterocytes | | control |
300±200 |
500±100 |
400±200 | | SOMAN |
400±200 |
700±200 |
500±100 | | control |
1300±300 |
1600±600 |
2600±1000 | | VX |
2000±1000 |
2800±1200 |
2000±700 | |
cryptal enterocytes | | control |
200±100 |
300±100 |
600±200 | | SOMAN |
300±100 |
300±100 |
700±200 | | control |
2900±700 |
3000±900 |
2200±400 | | VX |
2700±600 |
3100±1100 |
2700±600 |
Table 8. Immunoreactive score of protein expression and protein activation after nerve agent poisoning ± 2 x S.E.M.
|
4 h |
24 h |
72 h |
4 h |
24 h |
72 h | |
apical enterocytes |
cryptal enterocytes | | phospho-p53 |
control |
1.6±0.5 |
2.1±0.7 |
1.6±0.6 |
0.0±0.0 |
0.0±0.0 |
0.0±0.0 | |
soman |
2.2±0.4 |
1.8±0.5 |
2.6±0.8 |
0.0±0.0 |
0.0±0.0 |
0.0±0.0 | |
control |
3.0±1.2 |
2.4±0.8 |
4.7±1.2 |
0.0±0.0 |
0.0±0.0 |
0.0±0.0 | |
VX |
2.3±0.7 |
2.7±1.1 |
2.8±1.2 |
0.0±0.0 |
0.0±0.0 |
0.0±0.0 | | p21 |
control |
3.9±0.9 |
4.4±1.5 |
3.3±1.0 |
0.0±0.0 |
0.0±0.0 |
0.0±0.0 | |
soman |
4.0±1.0 |
4.1±1.5 |
4.3±1.4 |
0.0±0.0 |
0.0±0.0 |
0.0±0.0 | |
control |
2.3±1.2 |
3.3±1.2 |
3.9±0.8 |
0.0±0.0 |
0.0±0.0 |
0.0±0.0 | |
VX |
2.6±0.8 |
3.4±1.4 |
3.8±1.1 |
0.0±0.0 |
0.0±0.0 |
0.0±0.0 | | phospho-Elk-1 |
control |
11.6±0.3 |
11.8±0.4 |
12.0±0.0 |
11.9±0.2 |
11.6±0.4 |
11.9±0.0 | |
soman |
11.9±0.2 |
11.6±0.4 |
12.0±0.0 |
9.1±1.1* |
11.0±0.3 |
11.3±0.5 | |
control |
11.6±0.5 |
11.8±0.2 |
11.4±0.6 |
11.5±0.7 |
11.3±0.6 |
11.6±0.4 | |
VX |
11.5±0.6 |
11.0±0.7 |
11.3±0.6 |
11.4±0.8 |
10.9±0.7 |
11.3±0.6 | | phospho-CREB |
control |
2.9±0.6 |
3.1±0.7 |
3.8±1.2 |
2.6±0.8 |
2.3±1.0 |
2.0±0.9 | |
soman |
2.6±0.5 |
4.0±1.4 |
6.1±2.2* |
3.7±0.8 |
4.8±1.2 |
2.4±0.9 | |
control |
4.1±1.0 |
3.3±1.1 |
4.6±0.9 |
4.1±0.9 |
3.7±1.1 |
3.7±1.0 | |
VX |
4.6±1.6 |
4.8±1.2 |
4.3±1.6 |
4.0±0.8 |
4.1±0.9 |
3.3±0.6 | | phospho-ATF-2 |
control |
2.2±0.3 |
3.9±1.4 |
2.1±0.7 |
1.9±0.6 |
3.1±1.1 |
2.8±0.9 | |
soman |
2.9±0.5 |
3.0±0.4 |
3.0±1.2 |
2.6±0.6 |
3.4±0.9 |
3.3±1.7 | |
control |
1.8±0.4 |
1.9±0.8 |
3.4±0.7 |
3.6±0.6 |
3.4±1.1 |
3.1±0.5 | |
VX |
2.4±1.6 |
2.5±0.6 |
2.2±1.0 |
3.2±0.7 |
3.1±0.4 |
3.2±0.7 |
symbols as in Table 5
DISCUSSION
The in vivo model of gastrointestinal epithelium
provides a unique tool for assessing the effect of
soman and VX poisoning on undifferentiated and
proliferating cells localized at the base of the crypts
and on differentiated cells at the inner intestinal
surface. To evaluate the non-specific effects of nerve
agents on rat enterocytes, we focused on two groups
of proteins. The first group consists of two proteins -
cell cycle cyclin-dependent kinase inhibitor p21 and
its activated regulator p53, which is involved in the
pathogenesis of soman-induced cerebral damage
(Baille et al. 2005). The second group of proteins is
formed by Elk-1, CREB, and ATF-2, which are
regulated by MAPK signalling cascades. P38 MAPK
pathway participates in the regulation of the soman
response in the rat cerebelum (Pejchal et al. 2008,
2009).
The first group of proteins - p21 and activated
p53 - did not show any significant changes after
soman and VX poisoning. In the control as well as in
the soman and VX poisoned animals, we observed
slight p21 and phospho-p53Ser-15 positivity along
apical enterocytes and negative p21 and
phospho-p53Ser-15 expression in crypts. Both proteins
play an important role in processes such as intestinal
differentiation or regulation of intestinal homeostatis
in response to strong oxidative stressors such as
ionising radiation (Wilson et al. 1998, Tian and
Quaroni 1999). According to our results, these
processess do not seem to be affected by either nerve
agent in rat intestinal cells. It is likely that neither
nerve agent generates oxidative stress leading to
activation of p53/p21 signalling in enterocytes. This
is in contrast to Baille et al. (2005) who described
soman-induced expression of p53 in cerebral tissue.
Nevertheless, Baille et al. (2005) observed increased
p53 expression in the nuclei of injured or dying cells
which correlated with the duration of seizures and,
therefore, the difference between their study and ours
could be explained by the excitotoxicity of the
neurones and a higher susceptibility of cerebral cells
to hypoxia during convulsions (Morrison and
Kinoshita 2000, Guo et al. 2008).
In comparison with p21 and phospho-p53
expression, MAPK-regulated transcription factors
showed different activation patterns when the soman
or VX agent was applied. According to our results,
soman modulates the activation of Elk-1 and CREB,
while both proteins are unaffected by the VX agent.
The soman data are also supported by the findings
from other laboratories that organophosphorus agents
DFP and chlorpyrifos alter phosphorylation of CREB
(Schuh et al. 2002, Damodaran et al. 2009). Since the
symptoms of nerve agent intoxication were similarly
expressed in both groups, it is likely that the effect of
soman on Elk-1 and CREB activation is not related to
the basic mechanism of nerve agent-induced acute
toxicity (irreversible AChE inhibition). There are two
possible explanations. Either the effect is concen-tration-dependent and the dose of VX was too low to
affect Elk-1 and CREB activation or soman possesses
an additional mechanism through which it modulates
the biological outcome of poisoning.
As with the mechanism of the soman-induced
non-specific effect, its biological outcome is
uncertain. The transcription factor Elk-1 is a target of
ERK1/2 kinase (Kyriakis and Avruch 2001). In
enterocytes, ERK1/2 signalling supports cell survival
and it is required for S-phase entry and proliferation
(Rivard et al. 1999, Gauthier et al. 2001, Boucher et
al. 2004), in which Elk-1 may participate via
transcriptional regulation of growth stimulating
factors such as cyclin D1 (Shin et al. 2003). In
addition, in vitro experiments have shown that ERK
inhibition induced by cell-cell contacts could be a
critical step in initiating G1 cell cycle arrest and
induction of differentiation (Aliaga et al. 1999,
Laprise et al. 2004). Interestingly, we observed a
rather diffuse phospho-Elk-1 pattern in the crypts and
compartmentalization of phospho-Elk-1 into the
supranuclear cytoplasmatic space at the crypt-villus
junction and superficial enterocytes (Figs 1 and 2) in
both control and soman-poisoned animals. This
suggests that the relocation of phospho-Elk-1 into the
cytoplasm might be a regulatory mechanism
contributing to cell cycle arrest, differentiation and
maturation of enterocytes. Since soman poisoning
does not affect the Elk-1 activation pattern in
superficial enterocytes, the finding may support the
fact that soman does not influence the enterocyte
differentiation process based on p21 data. In contrast,
decreased phosphorylation of Elk-1 in crypts
measured 4 and 24 hours after the soman poisoning
indicates that soman temporarily downregulates
enterocyte proliferation at the regulatory protein level.
Phosphorylation of CREB at serine 133 is
regulated by ERK and p38 kinase pathways and other
kinases such as protein kinases A (Kyriakis and
Avruch 2001). In vitro experiments conducted on
enterocyte-derived cell lines implicate CREB in the
regulation of cell differentiation as well as survival
and proliferation (Paruchuri and Sjolander 2003, Lee
et al. 2010). Low control levels of activated CREB
may therefore maintain mitotic activity in crypts and
the differentiation process in the apical compartment.
Consequently, increased CREB phosphorylation
observed 4 and 24 hours after soman poisoning might
serve as a compensatory action for decreased Elk-1 activity in cryptal enterocytes. Nevertheless, this
hypothesis does not explain the increased activation
of CREB in both intestinal compartments 72 hours
after soman intoxication. A more likely explanation
associates the delayed CREB phosphorylation in
crypts and its shift to apical enterocytes correlating
with 3-5 day cycle of complete mucosal exchange
(Laprise et al. 2004) with the presence of DNA
damage. In vitro, soman is capable of rapid binding to
DNA and interference with DNA metabolism
(Ivanovic et al. 1985, Klein et al. 1987). Thus, we
argue that soman binds to DNA and forms DNA
adducts in vivo. According to our results, the amount
of DNA damage is not so extensive as to activate p53
and increase p21 expression but it seems to be
sufficient enough to impair the DNA replication
process, which leads to downregulation of
phospho-Elk-1 expression and activation of DNA
repair mechanisms, in which CREB may participate
via regulation of genes involved in the base and
nucleotide excision repair systems (Grosch and Kaina
1999, Lemee et al. 2007).
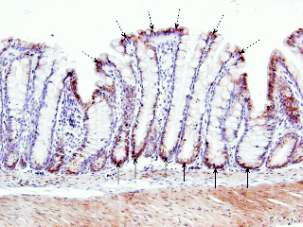
Fig. 1. Sample of control (saline, 4 hours after administration) rat colon transversum with immunohistochemical detection
of phospho-Elk-1Ser-383 at 200-fold magnification. In apical enterocytes (dashed arrows), phospho-Elk-1Ser-383
assumed a
supranuclear cytoplasmatic pattern, while in crypts (solid arrows), we observed a rather diffuse pattern. For publication purposes,
samples were counterstained with Harris heamatoxylin.
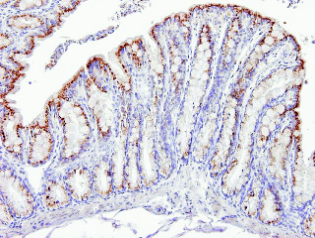
Fig. 2. Sample of soman-poisoned (single dose of 70 % LD50) rat colon transversum at 200-fold original magnification
4 hours after the poisoning. Immunohistochemical detection did not show any change of phospho-Elk-1Ser-383 pattern in apical
enterocytes but we observed significantly decreased phospho-Elk-1Ser-383 positivity in crypts. For publication purposes, samples
were counterstained with Harris heamatoxylin.
It is also noteworthy that changes in MAPK
signalling observed in rat colon transversum after
soman poisoning precede the delayed alteration of
MAPK cascades in the cerebellar tissue (Pejchal et al.
2008, 2009). Alteration of MAPK is associated with
many neurodegenerative diseases (Miloso et al. 2008)
and may play an important role in delayed toxicity of
nerve agents in CNS (Pejchal et al. 2008, 2009). From
this point of view, the detection of activated
transcription factors in enterocytes early after soman
poisoning may have a prognostic value. A
disadvantage is the invasive nature required for
accessing gut enterocytes. Therefore, blood or more
accessible proliferating cell lines such as skin
keratinocytes or epithelial cells from cavum oris
should be used. However, this suggestion has to be
verified by further experiments detecting the
prognostic markers in other tissues.
ACKNOWLEDGEMENTS
We would like to thank Mrs. Sarka Pruchova for her
skillful technical assistance. This work was supported
by the Ministry of Defence of the Czech Republic
through grant MO0FVZ0000501 (institutional
support No. 9079301306023) and grant
OVUOFVZ200812 - RADSPEC.
REFERENCES
Aliaga JC, Deschenes C, Beaulieu JF, Calvo EL, Rivard N. Requirement of the MAP kinase cascade for cell cycle progression and differentiation of
human intestinal cells. Am J Physiol. 277: 631-641, 1999.
[PubMed]
Baille V, Clarke PG, Brochier G, Dorandeu F, Verna JM, Four E, Lallement G, Carpentier P. Soman-induced convulsions: the neuropathology revisited.
Toxicology. 215: 1-24, 2005.
[CrossRef]
[PubMed]
Bajgar J. Organophosphate/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem. 38: 151-216, 2004.
[CrossRef]
Bajgar J, Hajek P, Zdarova JK, Kassa J, Paseka A, Slizova D, Krs O, Kuca K, Jun D, Fusek J, Capek L. A comparison of tabun-inhibited rat brain
acetylcholinesterase reactivation by three oximes (HI-6, obidoxime, and K048) in vivo detected by biochemical and histochemical techniques. J
Enzyme Inhib Med Chem. 25: 790-797, 2010.
[CrossRef]
[PubMed]
Boucher MJ, Jean D, Vezina A, Rivard N. Dual role of MEK/ERK signaling in senescence and transformation of intestinal epithelial cells. Am J Physiol
Gastrointest Liver Physiol. 286: 736-746, 2004.
[CrossRef]
[PubMed]
Damodaran TV, Gupta RP, Attia MK, Abou-Donia MB. DFP initiated early alterations of PKA/p-CREB pathway and differential persistence of beta-tubulin
subtypes in the CNS of hens contributes to OPIDN. Toxicol Appl Pharmacol. 240: 132-142, 2009.
[CrossRef]
[PubMed]
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53
tumor suppression. Cell. 75: 817-825, 1993.
[CrossRef]
Gauthier R, Harnois C, Drolet JF, Reed JC, Vezina A, Vachon PH. Human intestinal epithelial cell survival: differentiation state-specific control
mechanisms. Am J Physiol Cell Physiol. 280: 1540-1554, 2001.
[PubMed]
Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 12: 2973-2983, 1998.
[CrossRef]
Grosch S, Kaina B. Transcriptional activation of apurinic/apyrimidinic endonuclease (Ape, Ref-1) by oxidative stress requires CREB. Biochem Biophys
Res Commun. 261: 859-863, 1999.
[CrossRef]
[PubMed]
Guo Y, Korteweg C, McNutt MA, Gu J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 133: 4-12, 2008.
[CrossRef]
[PubMed]
Ivanovic V, Rapic V, Boskovic B. Pinacolyl methylphosphonochloridate: in vitro covalent binding to DNA and mutagenicity in the Ames test.
Mutat Res. 142: 9-12, 1985.
[CrossRef]
Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 298: 1911-1912, 2002.
[CrossRef]
[PubMed]
Jung YS, Qian Y, Chen X. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signal. 22: 1003-1012,
2010.
[CrossRef]
[PubMed]
Kassa J, Skopec F, Vachek J. The long term changes in liver DNA and total protein contents following low level sarin exposure in rats. Acta Medica
(Hradec Kralove). 43: 19-22, 2000.
[PubMed]
Klaidman LK, Adams JD, Jr., Cross R, Pazdernik TL, Samson F. Alterations in brain glutathione homeostasis induced by the nerve gas soman. Neurotox
Res. 5: 177-182, 2003.
[CrossRef]
[PubMed]
Klein AK, Nasr ML, Goldman M. The effects of in vitro exposure to the neurotoxins sarin (GB) and soman (GD) on unscheduled DNA synthesis by
rat hepatocytes. Toxicol Lett. 38: 239-249, 1987.
[CrossRef]
Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 81:
807-869, 2001.
[PubMed]
Lakin ND, Jackson SP. Regulation of p53 in response to DNA damage. Oncogene. 18: 7644-7655, 1999.
[CrossRef]
[PubMed]
Laprise P, Langlois MJ, Boucher MJ, Jobin C, Rivard N. Down-regulation of MEK/ERK signaling by E-cadherin-dependent PI3K/Akt pathway in
differentiating intestinal epithelial cells. J Cell Physiol. 199: 32-39, 2004.
[CrossRef]
[PubMed]
Lee HY, Crawley S, Hokari R, Kwon S, Kim YS. Bile acid regulates MUC2 transcription in colon cancer cells via positive EGFR/PKC/Ras/ERK/CREB,
PI3K/Akt/IkappaB/NF-kappaB and p38/MSK1/CREB pathways and negative JNK/c-Jun/AP-1 pathway. Int J Oncol. 36: 941-953, 2010.
[PubMed]
Lemee F, Bavoux C, Pillaire MJ, Bieth A, Machado CR, Pena SD, Guimbaud R, Selves J, Hoffmann JS, Cazaux C. Characterization of promoter regulatory
elements involved in downexpression of the DNA polymerase kappa in colorectal cancer. Oncogene. 26: 3387-3394, 2007.
[CrossRef]
[PubMed]
Marrs TC. Organophosphate poisoning. Pharmacol Ther. 58: 51-66, 1993.
[CrossRef]
Miloso M, Scuteri A, Foudah D, Tredici G. MAPKs as mediators of cell fate determination: an approach to neurodegenerative diseases. Curr Med Chem.
15: 538-548, 2008.
[CrossRef]
[PubMed]
Morrison RS, Kinoshita Y. The role of p53 in neuronal cell death. Cell Death Differ. 10: 868-879, 2000.
[CrossRef]
[PubMed]
Paruchuri S, Sjolander A. Leukotriene D4 mediates survival and proliferation via separate but parallel pathways in the human intestinal epithelial
cell line Int 407. J Biol Chem. 278: 45577-45585, 2003.
[CrossRef]
[PubMed]
Pazdernik TL, Emerson MR, Cross R, Nelson SR, Samson FE. Soman-induced seizures: limbic activity, oxidative stress and neuroprotective proteins. J
Appl Toxicol. 21: 87-94, 2001.
[CrossRef]
[PubMed]
Pejchal J, Osterreicher J, Kassa J, Tichy A, Mokry J. Activation of mitogen activated protein kinase (MAPK) pathways after soman poisoning in rat
cerebellar granule neurons. J Appl Toxicol. 28: 689-693, 2008.
[CrossRef]
[PubMed]
Pejchal J, Osterreicher J, Kassa J, Tichy A, Micuda S, Sinkorova Z, Zarybnicka L. Soman poisoning alters p38 MAPK pathway in rat cerebellar Purkinje
cells. J Appl Toxicol. 29: 338-345, 2009.
[CrossRef]
[PubMed]
Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection
(ER-ICA) in breast cancer tissue. Pathologe. 8: 138-140, 1987.
[PubMed]
Rivard N, Boucher MJ, Asselin C, L'Allemain G. MAP kinase cascade is required for p27 downregulation and S phase entry in fibroblasts and epithelial
cells. Am J Physiol. 277: 652-664, 1999.
[PubMed]
Schuh RA, Lein PJ, Beckles RA, Jett DA. Noncholinesterase mechanisms of chlorpyrifos neurotoxicity: altered phosphorylation of Ca2+/cAMP
response element binding protein in cultured neurons. Toxicol Appl Pharmacol. 182: 176-185, 2002.
[CrossRef]
[PubMed]
Shin HS, Lee HJ, Nishida M, Lee MS, Tamura R, Yamashita S, Matsuzawa Y, Lee IK, Koh GY. Betacellulin and amphiregulin induce upregulation of cyclin
D1 and DNA synthesis activity through differential signaling pathways in vascular smooth muscle cells. Circ Res. 93: 302-310, 2003.
[CrossRef]
[PubMed]
Steinboeck F, Hubmann M, Bogusch A, Dorninger P, Lengheimer T, Heidenreich E. The relevance of oxidative stress and cytotoxic DNA lesions for
spontaneous mutagenesis in non-replicating yeast cells. Mutat Res. 688: 47-52, 2010.
[CrossRef]
[PubMed]
Takekawa M, Adachi M, Nakahata A, Nakayama I, Itoh F, Tsukuda H, Taya Y, Imai K. p53-inducible wip1 phosphatase mediates a negative feedback
regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J. 19: 6517-6526, 2000.
[CrossRef]
[PubMed]
Tian JQ, Quaroni A. Involvement of p21(WAF1/Cip1) and p27(Kip1) in intestinal epithelial cell differentiation. Am J Physiol. 276: 1245-1258,
1999.
[PubMed]
Wilson JW, Pritchard DM, Hickman JA, Potten CS. Radiation-induced p53 and p21WAF-1/CIP1 expression in the murine intestinal epithelium: apoptosis and
cell cycle arrest. Am J Pathol. 153: 899-909, 1998.
[CrossRef]
Wittlinger M, Grabenbauer GG, Sprung CN, Sauer R, Distel LV. Time and dose-dependent activation of p53 serine 15 phosphorylation among cell lines
with different radiation sensitivity. Int J Radiat Biol. 83: 245-257, 2007.
[CrossRef]
[PubMed]
|
BACK
|



