Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 2, p 79-90
DOI 10.2478/v10136-012-0005-z
The antioxidant N-acetylcysteine in vitro improves several functions of peritoneal leucocytes from old mice approaching their values to those
of adult animals
Monica De la Fuente, Angel Hernanz
Address: Monica De la Fuente. Departamento de Fisiologia, Facultad de Ciencias Biologicas, Universidad Complutense, 28040 Madrid, Spain
mondelaf@bio.ucm.es
Received 6th September 2011.
Revised 6th November 2011.
Published online 9th November 2011.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
Acknowledgements
References
SUMMARY
The age-related deterioration of the function of immune cells, or immunosenescence, is based on oxidative stress (an imbalance between the levels of
oxidants and antioxidant defences with an increase of the former). Accordingly, the ingestion of a diet supplemented with thiolic antioxidants such
as N-acetylcysteine (NAC), a glutathione precursor, by aged subjects improved their leucocyte functions. The aim of the present study was to show if
NAC improves in vitro several functions of leucocytes from chronologically old mice and if this antioxidant is able to bring the values of
these functions to the levels of those of adult animals. Six concentrations of NAC (in a range from 0.001 mM to 2.5 mM) were investigated on several
functions of peritoneal leucocytes from old (78±2 weeks of age) BALB/c mice. These functions were those of the phagocytic process in macrophages,
namely: adherence to substrate, directed migration or chemotaxis, phagocytosis of inert particles and superoxide anion levels as a measure of
digestion capacity, as well as of the adherence and chemotaxis of lymphocytes. These functions were also studied in peritoneal leucocytes from adult
(18±2 weeks of age) mice. The results showed that NAC in vitro improves all the functions studied, especially at the highest concentrations,
which had shown impaired values in old mice, approaching those of adult animals. Since the immune functions studied are markers of health and
predictors of longevity, the administration to aged subjects of NAC, which shows a direct action in leucocytes, seems to be a good strategy to
improve their immune system and, therefore, to reach a healthy longevity.
KEY WORDS
N-acetylcysteine; immunosenescence; macrophages; lymphocytes; mice
INTRODUCTION
Ageing is accompanied by an impairment of the
physiological systems including the immune system.
In fact, it is well known that with the passage of time
there is a decrease in resistance to infections and an
increase in the autoimmune processes and cancer.
This indicates the presence of a less competent
immune system, which exerts a great influence on the
increasing morbidity and mortality observed in ageing
human subjects (Wayne et al. 1990). Moreover, the
high death rate found in aged populations is due in
great proportion to infectious processes (High 2004).
Thus, it is presently accepted that almost every
component of the immune system undergoes striking
age-associated re-structuring, leading to changes that
may include enhanced as well as diminished
functions; this fact being denominated immunosenescence (Aw et al. 2007, De la Fuente and Miquel
2009). We have studied the changes in several
immune cell functions through age of experimental
animals, such as rats and specially mice, and of
human beings, and we have observed a similar
age-related evolution of many of these functions in
immune cells from the peripheral blood of humans
and from the peritoneum of mice (De la Fuente 2002,
De la Fuente et al. 2004a, b, 2005, 2011a, De la
Fuente and Miquel 2009, Arranz et al. 2010). In
agreement with the oxidation theory of ageing
(Harman 1956, Miquel et al. 1980, Miquel 1998), we
have observed that those age-related changes of
immune cell functions have as their basis an oxidative
and inflammatory stress situation, which has among
its intracellular mechanisms the activation of the NF-kappaB in the immune cells (De la Fuente et al. 2005, De
la Fuente and Miquel 2009, Arranz et al. 2010).
Moreover, we have proposed a key involvement of
the immune system in the rate of ageing of each
organism, since there is a relation between the redox
state and functional capacity of the immune cells and
the longevity of individuals (De la Fuente and Miquel
2009, Alonso-Fernandez and De la Fuente 2011). A
confirmation of this role of the immune system in the
oxi-inflamm-ageing is that the administration of
adequate amounts of antioxidants in the diet, which
improves the immune cell functions, decreasing their
oxidative stress, in experimental animals and humans,
increases the longevity of mice (Arranz et al. 2008,
De la Fuente et al. 2008, 2011a, b, De la Fuente and
Miquel 2009, De la Fuente 2010).
In this context, we have especially studied the
effects of diet supplemented with thiol antioxidants
such as N-acetylcysteine (NAC). This antioxidant
neutralizes free radicals in a direct manner (Gressier
et al. 1994) and is a glutathione precursor (De Flora
et al. 1991). Glutathione (GSH) is the principal
nonenzymatic antioxidant of the cells and plays a
major role in the preservation of an adequate
intracellular redox state (Droge 2002). Moreover, an
optimal immune response will require adequate levels
of GSH (Droge and Breikreuz 2000). With ageing,
there is a decrease in this GSH content, which has
been observed in a variety of cells and tissues,
including those of the immune system (Hernanz et al.
2000, Droge 2005, Arranz et al. 2008). NAC has been
studied by many authors because of the wide range of
its effects at all cellular levels and with multiple
clinical applications (Dodd et al. 2008, Millea 2009).
In previous studies we have observed that the
ingestion of a diet supplemented with NAC improves
many leucocyte functions in adult prematurely ageing
mice (Puerto et al. 2002, Guayerbas et al. 2005). In
addition, in postmenopausal women NAC showed
similar effects (Arranz et al. 2008). The ingestion of
a diet supplemented with NAC and other thiolic
precursors namely thioproline, also improved many
leucocyte functions in adult, prematurely ageing mice
and old animals (Blanco et al. 1999, De la Fuente et
al. 2002, Guayerbas et al. 2002b, 2004, De la Fuente
2010). NAC in vitro stimulated several functions of
lymphocytes and macrophages from adult mice (Del
Rio et al. 1998, Pomaki et al. 2005, De la Fuente et al.
2011b) and human subjects (Karlsson et al. 2011).
However, the effects in vitro of NAC on functions of
peritoneal leucocytes from old mice have not yet been
studied. Moreover, since the amount of thiol
antioxidants in the diet that improve immune function
depends on the age of the animals (De la Fuente et al.
2002), the aim of the present work based on the above
was to test the effects of a wide range of
concentrations of NAC in vitro on several functions
of peritoneal macrophages and lymphocytes from old
mice, and to check if the NAC administration
approaches the values obtained in adult mice.
MATERIAL AND METHODS
Animals
Old (78±2 weeks of age) and adult (18±2 weeks of
age) female BALB/c mice (Mus musculus) (Iffa
Credo, France) were used. The mice were specific
pathogen free, as tested by Harlan according to
FELASA recommendations. Twelve mice of each age
were used. They were randomly divided in groups of
6, and each group was housed in a polyurethane box,
at a constant temperature (22±2 °C) in sterile
conditions inside an aseptic air negative-pressure
environmental cabinet (Flufrance, Cachan, France),
on a 12/12 h reversed light/dark cycle. All animals
were fed water and standard Sander Mus (A.04 diet
from Panlab L.S. Barcelona, Spain) pellets ad libitum.
The diet was in accordance with the recommendations
of the American Institute of Nutrition for laboratory
animals. The experimental protocol was approved by
the Animal Ethics Committee of the Complutense
University of Madrid (Spain).
Collection of peritoneal leucocytes
After being housed for 2 weeks, mice were sacrificed
by cervical dislocation according to the guidelines of
the European Community Council Directive 86/6091
EEC, between 8:00 and 10:00 h. The abdomen was
cleansed with 70% of ethanol, the abdominal skin
was carefully dissected without opening the
peritoneum and 4 ml of sterile Hank's solution was
injected intraperitoneally. Then, the abdomen was
gently massaged and peritoneal resident cells were
removed, allowing the recovery of 85-90% of the
injected volume. Macrophages, identified by
morphology and non-specific esterase staining, were
counted in Neubauer chambers and then adjusted by
dilution with Hank's solution to 5 x 105 macro-phages/ml. Lymphocytes were also identified by
morphology and adjusted to 5 x 105 lymphocytes/ml
Hank's medium. The cellular viability, determined in
each experiment using the trypan-blue exclusion test,
was in all cases higher than 95%.
Antioxidant
N-acetylcysteine (NAC) was purchased from Sigma
(St. Louis, USA) and the following concentrations
were used: 0.001 mM, 0.01 mM, 0.1 mM, 1 mM and
2.5 mM dissolved in Hank's solution.
Assays of phagocytic function in peritoneal
macrophages
In the peritoneal suspension, with macrophages
adjusted to 5 x 105 cells/ml Hank's solution, we
carried out a study of the different steps of the
phagocytic process, i.e., adherence to tissues,
mobility to infectious focus (chemotaxis),
phagocytosis of foreign inert material and digestion
capacity of this material through the production of
intracellular free radicals, namely the superoxide
anion, which is the first response in the respiratory
burst.
For the quantification of the adherence capacity to
the substrate, we observed the adherence to a smooth
plastic surface, because it resembles adherence to
animal tissue. The method was carried out as
previously described (Puerto et al. 2002). Briefly,
aliquots of 0.2 ml of the peritoneal suspensions were
placed in eppendorf tubes and incubated 10, 20 and
30 min at 37 °C, and after gentle shaking, the number
of non-adhered macrophages was determined in
Neubauer chambers. The adherence index, AI, was
calculated according to the following equation:
AI= (Mi-Mf/Mi) × 100, where Mi is the initial con-centration of macrophages (5 x 105 cells/ml) and Mf
the final concentration of macrophages in the
supernatant (non-adherent cells) after each incubation
time.
The chemotaxis assays were performed according
to a modification of Puerto et al. (2002) of the
original technique described by Boyden (1962),
which consists basically in the use of chambers with
two compartments separated by a filter (Millipore,
Bedford, MA) with a pore diameter of 3 microm. Aliquots
of 0.3 ml of the peritoneal suspension were deposited
in the upper compartment of the Boyden chambers.
F-met-leu-phe (Sigma, St. Louis, USA) (a positive
chemotactic peptide in vitro), at 10-8 M, was placed in
the lower compartment in order to determine
chemotaxis. The chambers were incubated for 3 h at
37 °C and 5% CO2, and after this time the filters were
fixed, stained and the chemotaxis index (C.I.) was
determined by counting in an optical microscope
(immersion objective) the total number of
macrophages in one third of the lower face of the
filters.
The latex phagocytosis assay was carried out
following a method previously described (Puerto et
al. 2002). Aliquots of 0.2 ml of peritoneal
suspensions were incubated in culture plates (Sterilin,
Teddington, England) for 30 min. To the adherent
monolayer, after being washed with PBS (phosphate
buffer saline), 0.02 ml latex beads (1.09 mm diluted
to 1% PBS, Sigma, St. Louis, MO) were added. After
30 min of incubation, the plates were washed, fixed
and stained and the number of particles ingested by
100 macrophages was counted, being expressed as a
phagocytic index (P.I.). The percentage of
macrophages with phagocytic capacity (ingesting at
least one particle) was also counted and expressed as
the phagocytic efficiency (P.E.).
Superoxide anion production was evaluated
assessing the capacity of this anion, produced by
macrophages, to reduce nitroblue tetrazolium (NBT).
This was carried out following the method described
by De la Fuente (1985) slightly modified as follows.
Aliquots of 0.25 ml of peritoneal suspension were
mixed with 250 ml of NBT (1 mg/ml in PBS, Sigma),
0.05 ml of a latex bead suspension were added to the
stimulated samples and 0.05 ml of PBS to the
non-stimulated samples. After 60 min of incubation,
the reaction was stopped, the samples were
centrifuged, and the intracellular reduced NBT was
extracted with dioxan (Sigma) and, after
centrifugation, the supernatant absorbance at 525 nm
was determined. The results were expressed as
nmol/106 cells using a pattern curve.
Assays of peritoneal lymphocyte functions
The two functions studied in macrophages that are
also carried out by lymphocytes, namely adherence
and chemotaxis, were studied in these cells of
peritoneal suspension adjusted to 5 x 105 lympho-cytes/ml Hank's medium, following similar methods
to those described for macrophages.
Statistical analysis
The data are expressed as the mean ±S.D of 10 values
corresponding to the same number of experiments.
Each value is the mean of the data from an assay
performed in duplicate. The data were examined
statistically by one-way analysis of variance
(ANOVA) for paired observations (NAC effects in
the aged mice), followed by the Scheffer's F post hoc
procedure. The ANOVA test for unpaired
observations was used for comparing adult and aged
mice, followed by the Scheffe's F test. The normality
of the samples was confirmed by the
Kolmogorov-Smirnov test. We used the significance
level 2alpha=0.05.
RESULTS
The percentages of macrophages and lymphocytes
obtained in the peritoneal suspensions from adult and
old mice were 39±11 and 30±13 for macrophages and
61±11 and 70±13 for lymphocytes, respectively.
The adherence and chemotaxis capacities of
macrophages are shown in Fig. 1. The adherence
indexes (Fig. 1A) at 10, 20 and 30 minutes of
incubation in macrophages from old mice were higher
(statistically significant) than those in cells from adult
animals. The presence of NAC decreased the
adherence indexes, the differences being statistically
significant at 10 and 20 minutes of incubation with
0.1 mM, 1 mM and 2.5 mM. Thus, the adherence
indexes with 2.5 mM (at 10 min of incubation) and
with 1 mM (at 10 and 20 min of incubation) were
similar to those in adult mice. The chemotaxis
indexes (Fig. 1B) in macrophages from old mice,
which in the controls were lower (statistically
significant) than those in adult animals, increased in
the presence of NAC. This antioxidant from 0.01 to
2.5 mM stimulated the chemotaxis, showing
statistically significant differences with respect to the
values of old controls. However, this increase did not
reach indexes similar to those in adult mice, since in
all cases the values were significantly lower than in
adult animals.
The results of the phagocytosis capacity, both
phagocytic index (P.I.) and phagocytic efficiency
(P.E.) (Fig. 2), show decreased values (statistically
significant) in macrophages from old mice in
comparison to those from adult animals. NAC
increased the P.I. (Fig. 2A) in macrophages from old
mice (with the exception of the concentration of
2.5 mM), showing statistically significant differences
with the concentrations of 0.01, 0.1 and 1 mM. The
levels of P.I. with the highest concentration of
2.5 mM were significantly lower than those with
1 and 0.1 and 0.01 mM of NAC. The values of P.I.
with all concentrations of NAC were significantly
lower than those in adult mice. With respect to the
P.E. (Fig. 2B) the concentration of 1 mM of NAC
was the only one that significantly increased this
index, however these values did not reach those of
macrophages from adult animals.
The levels of superoxide anion, both basal and
stimulated (Fig. 3) were significantly increased in
cells from old mice in comparison to those from adult
animals. However, the concentrations of 0.1 and
1 mM of NAC significantly increased these levels in
both basal and stimulated samples.
The adherence and chemotaxis capacities of
lymphocytes are shown in Fig. 4. The adherence
indexes (Fig. 4A) were higher (statistically
significant) in lymphocytes from old mice than in
those from adult animals at 10 min of incubation.
NAC significantly decreased the adherence indexes at
this time of incubation with 0.1 mM, 1 mM and
2.5 mM. With 1 mM of NAC the values at 10 min of
incubation were similar to those in lymphocytes from
adult mice. The chemotaxis of lymphocytes (Fig. 4B)
was significantly decreased in cells from old mice in
comparison with those from adult animals. No
statistically significant differences were found in the
presence of NAC.
DISCUSSION
NAC in vitro improved several functions of peritoneal
macrophages and lymphocytes from old mice, which
showed values quite similar to those in adult animals.
This fact suggests that the positive effects on these
immune functions caused by diet supplementation
with this antioxidant in adult prematurely ageing mice
(Guayerbas et al. 2005) are, at least in part, due to a
direct action on the immune cells. The results
obtained in the present study corroborate that the
deterioration of immune cells with ageing is linked to
oxygen stress and that the administration of adequate
amounts of the antioxidant NAC preserves an
appropriate function of the immune cells in the ageing
process (De la Fuente et al. 2005, 2011a, De la Fuente
and Miquel 2009).
The functions of macrophages studied in the
present work are the consecutive steps of the
phagocytic process, which is carried out by
phagocytic cells in their defensive activity against
infections. In this process the first step involves the
adherence of cells to tissue substrate, which is
followed by the migration of these cells to the focus
of the infection through a chemical gradient
(chemotaxis). With ageing, peritoneal macrophages
increase adherence capacity and decrease chemotaxis
(De la Fuente et al. 2004b, De la Fuente and Miquel
2009, Arranz et al. 2010). These changes have also been observed in the present study and show the
oxidative stress that old mice suffer. This is related to
the increase of adhesion molecules involving
activation of NF-kappaB (Lavie et al. 2005, Victor et al.
2005, Arranz et al. 2010). An oxidative stress
situation is also linked to a release of the migration
inhibitor factor (MIF) (Hirokawa et al. 1998, Victor
et al. 2005), which could explain the decrease of
chemotaxis in leucocytes from old mice.
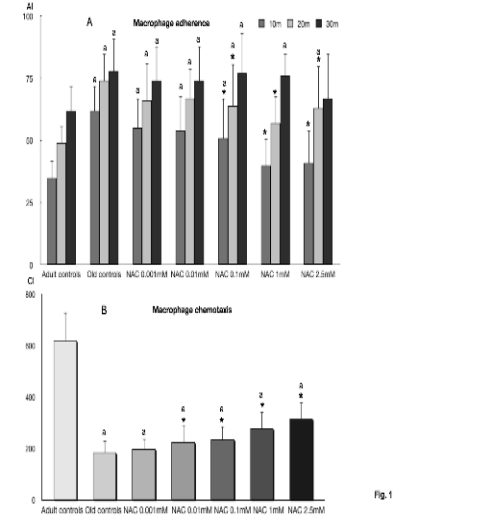
Fig. 1. Adherence of macrophages (A) and chemotaxis of macrophages (B) in peritoneal leukocytes from old BALB/c mice
incubated with 0.001, 0.01, 0.1,1 and 2.5 mM of N-acetylcysteine (NAC), as well as those indexes in cells from adult mice. Each
column represents the mean ± SD of 12 values corresponding to 12 animals, with each value being the mean of duplicate assays.
AI, adherence index following 10, 20 and 30 min of incubation; CI, chemotaxis index; * statistically significant as compared with
the corresponding old control values; a statistically significant versus the corresponding values in adult controls.
NAC decreases the adherence capacity in
macrophages from old mice at 0.1 mM, 1 mM and 2.5
mM, especially at 10 and 20 min of incubation, with
the values of this function being more similar to those
in adults. When we studied the effects of NAC (0.1 to
5 mM) in vitro on the adherence of macrophages from
adult mice, no change (Del Rio et al. 1998, Puerto et
al. 2002, Pomaki et al. 2005) or increase (Victor and
De la Fuente 2002) were found. Thus, NAC
modulates adherence of macrophages, not affecting or
increasing this function when cells are from adult
animals, but decreasing it in macrophages from old
mice, in which the oxidative stress situation of the
animals is shown with an increased adherence (De la
Fuente et al. 2005, De la Fuente and Miquel 2009).
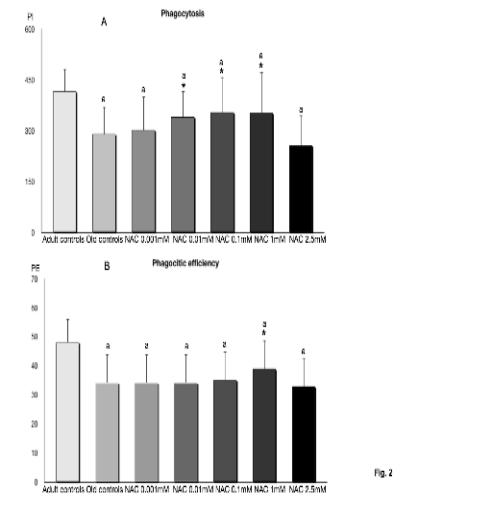
Fig. 2. Phagocytosis of latex particles (A) and phagocytic efficiency (B) in peritoneal leukocytes from old BALB/c mice
incubated with 0.001, 0.01, 0.1,1 and 2.5 mM of N-acetylcysteine (NAC), as well as those indexes in cells from adult mice. Each
column represents the mean ± SD of 12 values corresponding to 12 animals, with each value being the mean of duplicate assays. PI, phagocytosis index,
number of particles ingested by 100 macrophages; PE, number of macrophages ingesting at least one
particle per 100 macrophages; symbols as in Fig. 1.
Similar results, namely a decrease of the peritoneal
macrophage adherence capacity in the presence of
NAC, were obtained in cells from adult mice with
lethal endotoxic shock, a situation with an acute
oxidative stress that increases the adherence of these
phagocytes (Victor and De la Fuente 2002).
Chemotaxis was stimulated by NAC, especially in
the highest concentrations. Similar results were
obtained in the macrophages from adult mice (Del
Rio et al. 1998, Puerto et al. 2002, Victor and De la
Fuente 2002). Several studies have found a relation
between the supplementation and deficiency of
antioxidants, and the increase or decrease of
chemotaxis, respectively (Bendich 1989, De la Fuente
et al. 2000). In addition, NAC administration to
postmenopausal women, which showed a decreased
chemotaxis of peripheral blood neutrophils, increased
this function (Arranz et al. 2008). Moreover, in
prematurely ageing mice, with a decreased
chemotaxis in the peritoneal macrophages, the administration of diet with NAC increases this
function (Puerto et al. 2002, Guayerbas et al. 2005).
These effects could be due to the direct action of
NAC on phagocytic cells from old subjects, since in
the present study we have observed an increase of
chemotaxis in the presence of NAC in vitro. This
stimulation of chemotaxis in macrophages from
animals with an oxidative stress situation and a
decreased chemotaxis was also found in cells from
adult mice with a lethal endotoxic shock, in which
NAC acted decreasing the high activation of NF-B
caused by endotoxin in the immune cells (Victor and
De la Fuente 2002, 2003, Victor et al. 2005).
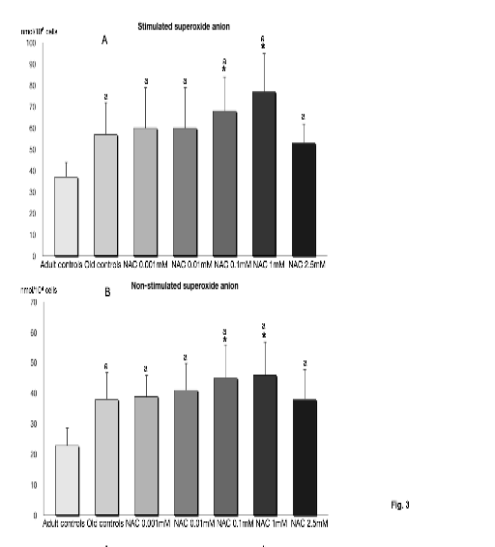
Fig. 3. Superoxide anion levels of samples stimulated with latex particles (stimulated superoxide anion) (A) and without latex
particles (non-stimulated superoxide anion) (B) in peritoneal leukocytes from old BALB/c mice incubated with 0.001, 0.01, 0.1,1
and 2.5 mM of N-acetylcysteine (NAC), as well as those indexes in cells from adult mice. Each column represents the mean ±
SD of 12 values corresponding to 12 animals, with each value being the mean of duplicate assays. Symbols as in Fig. 1.
An adequate chemotaxis capacity that allows the
phagocytes to reach the focus of the infection is
followed by the ingestion of foreign agents. The
phagocytic capacity decreases with ageing (De la
Fuente et al. 2004b, De la Fuente and Miquel 2009,
Arranz et al. 2010), and this fact is also observed in
the present work in both activities, the percentage of particles ingested (phagocytic index) and of
macrophages ingesting at least a particle (phagocytic
efficiency). Previous work showed that peritoneal
macrophages use their endogen antioxidants when
they are phagocyting (Hernanz et al. 1990). This
could explain why NAC and other antioxidants in
vitro increase the phagocytosis of macrophages from
adult mice (Del Rio et al. 1998, Puerto et al. 2002,
Victor and De la Fuente 2002, Pomaki et al. 2005). In
macrophages from old mice this effect is shown with
0.1 and 1 mM, but not with 2.5 mM, although this
concentration is effective in macrophages from adult
mice (Puerto et al. 2002, Victor and De la Fuente
2002). Previous studies also showed that a higher
concentration of a thiol antioxidant produces a lesser
effect on phagocytosis than other lower concentrations, as observed with GSH (Del Rio et al. 1998).
Moreover, no effect on phagocytosis was found with
5 mM of NAC in macrophages from adult mice, but
an increase of this function was found with lower
concentrations (Pomaki et al. 2005). Although the
amount of thiolic antioxidant such as NAC and
thioproline that adult and old animals have to ingest
with diet to improve phagocytic function was higher
in old mice than in adult animals (De la Fuente et al.
2002), this it is not observed in vitro. It is possible
that in vitro a high concentration of NAC neutralizes
so much of the reactive oxygen species (ROS) produced by the cell, that it
cannot carry out its function adequately. It is known
that leucocytes produce ROS as chemical weapons to
incapacitate pathogens and malignant cells and as
modulators of gene expression, regulating the
biosynthesis of many immune mediators (Knight
2000, Yoon et al. 2002). Moreover, macrophages are
the immune cells more clearly involved in the oxidant
generation, since they use free radicals in order to
perform their defensive functions such as the
phagocytic activity (De la Fuente 2008, De la Fuente
and Miquel 2009). It is possible that another
imbalance ROS/antioxidant appears with lower levels
of ROS than those that cell needs when there are high
concentrations of NAC in vitro. In addition, in a
recent study, moderate concentrations of NAC in
vitro (0.4-3.2 mM) increased alloantigen-induced
proliferation, the expression of activation markers
CD25 and CD71 on T cells, and the production of
IFN-gamma and IL-10, whereas high concentrations of
NAC (12.5-50 mM) were suppressive (Karlsson et al.
2011). The results in vivo on old subjects commented
on above, suggest that the alterations of the digestive
tract and in the passage of the antioxidants through
the gut, that appear with ageing, only allow adequate
levels of these compounds to get to the immune cells
in old animals when the amount of the antioxidant in
the diet is high.
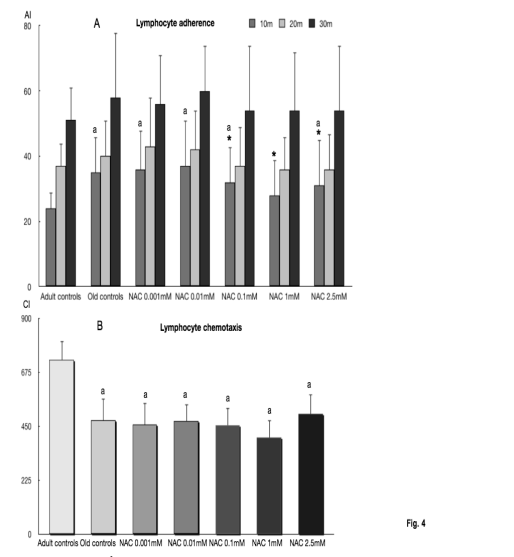
Fig. 4. Adherence (A) and chemotaxis of peritoneal lymphocytes (B) from old BALB/c mice incubated with 0.001,
0.01, 0.1,1 and 2.5 mM of N-acetylcysteine (NAC), as well as those indexes in cells from adult mice. Each column represents
the mean ± SD of 12 values corresponding to 12 animals, with each value being the mean of duplicate assays; AI, adherence
index following 10, 20 and 30 min of incubation; CI, chemotaxis index; other symbols as in Fig. 1.
In the presence of a phagocytic stimulus,
macrophages initiate what is known as the respiratory
burst, characterized by the production of free radicals,
the first of which is the superoxide anion. The levels
of this free radical in the phagocytes of old mice
increase in comparison to that of adult animals (De la
Fuente et al. 2002, Guayerbas et al. 2002a), although
we have found opposite results (De la Fuente et al.
2004b, 2011a). In previous work we have observed
increased levels of intracellular superoxide anion in
macrophages from adult mice after in vitro treatment
with NAC (Del Rio et al. 1998, Victor and De la
Fuente 2002). This effect was also found with GSH
(Del Rio et al. 1998), an antioxidant that also
increases neutrophil oxidative burst activity (Atalay
et al. 1996). These results show that the neutralizing
capacity of NAC does not interfere with the
generation of superoxide anion. Thus, it has been
observed that some antioxidants can efficiently
neutralize extracellular phagocyte-derived oxidants
without affecting the bactericidal oxygen radicals
inside the intracellular phagosomes (Jariwalla and
Harakeh 1996). Moreover, antioxidants such as
ascorbic acid increase the activity of the hexose
monophosphate shunt in neutrophils leading to the
synthesis of NADPH, which is needed for the
reduction of molecular oxygen to superoxide anion,
as well as increased NBT reduction (Anderson 1979).
This fact could explain the increments obtained in
both non-stimulated and stimulated cells in the
presence of NAC. Moreover, under appropriate
conditions, superoxide anion can be generated as a
consequence of radical scavenging by thiol
antioxidants like GSH in conditions usually found
intracellularly. Then, superoxide would act as a
radical sink being removed enzymatically by SOD
(Winterbourn 1993). In addition, ingestion of a diet
supplemented with thioproline and NAC increases the
levels of superoxide anion (De la Fuente et al. 2002)
or preserves these levels (Blanco et al. 1999) in the
peritoneal leukocytes from adult mice.
With respect to the adherence and chemotaxis of
lymphocytes - two functions that these cells share
with phagocytes - the changes with ageing were
similar to those in macrophages. In previous studies
we have also observed an age-related increase of
adherence and a decrease of chemotaxis in
lymphocytes from old mice, adult prematurely ageing
mice and old men and women (Guayerbas et al.
2002b, Viveros et al. 2007, Arranz et al. 2008, De la
Fuente et al. 2008, De la Fuente and Miquel 2009).
Similar changes have been shown in peritoneal
lymphocytes from mice with endotoxic shock, a
model of an acute oxidative stress situation (De la
Fuente and Victor 2000). The presence of NAC in
vitro, decreased the adherence, at least with 0.1 to
2.5 mM and at 10 min of incubation, but it did not
modify the chemotaxis in lymphocytes from old mice.
It is interesting to consider that in peritoneal
lymphocytes from adult mice, 1 mM of NAC in vitro
increases the adherence capacity of these cells, as
well as their chemotaxis (De la Fuente et al. 2011b).
The role as immuno-modulators of antioxidants,
such as NAC, bringing back altered immune function
to more optimum values, has been observed in
previous studies. Thus, in mice with lethal endotoxic
shock, in which the peritoneal lymphocytes show
increased adherence and depressed chemotaxis, NAC
decreased adherence and increased chemotaxis;
however, this antioxidant increased both functions in
control animals (De la Fuente and Victor 2000).
Moreover, in lymphocytes from chronologically adult
mice, but with premature ageing, which showed
higher adherence capacity than these cells from the
non prematurely ageing partners, NAC in vitro
decreased this function in cells from the prematurely
ageing but increased the function in those of
non-prematurely ageing animals (Puerto et al. 2002).
In conclusion, our results support the proposal that
NAC acts directly on the leucocytes and that the
positive effects on the immune cell functions shown
after administration of diet supplemented with NAC
could be due, at least in part, to this direct effect and
not only to the increase of intracellular GSH levels
that produce NAC (Arranz et al. 2008). Thus, NAC
may have benefits above other antioxidants, probably
because of both its direct and GSH-mediated effects.
Moreover, NAC increases in vitro, at least with
concentrations of 0.5 mM, the activity of antioxidant
enzymes such as catalase (Pomaki et al. 2005). This
capacity of up-regulation of intracellular antioxidant
defences has been proposed as a better way of
improving the antioxidant status of the organism than
the supplementation with higher amount of
antioxidants (Vina et al. 2007). Thus, the NAC
administration could be proposed as a good strategy
to slow down ageing and therefore, reach a healthy
longevity. However, more studies are required to
clarify if the administration of antioxidants to old
subjects is useful or not to control the rate of ageing.
The results, although in the case of NAC are not as
contradictory as with other antioxidants, are still not
conclusive, and the issue of the amount of antioxidant
effective (Halliwell 2009) need to be investigated
further.
ACKNOWLEDGEMENTS
This work was supported by MICINN
(BFU2008-04336 and BFU2011-30336); UCM
Research Group (910379ENEROINN) grants and
RETICEF (RD06/0013/0003) (ISCIII) of Spain.
REFERENCES
Alonso-Fernandez P, De la Fuente M. Role of the immune system in aging and longevity. Curr Aging Sci. 4: 78-100, 2011.
[PubMed]
Anderson R. Effects of ascorbate on leukocytes: Part II. Effects of ascorbic acid and calcium and sodium ascorbate on neutrophil phagocytosis and
post-phagocytic metabolic activity. S Afr Med J. 56: 401-404, 1979.
[CrossRef]
[PubMed]
Arranz L, Fernandez C, Rodriguez A, Ribera JM, De la Fuente M. The glutathione precursor N-acetylcysteine improves immune function in postmenopausal
women. Free Radic Biol Med. 45: 1252-1262, 2008.
[CrossRef]
[PubMed]
Arranz L, Caamano J, Lord JM, De la Fuente M. Preserved immune functions and controlled leukocyte oxidative stress in naturally long-lived mice:
Possible role of nuclear factor-kappa B. J Gerontol A Biol Sci. 65A: 941-950, 2010.
Atalay M, Marnila P, Lilius EM, Hanninen O, Sen CK. Glutathione-dependent modulation of exhausting exercise-induced changes in neutrophil function of
rats. Eur J Appl Physiol. 74: 342-347, 1996.
[CrossRef]
[PubMed]
Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 120: 435-446, 2007.
[CrossRef]
[PubMed]
Bendich A. Interaction between antioxidant vitamin C and E and their effect on immune responses. In Miquel J, Quintanilla AT, Weber H (eds.):
Handbook of Free Radicals and Antioxidants in Biomedicine. II. CRC press, Boca Raton 1989, pp. 153-160.
Blanco B, Ferrandez MD, Correa R, Del Rio M, Guaza C, Hernanz A, De la Fuente M. Changes in several functions of murine peritoneal macrophages by
N-acetylcysteine and thioproline ingestion. Comparative effect between two strains of mice. BioFactors. 10: 179-185, 1999.
[CrossRef]
[PubMed]
Boyden SV. The chemotaxis effect of mixtures of antibody and antigen on polymorphonuclear leukocytes. J Exp Med. 115: 453-466, 1962.
[CrossRef]
[PubMed]
De Flora S, Izzoti A, D’Agostini F, Cesarone CF. Antioxidant activity and other mechanisms of thiol involved in chemoprevention of mutation and
cancer. Am J Med. 91: 122-130, 1991.
[CrossRef]
De la Fuente M. Changes in the macrophage function with aging. Comp Biochem Physiol. 81 A: 935-938, 1985.
De la Fuente M. Effects of antioxidants on immune system ageing. Eur J Clin Nutr. 56: S5-S8, 2002.
[CrossRef]
[PubMed]
De la Fuente M. Role of neuroimmunomodulation in aging. Neuroimmunomodulation. 15: 213-223, 2008.
[CrossRef]
[PubMed]
De la Fuente M. Murine models of premature ageing for the study of diet-induced immune changes. Improvement of leukocyte functions in two strains of
old prematurely ageing mice by dietary supplementation with sulphur-containing antioxidants. Proc Nutr Soc. 69: 651-659, 2010.
[CrossRef]
[PubMed]
De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging. The involvement of the immune system in oxi-inflamm-aging. Curr
Pharm Design. 15: 3003-3026, 2009.
[CrossRef]
[PubMed]
De la Fuente M, Victor VM. Antioxidants as modulators of immune function. Immunol Cell Biol. 78: 49-54, 2000.
[CrossRef]
[PubMed]
De la Fuente M, Carazo M, Correa R, Del Rio M. Changes in macrophage and lymphocyte functions in guinea-pigs after different amounts of vitamin E
ingestion. Brit J Nutr. 84: 25-29, 2000.
[PubMed]
De la Fuente M, Guayerbas N, Catalan MP, Victor VM, Miquel J. The amount of thiolic antioxidant ingestion needed to improve the immune functions is
higher in aged than in adult mice. Free Radic Res. 36: 119-126, 2002.
[CrossRef]
De la Fuente M, Baeza I, Guayerbas N, Puerto M, Castillo C, Salazar V, Ariznavarreta C, F-Tresguerres JA. Changes with aging in several leukocyte
functions of male and female rats. Biogerontology. 5: 389-400, 2004a.
[CrossRef]
[PubMed]
De la Fuente M, Hernanz A, Guayerbas N, Puerto M, Alvarez P, Alvarado C. Changes with age in peritoneal macrophage functions. Implication of
leukocytes in the oxidative stress of senescence. Cell Mol Biol. 50: OL683-OL690, 2004b.
[PubMed]
De la Fuente M, Hernanz A, Vallejo MC. The immune system in the oxidation stress conditions of aging and hypertension. Favorable effects of
antioxidants and physical exercise. Antioxid Redox Signal. 7: 1356-1366, 2005.
[CrossRef]
De la Fuente M, Hernanz A, Guayerbas N, Victor VM, Arnalich F. Vitamin E ingestion improves several immune functions in elderly men and women. Free
Radic Res. 42: 272-280, 2008.
[CrossRef]
[PubMed]
De la Fuente M, Cruces J, Hernandez O, Ortega E. Strategies to improve the functions and redox state of the immune system in aged subjects. Curr
Pharm Des. 17: 3966-3983, 2011a.
De la Fuente M, Hernanz A, Viniegra S, Miquel J. Sulfur-containing antioxidants increase in vitro several functions of lymphocytes from mice.
Int Immunopharmacol. 11: 661-669. 2011b.
[CrossRef]
[PubMed]
Del Rio M, Ruedas G, Medina S, Victor VM, De la Fuente M. Improvement by several antioxidants of macrophage function in vitro. Life Sci. 63:
871-881, 1998.
[CrossRef]
Dodd S, Dean O, Copolov DL, Malhi GS, Berk M. N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expert Opin Biol Ther. 8:
1955-1962, 2008.
[CrossRef]
[PubMed]
Droge W. Aging-related changes in the thiol/disulfide redox state: implications for the use of thiol antioxidants. Exp Gerontol. 37: 1333-1345,
2002.
[CrossRef]
Droge W. Oxidative stress and ageing: is ageing a cysteine deficiency syndrome? Philos Trans R Soc B. 360: 2355-2372, 2005.
[CrossRef]
[PubMed]
Droge W, Breikreuz R. Glutathione and immune function. Proc Nutr Soc. 59: 595-600, 2000.
[CrossRef]
Gressier B, Cabanis A, Lebegue S, Brunet C, Dine T, Luyckx M, Cazin M, Cazin JC. Decrease of hypochlorous acid and hydroxyl radical generated by
stimulated human neutrophils: comparisons in vitro of some thiol-containing drugs. Methods Find Exp Clin Pharmacol. 16: 9-13, 1994.
[PubMed]
Guayerbas N, Catalan M, Victor VM, Miquel M, De la Fuente M. Relation of behaviour and macrophage function to life span in a murine model of
premature immunosenescence. Brain Behav Res. 134: 41-48, 2002a.
[PubMed]
Guayerbas N, Puerto M, Ferrandez MD, De la Fuente M. A diet supplemented with thiolic antioxidants improves leukocyte function in two strains of
prematurely ageing mice. Clin Exp Pharmacol Physiol. 29: 1009-1014, 2002b.
[CrossRef]
[PubMed]
Guayerbas N, Puerto M, Alvarez P, De la Fuente M. Improvement of the macrophage functions in premature ageing mice by a diet supplemented with
thiolic antioxidants. Cell Mol Biol. 50: OL677-OL681, 2004.
[PubMed]
Guayerbas N, Puerto M, Alvarado C, De la Fuente M. Effect of diet supplementation with N-acetylcysteine on leukocyte functions in prematurely ageing
mice. J Appl Biomed. 3: 199-205, 2005.
[JAB]
Halliwell B. The wandering of a free radical. Free Radic Biol Med. 46: 531-542, 2009.
[CrossRef]
[PubMed]
Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 2: 298-300,1956.
Hernanz A, Collazos ME, De la Fuente M. Effect of age, culture media and lymphocyte presence in ascorbate content of peritoneal macrophages from mice
and guinea pigs during phagocytosis. Int Arch Allergy appl Immunol. 91: 166-170, 1990.
[CrossRef]
Hernanz A, Fernandez-Vivancos E, Montiel C, Vazquez JJ, Arnalich F. Changes in the intracellular homocysteine and glutathione content associated with
aging. Life Sci. 67: 1317-1324, 2000.
[CrossRef]
High KP. Infection as a cause of age-related morbidity and mortality. Ageing Res Rev. 3: 1-14, 2004.
[CrossRef]
[PubMed]
Hirokawa J, Sakaue S, Furuya Y, Ishii J, Hasegawa A, Tagami S, Kawakami Y, Sakai M, Nishi S, Nishihira J. Tumor necrosis factor-alpha regulates the
gene expression of macrophage migration inhibitory factor through tyrosine kinase-dependent pathway in 3T3-L1 adipocytes. J Biochem. 123: 733-739,
1998.
[PubMed]
Jariwalla RJ, Harakeh S: Antiviral and immunomodulatory activities of ascorbic acid. Subcell Biochem. 25: 213-231, 1996.
[PubMed]
Karlsson H, Nava S, Remberger M, Hassan M, Ringden O. Eur J Immunol. 41: 1143-1153, 2011.
[CrossRef]
[PubMed]
Knight JA. Review: Free Radicals, Antioxidants, and immune system. Ann Clin Lab Sci. 30: 145-158, 2000.
[PubMed]
Lavie L. Sleep-disordered breathing and cerebrovascular disease: a mechanistic approach. Neurol Clin. 23: 1059-1075, 2005.
[CrossRef]
[PubMed]
Millea PJ. N-acetylcysteine: multiple clinical applications. Am Fam Physician. 80: 265-269, 2009.
[PubMed]
Miquel J. An update on the oxygen stress-mitochondrial mutation theory of aging: genetic and evolutionary implications. Exp Gerontol. 33: 113-126,
1998.
[CrossRef]
Miquel J, Economos AC, Fleming J, Johnson JE, Jr. Mitochondrial role in cell aging. Exp Gerontol. 15: 575-591, 1980.
[CrossRef]
Pomaki M, Mota MJ, De la Fuente M, Berger J. Effect of thiolic antioxidants on in vitro mouse peritoneal macrophage functions. Comp Clin Path.
13: 176-181, 2005.
[CrossRef]
Puerto M, Guayerbas N, Victor VM, De la Fuente M. Effects of N-acetylcysteine on macrophage and lymphocyte functions in a mouse model of premature
ageing. Pharmacol Biochem Behavior 73: 797-804, 2002.
[CrossRef]
Victor VM, De la Fuente M. N-acetylcysteine improves in vitro the function of macrophages from mice with endotoxin-induced oxidative stress.
Free Radic Res. 36: 33-45, 2002.
[CrossRef]
[PubMed]
Victor VM, De la Fuente M. Immune cells redox state from mice with endotoxin-induced oxidative stress. Involvement of NF-kappaB. Free Radic Res. 37:
19-27, 2003.
[CrossRef]
[PubMed]
Victor VM, Rocha M, Esplugues JV, De la Fuente M. Role of free radicals in sepsis: Antioxidant therapy. Curr Pharm Des. 11: 3141-3158, 2005.
[CrossRef]
[PubMed]
Vina J, Gomez-Cabrera MC, Borras C. Fostering antioxidant defences: up-regulation of antioxidant genes or antioxidant supplementation? British J
Nutr. 98: S36-S40, 2007.
[CrossRef]
[PubMed]
Viveros MP, Arranz L, Hernanz A, Miquel J, De la Fuente M. A model of premature ageing in mice based on altered stress-related behavioural response
and immunosenescence. Neuroimmunomodulation. 14: 157-162, 2007.
[CrossRef]
[PubMed]
Wayne SL, Rhyne RL, Garry PJ, Goodwin JS. Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. J Gerontol. 45:
M45-48, 1990.
[PubMed]
Winterbourn CC. Superoxide as an intracellular radical sink. Free Radic Biol Med. 14: 85-90, 1993.
[CrossRef]
Yoon SO, Yun ChH, Cheng AS. Dose effect of oxidative stress on signal transduction in aging. Mech Ageing Develop. 50: 1-8, 2002.
|
BACK
|





