Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 2, p 63-70
DOI 10.2478/v10136-011-0023-2
Effect of shRNA mediated Smad4 gene silencing on the fibrosis of C2C12 myoblasts
Shiqiu Chen, Jiwu Chen, Shiyi Chen, Hongyun Li, Jia Jiang
Address: Jiwu Chen, Department of Sports Medicine, Huashan Hospital, Fudan University Shanghai Medical College, Shanghai 200040, China
chen6786789@sina.com
Received 29th September 2011.
Revised 1st November 2011.
Published online 12th December 2011.
Full text article (pdf)
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
Acknowledgement
References
SUMMARY
Our present study aimed to investigate the effect of lentiviral-mediated RNAi using short hairpin RNA (shRNA) targeting Smad4 on TGF-beta1 induced fibrosis. shRNAs targeting Smad4 were designed and the most efficient shRNA was screened. This shRNA was introduced into a lentiviral vector which was used to infect C2C12 myoblasts, and then the Smad4 expression was detected. Cells were divided into: C2C12 cells group, TGF-beta1 induction group, transfection group, and transfection after TGF-beta1 induction group. C2C12 myoblasts were transfected with lentivirus carrying Smad4-shRNA and treated with TGF-beta1 to induce the differentiation into myofibroblasts. Fluorescence Real-time-PCR and the western blot assay were employed to detect the expressions of collagen I and alpha-SMA. The results showed that the protein and mRNA expression of Smad4 in the C2C12 cells transfected with Smad4-shRNA1 was significantly reduced when compared with C2C12 before transfection. In the TGF-beta1 induction group, the mRNA expressions of alpha-SMA and collagen I were significantly increased as compared to the C2C12 cells group. In the transfection after TGF-beta1 induction group, the mRNA expressions of alpha-SMA and collagen I were significantly increased compared to the transfection group, and the protein expressions significantly increased, respectively. In the transfection after TGF-beta1 induction group, the mRNA expressions of alpha-SMA and collagen I were significantly decreased compared to the TGF-beta1 induction group, and the protein expressions significantly reduced, respectively. The results indicate that suppression of Smad4 expression can efficiently inhibit the TGF-beta1 induced fibrosis in myoblasts. The findings suggest Smad4 may become a novel target for the treatment of skeletal muscle fibrosis.
KEY WORDS
skeletal muscle injury; fibrosis; transforming growth factor-beta1; C2C12 myoblast
INTRODUCTION
Skeletal muscle injury is one of the most common
sports injuries. However, the natural recovery of
skeletal muscle injury requires a relatively long time,
the quality of skeletal muscle after recovery is
unreliable, and local scars frequently form after
recovery. Scars have biomechanical defects which
may cause a second injury of the skeletal muscle
(Orchard 2001). There is evidence showing that the
local expression of the transforming growth factor-beta1
(TGF-beta1) is significantly increased following skeletal
muscle injury (Jackson et al. 2011). TGF-beta1 can
stimulate the synthesis of extracellular matrix (Cheng
and Grande 2002), and has been found to be a key
cytokine causing the development of fibrosis. Studies
also demonstrate that the inhibition of the TGF-beta1
signalling pathway may also counteract with the
fibrosis of the heart, lung, liver and kidney (Tse et al.
2004, Matsuzaki 2009, Bran et al. 2010). Smad2 and
Smad3 are the downstream substrates of the TGF-beta
receptor. Following activation, TGF-beta1 ineluctably
binds to the Smad4, forming a complex which then
enters the nucleus directly or indirectly inducing cell
differentiation (Li et al. 2010, 2011). We hypothesize
that lentiviral-mediated interference with Smad4-shRNA can significantly inhibit Smad4 expression in
myoblasts and thus the Smad2 and Smad3 can not
enter the nucleus of muscle cells (Kretschmer et al.
2003, Zhu et al. 2004), which then inhibit the TGF-beta1
induced skeletal muscle fibrosis.
MATERIALS AND METHODS
Materials
C2C12 myoblasts were purchased from the Wuhan
Cell Bank. The following reagents were used in the
present study: TRIzol (Invitrogen, Carlsbad, USA),
Real-time PCR kit (TaKaRa Biotechnology Co., Ltd.,
Dalian, China), ECL kit (Pierce Biotechnology, Inc.,
Rockford, USA), goat antimouse Smad4 monoclonal
antibody (Santa Cruz Biotechnology, Inc., Santa
Cruz, USA), GAPDH and anti-mouse IgG (Cell
Signalling Technology, Inc., Danvers, USA),
Lentivirus Package plasmid mix (System
Biosciences, Mountain View, USA), recombinant
lenti-siRNA1 plasmid expression vector (our lab),
293TN cells (System Biosciences), 0.25% trypsin
(GIBCO®), fetal bovine serum (FBS, GIBCO®),
DMEM high glucose (Invitrogen, Carlsbad, USA),
Opti-MEM (GIBCO®), LipofectamineTM 2000
(Invitrogen, Carlsbad, USA) and PVDF membrane
(Millipore, Billerica, USA).
Cell culture
C2C12 myoblasts were maintained in complete
medium (DMEM high glucose with 10% FBS, 100
U/ml penicillin and 100 U/ml streptomycin) at 37 °C
in a humidified atmosphere with 5% CO2.
Preparation of myofibroblasts
The TGF-beta1 was used to induce the differentiation of
C2C12 myoblasts into myofibroblasts according to
the method previous described by Ono et al. (2007).
The C2C12 myoblasts were grown in the complete
medium containing 5 ng/ml TGF-beta1 at 37 °C in a
humidified atmosphere with 5% CO2 for 72 h. The
expressions of alpha-SMA and collagen I were measured
by immunofluorescent staining daily. Light
microscopy was performed to observe whether these
cells were long-spindle shaped or polygonal
myofibroblasts, which help determine the
differentiation of myoblasts. Trypan blue staining was
performed to exclude the unviable cells.
Construction of lentivirus carrying Smad4-shRNA
and cell transfection
The siRNA targeting mouse Smad4 sequence
(GenBank accession No. NM_008540) and scramble
siRNA were designed according to the method
described in http://www.invitrogen.com/rnai. The
single-stranded DNA corresponding to siRNA was
used to synthesize double-stranded oligonucleotide.
The shRNA1, shRNA2, shRNA3 and scramble
shRNA are shown in Table 1.
The NIH3T3 cells were seeded into 6-well plates
(3x105 cells/well) followed by incubation for 24 h and
subsequent transfection. The siRNA expression
vectors were used to transfect NIH3T3 cells using
Lipofectamine TM 2000 according to the
manufacturer's instructions. Two days after
transfection, the total RNA was extracted using TRIzol
according to the manufacturer's instruction and then
reverse transcribed into cDNA. Then, the cDNA was
used for amplification using the Real-time PCR kit to
measure the mRNA expression of Smad4, and beta-actin
served as an internal reference. The primers were as
follows:
Smad4: 5'-CGGCCGTGGCAGGGAACA-3'
(forward);
5'-CTGCAGAGCTCGGTGAAGGTGAAT-3'
(reverse);
beta-actin: 5'-CCTCTATGCCAACACAGTGC-3'
(forward);
5'-GTACTCCTGCTTGCTGATCC-3' (reverse).
PCR was carried out in 20 microl of reaction mixture
and the conditions for PCR were as follows: 40 cycles
of denaturation at 95 °C for 1 min, annealing at 60 °C
for 20 s and extension at 72 °C for 20 s. The products
were then subjected to 1% agarose gel
electrophoresis.
Two days after transfection, cells were lysed in the
buffer containing 20 nM Tris-HCl (pH 7.4), 150 mM
NaCl and 1% PMSF. The extracted protein of equal
content was loaded onto 8% Tris glycine SDS buffer
followed by SDS-PAGE. Then, the proteins were
transferred onto PVDF membranes which were
blocked in 5% slim milk in TBST for 1 h.
Subsequently, the membranes were treated with
Smad4 antibody (1:200, Santa Cruz Biotechnology,
Inc., Santa Cruz, USA) or GAPDH (1:200, Cell
Signalling Technology, Inc., Danvers, USA) in TBST at 4 °C overnight and then with
HRP conjugated anti-mouse IgG (1:6000, Cell
Signalling Technology) at room
temperature for 2 h. Visualization was carried out with ECL kit to detect the protein expression of
Smad4. The most efficient shRNA1 was used to
construct lentivirus for the transfection of 293TN
cells according to the description in Lentivirus
Package plasmid mix (System Biosciences, Mountain
View, USA).
Table 1. shRNA sequence for interfering.
| shRNA |
Oligonucleotide sequences | | shRNA1 |
Forward:
5'-GATCCGGATGAGTACGTTCACGACCTTCCTGTCAGAGTCGTGAACGTACTCATCCTTTTTG | |
Reverse:
5'-AATTCAAAAAGGATGAGTACGTTCACGACTCTGACAGGAAGGTCGTGAACGTACTCATCCG | | shRNA2 |
Forward:
5'-GATCCGTAATCGCGCATCAACGGACTTCCTGTCAGATCCGTTGATGCGCGATTACTTTTTG | | : |
Reverse:
5'-AATTCAAAAAGTAATCGCGCATCAACGGATCTGACAGGAAGTCCGTTGATGCGCGATTACG | | shRNA3 |
Forward:
5'-GATCCGTAGGACTGCACCATACACCTTCCTGTCAGAGTGTATGGTGCAGTCCTACTTTTTG | |
Reverse:
5'-AATTCAAAAAGTAGGACTGCACCATACACTCTGACAGGAAGGTGTATGGTGCAGTCCTACG | | Scr |
Forward:
5'-GATCCCGTTTAACTCTCCCAACCACTTCCTGTCAGATGGTTGGGAGAGTTAAACGTTTTTG | |
Reverse:
5'-AATTCAAAAACGTTTAACTCTCCCAACCATCTGACAGGAAGTGGTTGGGAGAGTTAAACGG |
Notes: a total of 3 shRNAs and a scramble shRNA were designed (Scr: scramble shRNA).
Construction of cell line with stable Smad4-shRNA
expression
C2C12 cells were seeded into 96-well plates followed
by incubation for 24 h. Then, lentivirus was added to
these cells at the MOI of 6 followed by incubation at
37 °C in an atmosphere with 5% CO2. Three days
later, fluorescence microscopy was carried out to
detect the expression of green fluorescent protein.
According to the fluorescence intensity, the colonies
with stable expression were selected for passaging.
Effect of lentiviral-mediated RNA interference against
Smad4 on TGF-beta1 induced fibrosis of
myofibroblasts
Cells were divided into groups A, B, C and D. In
group A, the C2C12 cells were untreated. The C2C12
cells in the group received TGF-beta1 induction. In
group C, the C2C12 cells were transfected with
lentivirus. In group D, the C2C12 cells underwent
TGF-beta1 induction and were then transfected with
lentivirus.
Three days after culture, the GFP expression in
group C was observed under a fluorescence
microscope. Real-time PCR and Western blot were
employed to detect the mRNA and protein
expressions of Smad4 in the cells of groups A and C
which had high expression of GFP. The method and
procedures were similar to those described in the
"Construction of lentivirus carrying Smad4-shRNA
and cell transfection". In addition, Real-time PCR,
Western blot and immuno-fluorescence staining were
carried out to detect the expressions of alpha-SMA and
collagen I in the four groups.
1) Real-time PCR:
The primers were as follows: alpha-SMA:
5'-CTGAAGAGCATCCCACCCT-3' (forward),
5'-TCTCCAGAGTCCAGCACGAT-3' (reverse).
Collagen I:
5'-GAGCGGAGAGTACTGGATCGA-3'
(forward),
5'-CTGACCTGTCTCCATGTTGCA-3' (reverse).
2) Western blot assay:
The primary antibodies were alpha-SMA (1:1,500,
Thermo) and collagen I (1:200, Merck). The
secondary antibody was anti-mouse IgG (1:1000,
Cell Signaling).
3) Immunofluorescence staining: cells were seeded
into 6-well plates and washed in PBS. Then, these
cells were fixed in 4% paraformaldehyde at room
temperature for 15 min followed by perforation
with TritonX-100 for 30 min. These cells were
subsequently treated with 5-7% goat serum at
room temperature for 30 min followed by
incubation with alpha-SMA antibody (1:1,500,
Thermo) or collagen I (1:200, Merck) overnight.
After washing in PBS, cells were treated with
anti-mouse IgG (1:1000, Cell Signalling) and
Topro-3 in 0.01% Triton X-100 in PBS (pH 8.0) at
room temperature for 2 h. After washing in PBS,
these cells were observed under confocal
microscope.
Statistical analysis
Data were expressed as mean ± standard deviation
(SD). Statistical analysis was carried out with SPSS
version 11.0 (IBM, Armonk, USA). Comparisons
between two groups were carried out with t test at the
significance level of 2alpha=0.05.
RESULTS
Effect of different shRNAs on the Smad4 expression
The Real-time PCR and Western blot assay showed
that three shRNAs could significantly inhibit the
protein and mRNA expressions of Smad4 which
however remained unchanged in the scramble shRNA
group (Fig. 1). As shown in Fig. 1B, shRNA1
inhibited the Smad4 expression by 60% (statistically
significant). Considering that the transfection efficacy
was about 75%, the actual silencing efficacy of
shRNA could reach higher than 80%. Thus, shRNA1
was used for the construction of lentivirus.
Lentiviral-mediated Smad4-shRNA1 transfection
and suppressed protein expression of Smad4
Immunofluorescence staining showed that about 98%
of C2C12 cells were transfected with lentivirus
carrying Smad4-shRNA at 2 days after transfection at
a MOI of 6. Furthermore, the GFP expression was not
significantly reduced in these transfected cells of
passage 3. Real-time PCR and Western blot assay
showed the cells with high GFP expression had low
Smad4 expression when compared with cells in the
control group. Furthermore, the mRNA expression
was decreased by 85% and the protein expression
reduced by 86.93% in the lentiviral mediated
Smad4-shRNA1 transfection group (statistically
significant) (Fig. 2).
Effect of Smad4-shRNA1 on the TGF-beta1 induced
expressions of alpha-SMA and collagen I
In the present study, TGF-beta1 was used to induce the
differentiation of myoblasts into myofibroblasts (Ono
et al. 2007). Immunohistochemistry was employed to
detect the expressions of alpha-SMA and collagen I. In
this experiment, cells were divided into groups A, B,
C and D which were untreated C2C12 cells, C2C12
cells receiving TGF-beta1 induction, C2C12 cells which
were transfected with lentivirus carrying
Smad4-shRNA and the C2C12 which were
transfected with lentivirus carrying Smad4-shRNA
and underwent TGF-beta1 induction, respectively. The
results showed that the mRNA expressions of alpha-SMA
and collagen I in group B were increased by 34.41%
and 29.04% (statistically significant), respectively,
when compared with group A, and the protein
expression increased by 72.14% and 79.33%
(statistically significant), respectively. In the group D,
the mRNA expressions of alpha-SMA and collagen I
were increased by 6.36% and 7.22% (statistically
significant), respectively, compared to group C and
the protein expressions elevated by 5.12% and 7.31%
(statistically significant), respectively. When
compared with group B, the mRNA expressions of
alpha-SMA and collagen I in group D were decreased by
32.51% and 35.17% (statistically significant),
respectively, and the protein expressions of alpha-SMA
and collagen I reduced by 65.06% and 72.02%
(statistically significant), respectively (Fig. 3).
DISCUSSION
Skeletal muscle injury is a common disease in sports
medicine. According to recent statistics, skeletal
muscle injury accounts for 31% of all sports injuries
(Ekstrand et al. 2011). Studies show that the satellite
cells play an important role in the repair following
skeletal muscle injury. Normally, the satellite cells are
in a quiescent state and have no expression of muscle
proteins. Following skeletal muscle injury, these
satellite cells are activated, express a lot of muscle
regulatory factors in the presence of integrate basal
lamina, and synthesize the functional proteins in the
muscles leading to the formation of muscle fibers
(Charge and Rudnicki 2004, Wagers and Conboy
2005).
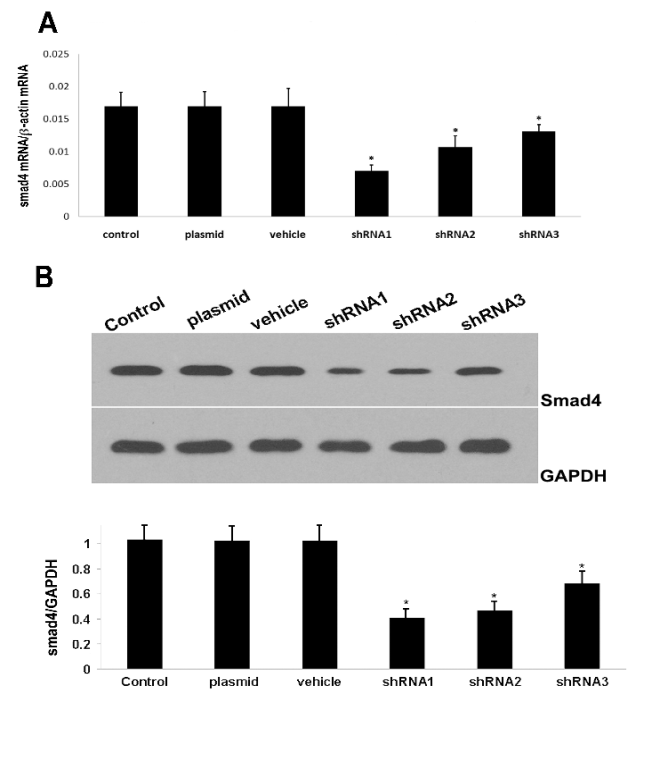
Fig. 1. Effect of Smad4-shRNA on the expression of Smad4. shRNA was used to transfect C2C12 cells using Lipofectamine
2000. Two days later, RT-PCR (A) and Western blot assay (B) were employed to detect the silencing efficacy. The mRNA and
protein expressions of Smad4 were significantly reduced. *Statistically significant as compared with normal C2C12 cells.
Repair following skeletal muscle injury is a hot
topic in sports medicine. It has been found that some
cytokines can promote the regeneration of skeletal
muscle or inhibit skeletal muscle fibrosis following
injury, and thus the gene therapy of skeletal muscle
injury has attracted increasing attention from
researchers. However, in studies on skeletal muscle
injury, the therapeutic efficacy of various measures to
promote the recovery of skeletal muscle injury is still
unsatisfactory and treatment with these measures is
often accompanied by fibrosis of the injured skeletal
muscles and the formation of scars (Musaro 2005,
Urish et al. 2005). Li and Huard (2002) were the first
to report the differentiation of myoblasts into the
precursors of myofibroblasts following skeletal
muscle injury in a mouse model. Therefore, the
activation of myoblasts is usually accompanied by the
development of abnormal fibrosis. Thus, to block the
differentiation of myoblasts into myofibroblasts may
be an effective way to prevent skeletal fibrosis
following skeletal muscle injury.
Over deposition of extracellular matrix including
type I and III collagen may cause tissue fibrosis.
TGF-beta1 is a critical cytokine that can promote the
synthesis and deposition of extracellular matrix.
TGF-beta1 can induce the differentiation of different mesenchymal cells (fibroblasts, stromal cells, satellite
cells and myoblasts) into myofibroblasts (Powell et
al. 1999, Leask and Abraham 2004, Li et al. 2004). In
the present study, TGF-beta1 was used to induce the
differentiation of C2C12 cells. In group B, C2C12
myoblasts received differentiation induction with
TGF-beta1 for 72 h, and results showed the expressions
of alpha-SMA and collagen I, two markers of
differentiation into myofibroblasts, at both mRNA
and protein levels (statistically significant). This
demonstrates that TGF-beta1 can inhibit the myogenic
differentiation of C2C12 cells and induce the
differentiation of C2C12 myoblasts into
myofibroblasts (Wicik et al. 2010).
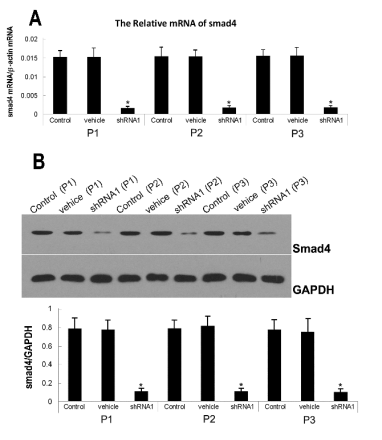
Fig. 2. Effect of lentiviral mediated Smad4-shRNA1 transfection on the protein expression of Smad4. Three days after
transfection, the Real-time PCR(A) and Western blot analysis(B) showed a lower expression of Smad4 that was mantained in three
generations in the lentiviral mediated Smad4-shRNA1 transfected group. *Statistically significant as compared with the
control (non-tranfected) and vehicle groups.
The signal transduction of TGF-beta1 depends on
the serine kinase phosphorylation of its receptor as
well as the activation of transcriptional factors
including Smad, MAPK and PI3K/Akt. Ghosh et al.
(2001) reported that TGF-beta1 could regulate the
human a2(I) collagen promoter via the Smad
signalling pathway which resulted in the abnormal
synthesis and deposition of collagens and a fibrotic
response. By using RNA interference, the expressions
of down-stream proteins of TGF-beta1 signal (Smad2
and Smad3) are inhibited, exhibiting an anti-fibrotic
effect, which has been confirmed to be effective in
some studies. Gressner et al. (2009) reported that to
decrease the Smad2 expression could effectively
inhibit the hepatic fibrosis, and Wang et al. (2007)
also reported that down-regulation of Smad3 could
suppress the synthesis of collagens in skin scars.
Following the activation of Smad2 and Smad3 signals, the initiation of gene transcription depends on
the translocation of these proteins into the nucleus.
Smad4 is a type of coordination Smad protein, and
can bind to the activated Smad 1, 2, 3, 5 or 8
following phosphorylation forming heterodimers,
which then translocate into the nucleus and
subsequently initiate the gene transcription resulting
in cell differentiation. Thus, to inhibit the Smad4
expression may be beneficial for the treatment of
fibrosis.
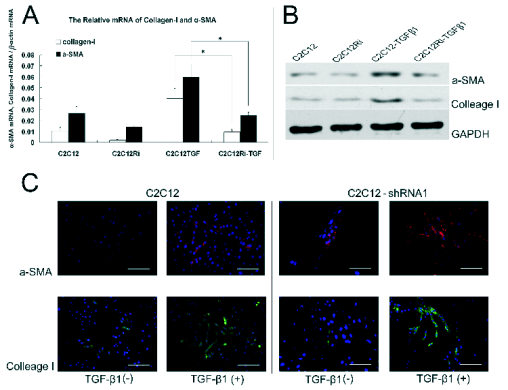
Fig. 3. Expressions of alpha-SMA and collagen I following transfection of lentivirus carrying Smad4-shRNA1. Smad4 could
down-regulate the TGF-beta1 induced differentiation of C2C12 myoblasts into myofibroblasts. C2C12 cells transfected with
lentivirus carrying shRNA and those transfected with blank lentivirus were maintained in the presence of TGF-beta1 for 72 h.
Fluorescent quantitative PCR (A), Western blot (B) and immunofluorescence (C) were employed to detect the expressions of
alpha-SMA and collagen I. The expressions of alpha-SMA and collagen I of Smad4-lentivirus mediated C2C12 cells significantly
decreased. *Statistically significant as compared with bland letivirus mediated C2C12 cells under TGF-beta1 induction, scale:
200 microm.
Short hairpin RNA can induce the degradation of
mRNAs and silence the gene expression (Nishikawa
and Sugiyama 2010). Our results also demonstrate the
mRNA and protein expressions of Smad4 in the
C2C12 cells transfected with lentivirus carrying
Smad4-shRNA were reduced by 85% and 86.93%,
respectively, which suggests the Smad4 is
significantly down-regulated. In addition, the alpha-SMA
and collagen I expressions in the C2C12 cells
transfected with lentivirus carrying Smad4-shRNA in
the presence of induction with TGF-beta1 are reduced by
65.06% and 72.02% when compared with those in
normal C2C12 cells following induction with
TGF-1, which indicates that Smad4 plays a crucial
role in TGF-beta1 induced fibrosis. Together with the
aforementioned, we speculate that Smad4 is also a
critical factor in TGF-beta1 induced fibrosis. However,
the specific mechanism and the role of other
signalling pathways are required to be elucidated.
Taken together, our results demonstrate that
inhibition of Smad4 expression by using shRNA can
effectively inhibit the TGF-beta1 induced fibrosis of
C2C12 myoblasts. Thus, Smad4 may become a novel
target for the treatment of the fibrosis of skeletal
muscle.
ACKNOWLEDGEMENT
This work was supported by the National Natural
Science Foundation of China (30800543).
REFERENCES
Bran GM, Sommer UJ, Goessler UR, Hormann K, Riedel F, Sadick H. TGF-ss1 antisense impacts the SMAD signalling system in fibroblasts from keloid
scars. Anticancer Res. 30: 3459-3463, 2010.
[PubMed]
Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 84: 209-238, 2004.
[CrossRef]
[PubMed]
Cheng J, Grande JP. Transforming growth factor-beta signal transduction and progressive renal disease. Exp Biol Med (Maywood). 227: 943-956,
2002.
Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (Soccer). Am J Sports Med. 39: 1226-1232, 2011.
[CrossRef]
[PubMed]
Ghosh AK, Yuan W, Mori Y, Chen S, Varga J. Antagonistic regulation of type I collagen gene expression by interferon-gamma and transforming growth
factor-beta. Integration at the level of p300/CBP transcriptional coactivators. J Biol Chem. 276: 11041-11048, 2001.
[CrossRef]
[PubMed]
Gressner OA. Less Smad2 is good for you! A scientific update on coffee's liver benefits. Hepatology. 50: 970-978, 2009.
[CrossRef]
[PubMed]
Jackson WM, Aragon AB, Onodera J, Steven M, Koehler SM, Ji Y, Bulken-Hoover JD, Vogler JA, Tuan RS, Nesti LJ. Cytokine expression in muscle following
traumatic injury. J Orthop Res. 29: 1613-1620, 2011.
[CrossRef]
[PubMed]
Kretschmer A, Moepert K, Dames S, Sternberger M, Kaufmann J, Klippel A. Differential regulation of TGF-beta signaling through Smad2, Smad3 and Smad4.
Oncogene. 22: 6748-6763, 2003.
[CrossRef]
[PubMed]
Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 18: 816-827, 2004.
[CrossRef]
[PubMed]
Li J, Tang X, Chen X. Comparative effects of TGF-beta2/Smad2 and TGF-beta2/Smad3 signaling pathways on proliferation, migration, and extracellular
matrix production in a human lens cell line. Exp Eye Res. 92: 173-179, 2011.
[CrossRef]
[PubMed]
Li TT, Si GM, Chen FC. Effects of Shenqi Jiedu Decoction on expressions of transforming growth factor-beta1, Smad2 and Smad3 in renal tissues of rats
with chronic renal failure induced by adenine. Zhong Xi Yi Jie He Xue Bao. 8: 263-268, 2010.
[CrossRef]
[PubMed]
Li Y, Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol. 161: 895-907, 2002.
[CrossRef]
Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. Transforming growth factor-beta1 induces the differentiation of myogenic cells
into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 164: 1007-1019, 2004.
[CrossRef]
Matsuzaki K. Modulation of TGF-beta signaling during progression of chronic liver diseases. Front Biosci. 14: 2923-2934, 2009.
[CrossRef]
[PubMed]
Musaro A. Growth factor enhancement of muscle regeneration: a central role of IGF-1. Arch Ital Biol. 143: 243-248, 2005.
[PubMed]
Nishikawa Y, Sugiyama T. A shRNA library constructed through the generation of loop-stem-loop DNA. J Gene Med. 12: 927-933, 2010.
[CrossRef]
Ono Y, Sensui H, Okutsu S, Nagatomi R. Notch2 negatively regulates myofibroblastic differentiation of myoblasts. J Cell Physiol. 210: 358-369,
2007.
[CrossRef]
[PubMed]
Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 29: 300-303, 2001.
[PubMed]
Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol.
277: C1-9, 1999.
[PubMed]
Tse R, Howard J, Wu Y, Gan BS. Enhanced Dupuytren's disease fibroblast populated collagen lattice contraction is independent of endogenous active
TGF-beta2. BMC Musculoskelet Disord. 5: 41, 2004.
[CrossRef]
[PubMed]
Urish K, Kanda Y, Huard J. Initial failure in myoblast transplantation therapy has led the way toward the isolation of muscle stem cells: potential
for tissue regeneration. Curr Top Dev Biol. 68: 263-280, 2005.
[CrossRef]
Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration. current concepts and controversies in adult myogenesis. Cell. 122:
659-667, 2005.
[CrossRef]
[PubMed]
Wang Z, Gao Z, Shi Y, Sun Y, Lin Z, Jiang H, Hou T, Wang Q, Yuan X, Zhu X, Wu H, Jin Y. Inhibition of Smad3 expression decreases collagen synthesis
in keloid disease fibroblasts. J Plast Reconstr Aesthet Surg. 60: 1193-1199, 2007.
[CrossRef]
Wicik Z, Sadkowski T, Jank M, Motyl T. Transcriptional pattern of TGF-beta1 inhibitory effect on mouse C2C12 myoblasts differentiation. Pol J Vet
Sci. 13: 629-638, 2010.
[CrossRef]
[PubMed]
Zhu X, Topouzis S, Liang LF, Stotish RL. Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative
feedback mechanism. Cytokine. 26: 262-272, 2004.
[CrossRef]
[PubMed]
|
BACK
|




