Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 1, p 29-34
DOI 10.2478/v10136-011-0019-y
The role of the light/dark cycle in the daily rhythm of serum proteins in Equus caballus
Giuseppe Piccione, Simona Marafioti, Claudia Giannetto, Caterina Faggio, Michele Panzera, Francesco Fazio
Address: Giuseppe Piccione, Department of Experimental Sciences and Applied Biotechnology, Faculty of Veterinary Medicine, University of Messina,
Polo Universitario dell'Annunziata, 98168, Messina, Italy
giuseppe.piccione@unime.it
Received 23rd May 2011.
Revised 2nd September 2011.
Published online 3rd November 2011.
Full text article (pdf)
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
References
SUMMARY
This research was carried out on five clinically healthy Sella Italiana horses to determine the daily rhythm of total proteins and their fractions,
to establish if these rhythms are endogenously generated and to assess the role of light as synchroniser of these rhythms. Blood samples were
collected from each subject every 3 h over a period of 48 h, starting at 9:00 on day 1 and finishing at 9:00 on day 3, into vacutainer tubes
without an anticoagulant via intravenous cannulas inserted into the jugular vein. Total serum proteins, albumin, alpha1-,
alpha2-, beta1-, beta2- and gamma- globulin concentrations were assessed in all samples. The application of
two-way ANOVA showed a significant effect of the time of day on total proteins, albumin, beta1- and beta2- globulins, and of
the experimental conditions on total proteins alpha1-, alpha2-, beta1-, beta2- and gamma- globulins. No
statistical modifications were observed on the A/G ratio. Daily rhythmicity was exhibited only by total proteins and albumin during the L/D cycle.
We can claim that the fluctuation of serum total proteins and albumin concentrations are daily and not circadian and that they are driven by the
L/D cycle.
KEY WORDS
total proteins; electrophoresis profile; daily rhythm; horse; photoperiod
INTRODUCTION
In clinical medicine the protein status of the organism
is usually investigated by the assessment of
serum/plasma total proteins. Total proteins are
constituted by various fractions that are different
among species. The two major fractions are albumin
and globulins. Albumin is the most osmotically active serum protein used by many substances as principal
carrier, and globulins are a heterogeneous group of
proteins including vitamins, hormones, carriers of
lipids, antibodies, and inflammatory, haemostatic and
fibrinolytic molecules (Alberghina et al. 2010).
In physiological conditions total proteins levels
could be influenced by many factors, such as age,
body weight, hormones, pregnancy, lactation, oestrus
condition, change in ambient temperature, or the
nutritive state of the animal (Batavani et al. 2006).
Moreover in mammals, a multitude of pathological
conditions can cause changes in albumin and globulin
concentrations. In horses, changes in concentrations
of total proteins and their fractions could be attributed
to poor performance, depression, fever, weight loss,
diarrhoea, abdominal pain, and polyuria (Colhan et al.
1999).
Serum protein electrophoresis is the standard
method of evaluating the concentrations of various
protein fractions. As electrophoretic mobility differs
among species, it has been essential to determine the
"normal" electrophoretic pattern of each species in
order to develop reference intervals that are used to
identify abnormalities in patients.
One important factor usually ignored in the
conventional "normal range" is the time of a
biomedical measurement in relation to biological
rhythms. In some cases, reference intervals
determined irrespective of time are much too broad
and indiscriminate. A "normal" value in conventional
terms might be a false positive or a false negative,
depending on when the measurement was performed
in relation to a rhythm in the variable concerned.
Alteration of one or several rhythm characteristics
may reveal and quantify vulnerability or risk, prior to
the occurrence of a given pre-disease or disease. So,
the analysis of rhythm can show significant changes
in parameters at determined times and it can give new
information suggesting pre-symptomatic conditions
(Tarquini et al. 1993). It is well known that
haematological characteristics must be compared to
the non-pathological reference values of the
appropriate time of day, while several characteristics
seem to be stable during the day since their circadian
variations are not detectable or do not have any
clinical significance (Berger 2004). For example,
knowledge of the daily rhythm of serum creatinine in
the dog allows us to predict progressive renal disease
and the drugs treatment that could alter the kidney
function (Giudice et al. 2009). In the same way, a
better understanding of the circadian rhythm of the
physiological functions of the eye such as tear
production or intraocular pressure, could be useful in
diagnosing a deficiency of the lachrymal system,
corneal inflammation or damage that may lead to
blindness (Piccione et al. 2008, 2009, Giannetto et al.
2009a, b). The circadian rhythms are most often
described in terms of their phases and amplitudes, and
how these respond, in both health and desease, to a
single exposure to synchronisers (Berger 2008).
In literature little information is available about
the circadian rhythm of total proteins and their
fractions. The circadian variations of total protein,
albumin, 2 and gamma-globulins have been observed in
adult rats (Valli et al. 1979) but in horses and sheep,
only the daily variation of total protein and albumin
has been investigated (Piccione et al. 2005a). A
different photoperiod has been observed to influence
the values of total protein, albumin and alpha2-globulins,
whereas it has been suggested that light is not the
most important factor affecting alpha1- beta and gamma-globulins
in the common vole (Dobrowolska and Gromadzka-Ostrowska 1983). On the other hand, seasonal
changes in total serum proteins and their fractions in
horses, have been connected with the changes of light
and temperature throughout the year (Gill et al. 1985).
Because of the important role that serum proteins
plays in the organism, it is undoubtedly useful to
know their circadian rhythm; primarily, because they
can affect the interpretation of the concentrations of
the bound and unbound fractions of a physiological
and pharmacological agent; and secondly because
they should be taken into account in designing a
therapeutic protocol that optimizes the tolerance and
expected effects of drugs and diminishes their side
effects.
The aim of this study was to investigate the daily
rhythm of total proteins and their fractions in horses
in order to establish if these rhythms are
endogenously generated and to assess the role of
light/dark cycle on these parameters.
MATERIALS AND METHODS
Five clinically healthy and regularly trained Italian
Saddle horses (females, 6-10 years old, 530±20 kg
body weight) were used in our study carried out in
Messina, Italy (Latitude: 38°, 26' Longitude: 15°, 59').
All the horses were subjected to the same
management regime and were housed in individual
boxes (3.00 x 3.00 meters, equipped with big
windows). Water was available ad libitum and hay
and oats were provided 3 times a day (6:00; 12:00;
18:00). The animals were subjected to natural 13/11
Light/Dark (L/D) cycle (sunrise at 06:20 h, sunset at
19:20 h) and constant darkness (D/D). The D/D
regime was the same as in the L/D cycle. Thermal and
hygrometric records were carried out inside the box
for the whole study by means of a data logger
(Gemini, UK).
Blood samples (10 ml) were collected every 3 h
over a period of 48 h, starting at 9:00 on day 1 and
finishing at 9:00 on day 3, in vacutainer tubes without
an anticoagulant (Terumo Corporation, Japan) via
intravenous cannulas inserted into the jugular vein
(MILA International, Florence, KY). The tubes were
kept at room temperature for 20 min, then centrifuged
at 3.000 rpm for 10 min and the serum obtained was
stored at -25 °C until analysed. The concentration of
serum total proteins was determined by the biuret
method using an automated analyser UV
Spectrophotometer (SEAC, Slim, Florence, Italy).
The protein fractions were performed using an
automated system (Sel Vet 24, SELEO Enginering,
Naples, Italy) according to the procedures described
by the manufacturer. For each sample, 25 microl of serum
were applied to numbered sample wells. Each holder
accommodated up to 24 samples. The films were
electrophoresed for 28 minutes at 450 V. After
electrophoresis, the films were simultaneously fixed
using an automated system, stained in red stain acid
solution for 10 minutes, and then dried at 37 °C. After
destaining in acetic acid and drying completely for
15 minutes the films were scanned on a densitometer,
and electrophoretic curves plus related quantitative
specific protein concentrations for each sample were
displayed. Relative protein concentrations within each
fraction were determined as the optical absorbance
percentage, and absolute concentrations (g/l) were
calculated using the total protein concentration. The
major protein fractions were divided, according to the
recommendation by the manufacturer, from cathode
to anode as albumin alpha1, alpha2, beta1, beta2, gamma-globulins,
respectively. All housing and care conformed to the
standard recommended by the Guide for the Care and
Use of Laboratory Animals and Directive 86/609
CEE.
All results were expressed as mean ± standard
deviation (SD). Two-way repeated measures analysis
of variance (ANOVA) was used to determine
significant differences due to the time of day and
experimental conditions on all parameters studied at
the significant level 2alpha=0.05. The data were analysed
using the STATISTICA 8 (Stat Soft Inc., Tulsa, USA)
software. Using cosinor rhythmometry (Nelson et al.
1979), four rhythmic parameters were determined:
mesor (mean level), amplitude (half of the range of
oscillation), acrophase (time of peak), and robustness
(a stationary rhythm). The robustness of the rhythms
was computed as the quotient of the variance
associated with sinusoidal rhythmicity and the total
variance of the time series (Refinetti 2004).
Robustness greater than 40% is above noise level and
indicates statistically significant rhythmicity.
RESULTS
During the experimental period, minimum and ma-ximum temperatures were 21 and 26 °C respectively,
and the relative humidity was 65%. The application of
two-way ANOVA showed a significant effect of the
time of day on total proteins, albumin, beta1- and beta2-
globulins and of experimental conditions on total
proteins, alpha1-, alpha2-, beta1-, beta2- and gamma-globulins (Table 1).
No statistical modifications were observed on A/G
ratio.
The application of the periodic model and the
statistical analysis of cosinor enabled us to define the
periodic parameters and their acrophases during the
24 h of monitoring for both photoperiods.
Daily rhythmicity was exhibited only by total
proteins and albumin during the L/D cycle. These
rhythms are characterized by diurnal acrophases
(16:28 and 17:20 respectively), amplitudes of rhythms
of 0.50 for total proteins and 0.22 for albumin, and
the robustness of rhythms were 48.2% for total
proteins and 70% for albumin. Figs 1-2 show a mean
pattern and mean values of four rhythmic parameters
of serum total protein and albumin recorded during
the experimental period.
DISCUSSION
Serum total proteins and their fraction in all the data
points tested were within the physiological range
suggested for horses (Kaneko et al. 1997), and all
serum electrophoreses were characterized by the
absence of a pre-albumin region and by six different
bands: albumin, alpha1, alpha2, beta1, beta2, gamma-globulins (Kohn
1957).
In contrast to the observations of Flisinska-
Bojanowska et al. (1991), in which study serum total
proteins showed no daily rhythmicity, but only small
and insignificant changes during the day, our results
reported a daily rhythmicity of serum total proteins
and albumin during the L/D cycle.
The time series studied showed a central tendency
of oscillation (mean level) of 64.9 and 33.5 for total
protein and albumin respectively, that represent the
point of balance of distribution of the studied
parameters. The range of oscillation, which is twice
this amplitude, identifies the boundaries of the
oscillation. Therefore the amplitude of circadian
rhythms are not necessary symmetrical so that the
amplitude below the mean level may be different
from the amplitude above the mean level. This
condition is evident in Figs 1-2. The total protein
range of oscillation is higher above the mean level
than below. In contrast, the range of oscillation of the
albumin is higher below the mean level than above.
Both rhythms were diurnal (Figs 1-2), their
acrophases were similar, and were observed at 16:28
and 17:20 respectively for total proteins and albumin.
This finding is challenged by a previous study
conducted by Piccione et al. (2005a) in which
nocturnal acrophases at the beginning of day (04:32)
for total proteins and at the end of day (20:30) for
albumin were observed. This finding also underlines
the importance of exogenous factors - primarily the L/D cycle - and suggests that their circadian pattern
is under L/D control, as previously observed on
haematological parameters in this species (Piccione et
al. 2005b), and excludes the influence of protein
levels of the diet on daily rhythms (Greppi et al.
1996).
Table 1. Serum total protein, protein fractions and A/G ratio recording during the experimental period.
| Parameters (g/l) |
Day 1 (L/D cycle) M±SEM |
Day 2 (D/D cycle) M±SEM | | Total proteins |
64.8±1.49 |
65.9 ± 0.79 | | Albumin |
33.4±0.60 |
33.8±0.27 | | alpha1-globulins |
1.98±0.09 |
1.72±0.13 | | alpha2-globulins |
8.10±0.25 |
7.28±0.17 | | beta1-globulins |
6.78±0.40 |
7.91±0.27 | | beta2-globulins |
5.23±0.31 |
4.25±0.20 | | gamma-globulins |
9.32±0.37 |
10.78±0.34 | | A/G ratio |
1.88±0.30 |
10.81±0.31 |
M = mean values, SEM = standard error of the mean
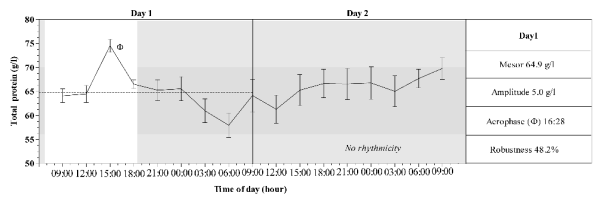
Fig. 1. Mean pattern of serum total protein recorded during day 1 (L/D) and 2 (D/D) in horses. Grey area represent the dark
phase of the photoperiod. Dashed line indicates the Mesor value and phi indicates the acrophase observed in day 1.
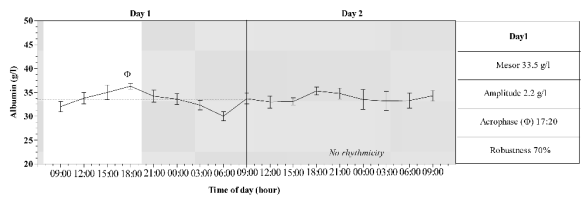
Fig. 2. Mean pattern of serum albumin recorded during day 1 (L/D) and 2 (D/D) in horses. Grey area represent the dark
phase of the photoperiod. Dashed line indicates the Mesor value and phi indicates the acrophase observed in day 1.
Even if the albumin daily rhythm was more robust
than the serum total proteins daily rhythm, the
influence of the first on the second rhythm is probably
not linked to the concept that a rhythm with low
robustness cannot be the cause of a rhythm with high
robustness (Piccione et al. 2005a), but to the fact that
the albumin constitutes about 50% of the serum total
protein has a high impact on the serum total protein
daily rhythm. Constant darkness is considered a "free
running" circadian state. In the absence of temporal
environmental cues the rhythm persists with a period
approximately 24-hours. In this case the rhythm is
defined as circadian. When the rhythm cycle is in
24 hour intervals and is not endogenously generated
but susceptible by 24 hour environmental cycles it is
called daily. Therefore, we can claim that the
fluctuation of serum total proteins and albumin
concentrations are daily and peak during the day and
decrease in the morning during the natural L/D cycle.
The results support the hypothesis that these rhythms
are driven by an L/D cycle, considering that the other
experimental conditions were constant through the
study. Single samples from individuals are of little
value for monitoring changes in total proteins and
albumin due to pathological conditions; a series of
measurements should be taken over a period of time,
or samples should be collected at precise times for
results to be meaningful. However, further studies are
necessary to better understand the roles of light as a
synchroniser of these rhythms.
REFERENCES
Alberghina D, Casella S, Vazzana I, Ferrantelli V, Giannetto C, Piccione G. Analysis of serum proteins in clinically healthy goats (Capra
hircus) using agarose gel electrophoresis. Vet Clin Pathol. 39: 317-321, 2010.
[CrossRef]
[PubMed]
Batavani RA, Ansari MH, Asri S. Concentrations of serum total protein and protein fractions during diestrus and pregnancy in Makuii ewes. Comp Clin
Pathol. 15: 227-230, 2006.
[CrossRef]
Berger J. Chronohaematology. J Appl Biomed. 2: 179-185, 2004.
[JAB]
Berger J. Advances in chronohaematology. J Appl Biomed. 6: 65-72, 2008.
[JAB]
Colhan PT, Merrit AM, Moore JN, Mayhew G. Equine Medicine and Surgery. Vol II, 5th ed. St Louis, MO: Mosby Inc. 1999, pp.
1987-1989.
Dobrowolska A, Gromadzka-Ostrowska J. Influence of photoperiod on morphological parameters, androgen concentration, haematological indices and serum
protein fractions in common vole (Microtus arvalis, Pall.). Comp Biochem Physiol A. 74: 427-433, 1983.
[CrossRef]
Flisinska-Bojanowska A, Komosa M, Gill J. Influence of pregnancy on diurnal and seasonal changes in cortisol, T3 and T4 levels
in the mare blood serum. Comp Biochem Physiol A. 98: 23-30, 1991.
[CrossRef]
Giannetto C, Assenza A, Fazio F, Casella S, Piccione G. Circadian intraocular pressure and tear production in horse. Arch Vet Ital. 60: 47-52,
2009a.
Giannetto C, Piccione G, Giudice E. Daytime profile of the intraocular pressure and tear production in normal dog. Vet Ophtal. 12: 302-305,
2009b.
[CrossRef]
[PubMed]
Gill J, Jakubow K, Kompanowska-Jezierska E, Kott A, Szumska D. Seasonal changes in blood serum protein factions and in activity of AspAT and AlAT in
Arabian brood mares and their foals. Comp Biochem Physiol A. 82: 167-178, 1985.
[CrossRef]
Giudice E, Giannetto C, Fazio F, Piccione G. Daily rhythm of creatinine in dog: clinical and diagnostic significance. Biol Rhythm Res. 40: 181-187,
2009.
[CrossRef]
Greppi GF, Casini L, Gatta D, Orlandi M, Pasquini M. Daily fluctuations of haematology and blood biochemistry in horses fed varying levels of
protein. Equine Vet J. 28: 350-353, 1996.
[CrossRef]
[PubMed]
Kaneko JJ, Harvey JW, Bruss ML. Serum proteins and the dysproteinemias. In: Clinical Biochemistry of Domestic Animals, 5th ed. (Kaneko JJ
(ed.). Academic Press. San Diego, pp. 117-138, 1997.
[CrossRef]
Kohn J. A cellulose acetate supporting medium for zone electrophoresis. Clin Chim Acta. 2: 297-303, 1957.
[CrossRef]
Nelson K, Tong JL, Lee JK, Halberg F. Methods for cosinor rhythmometry. Chronobiologia. 6: 305-323, 1979.
[PubMed]
Piccione G, Caola G, Refinetti R. Temporal relationships of 21 variables in horse and sheep. Comp Biochem Physiol A. 142: 389-396, 2005a.
[CrossRef]
Piccione G, Fazio F, Giudice E, Grasso F, Morgante M. Nycthemeral change of some haematological parameters in horses. J Appl Biomed. 3: 123-128,
2005b.
[JAB]
Piccione G, Giannetto C, Fazio F, Giudice E. Daily rhythm of tear production in normal horse. Vet Ophthal. 1: 57-60, 2008.
[CrossRef]
[PubMed]
Piccione G, Giannetto C, Fazio F, Assenza A, Caola G. Daily rhythms of tear production in normal dog maintained under different light/dark cycles.
Res Vet Sci. 86: 521-524, 2009.
[CrossRef]
[PubMed]
Refinetti R. Non-stationary time series and the robustness of circadian rhythms. J Theor Biol. 227: 571-581, 2004.
[CrossRef]
[PubMed]
Tarquini B, Tarquini R, Perfetto F, Tapparini L, Lombardi A, Pignone A. Chronobiology in epidemiology and preventive medicine. Ann Ist
Super Sanita 29: 559-567, 1993.
[PubMed]
Valli M, Jadot G, Bruguerolle B, Bussiere H, Bouyard P. Circadian variations of the plasma proteins in the adult male rat under natural
synchronisation. J Physiol (Paris). 75: 811-814, 1979.
|
BACK
|



