Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 2, p 91-101
DOI 10.2478/v10136-011-0014-3
Modulation of antioxidant enzymes activities and lipid peroxidation products in diet-induced hypercholesterolemic rats fed ortanique peel
polymethoxylated flavones extract
Curtis Omar Green, Andrew O’Brien Wheatley, Donovan Anthony McGrowder, Lowell Lloyd Dilworth, Helen Nosakhare Asemota
Address: Curtis Omar Green, Tropical Medicine Research Institute, University of the West Indies (Mona), Kingston 7, Jamaica W.I.
curtis.green@uwimona.edu.jm
Received 3rd August 2011.
Revised 7th September 2011.
Published online22nd September 2011.
Full text article (pdf)
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
Acknowledgements
References
SUMMARY
The primary aim of this study was to investigate the effects of ortanique peel polymethoxylated flavones extract (PMFort) on antioxidant
enzymes activities and lipid peroxide levels in organs of hypercholesterolemic and normal rats. Thirty Sprague-Dawley rats were fed high cholesterol
diets supplemented with 1.5% PMFort and niacin respectively for 49 days. Hypercholesterolemic rats fed PMFort had significant
reductions in malondialdehyde levels in the liver and brain compared to untreated hypercholesterolemic control rats. This reduction also occurred in
the brain of the rats fed niacin. The activities of catalase, glutathione reductase, transferase and peroxidase were significantly reduced in the
spleen, brain and liver of hypercholesterolemic rats fed PMFort compared to control. The activities of these enzymes were only reduced in
the brain and liver of rats fed niacin. The results would suggest that PMFort modulates hypercholesterolemia-associated organ injury and
oxidative stress in rat organs. PMFort could therefore be a suitable candidate of natural origin for prophylactic and therapeutic
treatment of hypercholesterolemia-associated oxidative stress and organ injury.
KEY WORDS
hypercholesterolemia; ortanique; antioxidant enzymes; polymethoxylated flavones
INTRODUCTION
We have demonstrated in previous publications that
Ortanique peel PMF extract (PMFort) is a very potent
hypolipidemic supplement that significantly reduced
serum total, LDL, VLDL and triglycerides levels and
increased HDL cholesterol levels (Green et al.
2011a). We also derived the mechanism of action
which included reductions in the hepatic activity of
the rate-limiting cholesterol synthesizing enzyme
3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA
HMG-CoA) reductase as well as absorption of
cholesterol in the small intestines due to reductions in
villi length and goblet cell proliferation. Our
histopathological findings also demonstrated that
PMFort ameliorated hypercholesterolemic-associated
organ injury (Green et al. 2011b). Therefore another
objective that warrants further exploration is to
investigate the effects of PMFort on antioxidant
enzymes activity and organ injury. This is important
as several studies have shown that hypercholesterolemia usually results in oxidative stress and
since flavonoids are potent antioxidants they could be
explored as possible supplements for treating
hypercholesterolemia-induced oxidative stress.
Flavonoids are a ubiquitous family of
phytochemicals, inclusive of polymethoxylated
flavones, that exhibit a broad spectrum of
pharmacological properties (Harborne 1993,
Middleton et al. 2000, Manthey and Grohmann 2001,
Green et al. 2007). They represent a highly diverse
class of secondary plant metabolites with about 9000
known structures (Martens and Mithofer 2005).
Flavonoids are common in fruits and vegetables and
may function as natural antioxidants through their
ability to suppress reactive oxygen species (ROS)
formation and due to their lower redox potentials
(Affany et al. 1987, Halliwell and Gutteridge 1999,
Pietta 2000). This is an important property as
antioxidants can act as free radical scavengers and
may also chelate peroxidant metals, reducing their
capacity to produce free radicals. ROS are produced
as normal products of aerobic metabolism but can be
elevated under certain pathophysiological conditions
(Sies 1997, Lee et al. 1998). Reactive oxygen
intermediates such as hydroxyl radicals (HO·),
superoxide anions (O2-) and hydrogen peroxide
(H2O2) at low concentrations may be beneficial or
even indispensable in processes such as intracellular
messaging and defense against micro-organisms
(Schulze-Osthoff et al. 1997, Sun et al. 1998, Vogt et
al. 1998). On the other hand high doses and/
inadequate removal of ROS result in oxidative stress,
which may cause severe metabolic malfunctions and
damage to biological macromolecules (Lledias et al.
1998). A plethora of non-enzymatic and enzymatic
antioxidant defenses exists, including superoxide
dismutase (SOD), glutathione peroxidase (GPX) and
catalase (CAT).
Pathological conditions that predispose to
cardiovascular events, such as hypertension,
hypercholesterolemia, and diabetes, are associated
with oxidative stress (Mataix et al. 1998, Wassmann
et al. 2004). There is considerable evidence that
hypercholesterolemia is a major risk factor for
progression of coronary atherosclerosis and is
associated with an increase in the incidence of
myocardial ischemia and cardiac events (Kannel et al.
1971, Smith et al. 1992). One mechanism that may
underlie this degradation in coronary vascular
function is an alteration in oxidative status. Increased
production of several oxidants such as peroxynitrites,
as well as peroxidation compounds and oxidative
end-products such as PGF2alpha-isoprostanes as well as
a shift in oxidative status with a decrease in nitric
oxide (NO) and an increase in oxygen radicals may
have a deleterious effect on vascular permeability
(Chait et al. 1993, Napoli et al. 1995, Davi et al.
1997). The primary aim of this study was to
determine the effect of PMFort on lipid peroxide levels
and the activities of the endogenous antioxidant
enzymes in the major organs of rats to determine
whether or not they ameliorate hypercholesterolemia-associated organ injury and oxidative
stress.
MATERIALS AND METHODS
Materials
Chemical reagents were purchased from Sigma (St.
Louis, USA). PMFs extract containing tangeretin -
TAN (29%), nobiletin - NOB (24%), tetramethylscutellarein - TMS (23%), sinensetin - SIN (10%),
hexamethyl-o-quercetagetin - QUE (10%) and
heptamethoxyflavone - HMF (4%) was isolated from
Ortanique peel in reagent grade methanol using a
method previously described (Green et al. 2007). The
extract was collected and washed extensively with
hexane to remove the volatile oil constituents and
other hexane soluble contaminants. The hexane-
washed residue was dissolved in chloroform, filtered,
dried by rotary evaporation, and analyzed for PMF
content.
Animals and diets
The animal protocol was approved by the University
of the West Indies (Mona) Ethics Committee. Thirty
adult Sprague-Dawley rats were obtained from the
Animal House of the University of the West Indies,
Mona Campus. They were housed in stainless steel
cages that were maintained daily. The cages were
placed in a room that was kept on a 12/12-hour
light/dark cycle and were fed normal rat chow for one
week. The rats were weighed (average body weight,
343 g), labeled and placed into five groups of six rats
each, as follows: 1. Normal rats fed rat chow, 2.
Normal rats fed 1.5% PMFort, 3. Hypercholesterolemic rats fed a high cholesterol diet (4%
cholesterol and 1% cholic acid), 4. Hypercholesterolemic rats fed a high cholesterol diet
supplemented with 1.5% PMFs extract and, 5.
Hypercholesterolemic rats fed a high cholesterol diet
supplemented with the cholesterol reducing drug
niacin at a dose of 1.5%. Food and water were
provided ad libitum. Body weights were monitored
weekly, and food consumption was estimated daily
for 49 days by giving rats a known amount of food
and by re-weighing food each day and calculating the
difference. At the end of the feeding period, rats were
sacrificed and internal organs were excised, weighed
and frozen in liquid nitrogen and stored at -80 °C
until required for analysis. Antioxidant enzymes
extraction was done according to a modified method
of Ibrahim et al. (1997). Weighed frozen tissue
samples were partially thawed in ice-cold 1.55 mol/l
KCl in 0.05 mol/l phosphate buffer, pH 7.4 using
Teflon Potter-Elvehjem homogenizer. The homogenates were then centrifuged for thirty minutes at
4 °C at 14,000 rpm and supernatant stored -80 °C for
determination of antioxidant enzyme activity
(glutathione-S-transferase, glutathione reductase,
glutathione peroxidase, superoxide dismutase and
catalase). The activity of glutathione-S-transferase
was determined according to the method of Habig et
al. (1974). Glutathione peroxidase activity was
determined according to a modified coupled assay
procedure of Paglia and Valentine (1967).
Glutathione reductase activity was determined by the
method of Carlberg and Mannervik (1985). The
activity of catalase was measured by monitoring the
decomposition of H2O2 as described by Stern (1937)
and Beers and Siger (1952). The activity of
superoxide dismutase (SOD) was measured by using
a SOD assay kit obtained from Dojindo Molecular
Technologies, Inc. (Rockville, USA) according to
instructions within the technical manual, 2006. Lipid
peroxidation was determined by the method of
Okhawa et al. (1979).
Protein was determined according to the method
of Bradford (1976).
Statistical analysis
The results were expressed as mean value ± the
standard error of the mean (SEM). Analysis of the
variance (ANOVA) was used to test for the
differences among all the groups at the significance
level 2=0.05. To find out where the significant
difference occurred among the groups, the Duncan's
multiple range test was used to compare the means.
All statistical analyses were done using the statistical
program SPSS version 16 (2007).
RESULTS
The effects of PMFort on lipid peroxidation in
organs of hypercholesterolemic rat
Fig. 1 shows the lipid peroxide levels in the organs of
rats fed different diets. Rats in the untreated hypercholesterolemic group had significant increases in
liver lipid peroxide levels (3382±480 nmol/ MDAg/g
wet wt). This increase was however significantly
reduced in the groups fed niacin and PMFort. The
reduction in lipid peroxides level in the liver of
hypercholesterolemic rats fed niacin (2000±202.7
nmol/MDAg/g wet wt) was significantly less than that
exhibited by the hypercholesterolemic rats fed PMFort
(741±128 nmol/MDAg/g wet wt). Hypercholesterolemic rats fed PMFort (809±293 nmol/ MDAg/g
wet wt) and niacin (825±68.76 nmol/MDAg/g wet
wt) had significant decreases in the lipid peroxide
levels in the brain relative to the untreated
hypercholesterolemic group (2873±367.2 nmol/
MDAg/g wet wt. No significant changes were
however observed in the lipid peroxide levels in the
spleen, heart and kidneys of rats fed niacin or PMFort.
The effects of PMFort on catalase activity in organs
of hypercholesterolemic rat
Table 1 shows the results of the activity of the
antioxidant enzyme catalase in the organs of
hypercholesterolemic and normal rats fed their
respective diets. Hypercholesterolemic rats whose
diets were supplemented with PMFort and niacin had
significant reductions in the activity of catalase in the
heart (3.09±0.09, 18.07±0.13 micromol H2O2/min/mg
protein), liver (1.01±0.04, 19.94±0.13 micromol
H2O2/min/mg protein), brain (1.13±0.11, 2.23±0.13
micromol H2O2/min/mg protein) and kidney (8.48±0.31,
5.5±0.69 micromol H2O2/min/mg protein) compared to the
untreated hypercholesterolemic group.
The effects of PMFort on glutathione transferase
activity in organs of hypercholesterolemic rat
Table 2 shows the results of the activity of the
antioxidant enzyme glutathione transferase in the
organs of hypercholesterolemic and normal rats fed
their respective diets. Hypercholesterolemic rats
whose diets were supplemented with PMFort had
significant reductions in the activity of glutathione
transferase in the spleen (0.07±0.002 nmol/min/mg
protein), liver (2.05±0.12 nmol/min/mg protein), brain
(0.26±0.01 nmol/min/mg protein) and kidney
(0.014±0.02 nmol/min/mg protein) compared to the
untreated hypercholesterolemic group. Significant
reductions were observed in glutathione transferase
activity in the brain (0.28±0.04 nmol/min/mg protein),
liver (2.37±0.42 nmol/min/mg protein) and kidney
(0.02±0.07 nmol/min/mg protein) but not the spleen
(0.16±0.07 nmol/min/mg protein) of rats fed niacin
compared to the untreated hypercholesterolemic rats.
There were however, no significant reductions in
glutathione transferase activity in the heart in any of
the treatment groups.
The effects of PMFort on superoxide dismutase
activity in organs of hypercholesterolemic rat
Fig. 2 shows the effect of the treatment diets on SOD
activity in the organs. Hypercholesterolemic rats fed
PMFort and niacin had significant reductions in the
activity of SOD in the heart (36.7±0.33, 36.9±1%
inhibition) and liver (35.01±1.89, 37.9±1.05%
inhibition) compared to the untreated hyper-cholesterolemic rats. No significant changes in the
SOD activity were observed in the spleen, kidney and
brain of the hypercholesterolemic rats whose diets
were supplemented with PMFort relative to the
untreated hypercholesterolemic rats.
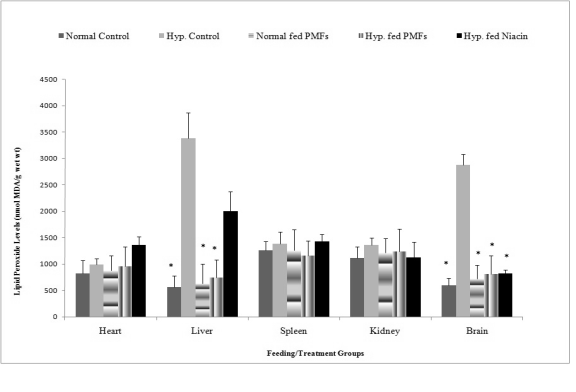
Fig. 1. Effects of ortanique peel PMFs extract on lipid peroxide levels in organ homogenates.
* statistically significant versus Hyp. fed H.C diet, H.C = high cholesterol, Hyp. = hypercholesterolemia
Table 1. Effect of PMFort on the activity of catalase in rat organs.
|
Catalase activity (mol H2O2/min/mg
protein) | | Feeding/treatment groups |
Spleen |
Brain |
Liver |
Heart |
Kidney | | Normal fed normal diet |
172±10.45* |
2.95±0.14* |
5.22±0.22* |
10.50±0.56* |
5.82±0.47* | | Normal fed PMFs extract |
158.21±9.62* |
3.18±0.10* |
2.37±0.36* |
19.4±0.10* |
4.38±0.21* | | Hyp. fed H.C. diet |
223.87±5.85 |
21.49±0.14 |
114.21±9.21 |
216.98±20.14 |
15.45±0.84 | | Hyp. fed PMFs extract |
188.73±9.77* |
1.13±0.11* |
1.01±0.04* |
3.09±0.09* |
8.48±0.31* | | Hyp. fed niacin |
229.75±13.34 |
2.23±0.12* |
19.94±0.13* |
18.07±0.13* |
5.51±0.69* |
* statistically significant as compared with Hyp. fed H.C diet. H.C = high cholesterol, Hyp. = hypercholesterolemia
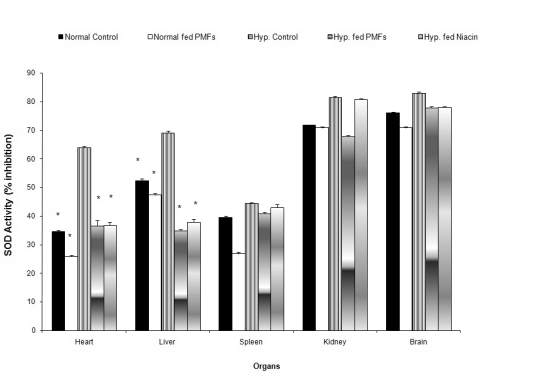
Fig. 2. Effect of PMFort on the activity of superoxide dismutase in organs of rats.
* symbols as in Fig. 1
Table 2. Effect of PMFort on the activity of glutathione transferase in rat organs
|
Glutathione transferase activity (nmol/min/mg
protein) | | Feeding/treatment groups |
Spleen |
Brain |
Liver |
Heart |
Kidney | | Normal fed normal diet |
0.08±0.002* |
0.25±0.03* |
1.46±0.55* |
0.21±0.013 |
0.01±0.001* | | Normal fed PMFs extract |
0.09±0.02* |
0.28±0.02* |
1.5±0.41* |
0.19±0.013 |
0.016±0.18* | | Hyp. fed H.C. diet |
0.12±0.02 |
0.44±0.05 |
3.54±0.68 |
0.22±0.038 |
0.053±0.03 | | Hyp. fed PMFs extract |
0.07±0.002* |
0.26±0.01* |
2.05±0.17* |
0.18±0.12 |
0.014±0.018* | | Hyp. fed niacin |
0.16±0.07 |
0.28±0.04* |
2.37±0.42* |
0.21±0.057 |
0.020±0.065* |
* symbols as in Table 1
The effects of PMFort on glutathione reductase
activity in organs of hypercholesterolemic rat
Fig. 3 shows the results of the activity of the
antioxidant enzyme glutathione reductase in the
organs of hypercholesterolemic and normal rats fed
their respective diets. Hypercholesterolemic rats
whose diets were supplemented with PMFort had
significant reductions in the activity of glutathione
reductase in the liver (18.2±1.15 nmol/min/mg
protein), brain (11.45±2.25 nmol/min/mg protein), spleen (20.56±2.89 nmol/min/mg protein) and heart
(10.24±1.59 nmol/min/mg protein) when compared
with the untreated hypercholesterolemic group. There
were however, no significant reductions in
glutathione reductase activity in the liver (47.23±1.33
nmol/min/mg protein), spleen (39.48±1.66 nmol/
min/mg protein) or brain (18.95±3.33 nmol/min/mg
protein) except in the heart as a result of niacin
supplementation. Also, no significant increase was
observed in the activity of glutathione transferase in
the kidney in any of the treatment groups.
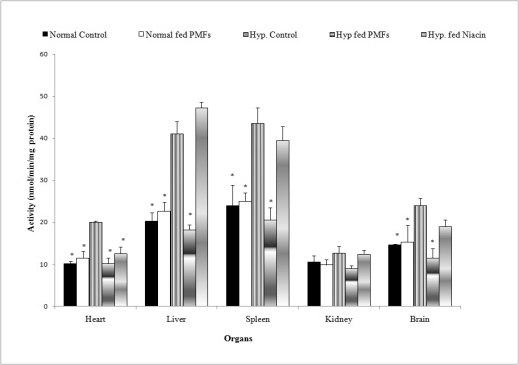
Fig. 3. Effect of PMFort on the activity of glutathione reductase in organs of rats.
* symbols as in Fig. 1
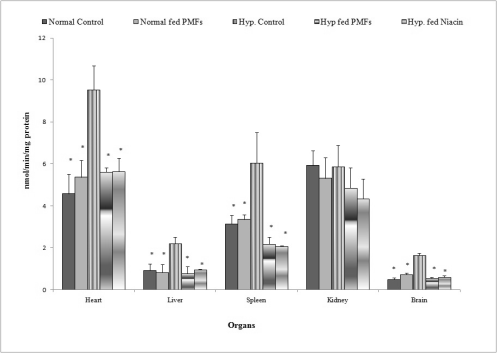
Fig. 4. Effect of PMFort on the activity of glutathione peroxidase in organs of rats.
* symbols as in Fig. 1
The effects of PMFort on glutathione peroxidase
activity in organs of hypercholesterolemic rat
Fig. 4 shows the results of the activity of the
antioxidant enzyme glutathione peroxidase in the
organs of hypercholesterolemic and normal rats fed
their respective diets. Hypercholesterolemic rats
whose diets were supplemented with PMFort and
niacin had significant reductions in the activity of
glutathione peroxidase in the liver (0.78±0.33,
0.95±0.02 nmol/min/mg protein), brain (0.54±0.07,
0.59±0.09 nmol/min/mg protein), spleen (2.18±0.33,
2.07±0.034 nmol/min/mg protein) and heart
(5.61±0.22, 5.64±0.63 nmol/min/mg protein)
compared to the untreated hypercholesterolemic
group. There were however no significant increase in
the activity of glutathione transferase in the kidney in
any of the treatment groups.
DISCUSSION
In hypercholesterolemia, one of the mechanisms that
might be activated and might hinder coronary
vascular function is a shift in scavenging activity and
redox status, a state known as increased oxidative
stress (Montilla et al. 2004). Numerous studies show
that a close relationship exists between high blood
cholesterol and atherosclerosis, it has also been
suggested that this relationship may be dependent on
enhanced oxidative stress (Domagala et al. 1989,
Ohara et al. 1993, Prasad et al. 1997). Studies have
also shown that cholesterol rich diet increases the
formation of peroxynitrile, a toxic reaction product of
superoxide and nitric oxide in the rat myocardium
(Onody et al. 2003). Work done by Eddy et al. (1998)
showed that rats that develop hypercholesterolemia
consuming a high fat diet had elevations in renal lipid
peroxidation products compared to rats that were fed
normal rat chow.
Parmar and Kar (2007) reported that citrus peel
extract reduced lipid peroxidation levels in the liver
and kidneys of animal model of diet-induced
atherosclerosis and thyroid dysfunctions. To our
knowledge, this study is the first report on the effect
of PMFort on lipid peroxide levels in hyper-cholesterolemic rats. The results showed that
supplementation of the diet of hypercholesterolemic
rats with PMFort resulted in significant reductions in
the lipid peroxide levels in the liver and brain
compared to the untreated hypercholesterolemic
group. Supplementation of the diet of hyper-cholesterolemic rats with niacin however significantly
increased lipid peroxide levels in the liver relative to
the hypercholesterolemic group fed PMFort. No
significant changes were however observed in the
lipid peroxide levels in the kidney, spleen and heart in
any of the groups studied.
Studies have shown that antioxidant therapy
attenuates elevations in lipid peroxidation products
(Eddy et al. 1998). The PMFs extract may have
reduced lipid peroxidation by acting as antioxidants
and hence aiding the endogenous antioxidant
enzymes (e.g. superoxide dismutase, glutathione
peroxidase and catalase) involved in the
scavenging/inactivation of the reactive oxygen
species or redox metal ions before lipid peroxidation
takes place (Sung and Park 1999).
Several studies show that in nearly all regions of
the world heart failure is both common and on the rise
(McMurray and Stewart 2000, Mendez and Cowie
2001). Cardiovascular diseases such as hypertension,
diabetes and hypercholesterolemia are risk factors for
heart failure and hence studies aimed at reducing or
preventing the occurrence of these disorders have
increased in recent years (Sanderson and Tse 2003).
Several studies show that feeding animals a high
cholesterol diet results in intracellular lipid
accumulation in cardiomyocyte as well as alterations
in the structural and functional properties of the
myocardium (Melax and Leeson 1975, Hexeberg et
al. 1993, Onody et al. 2003). Work done by Prasad et
al. (1997) showed that diet-induced hypercholesterolemia produces oxidative stress in the myocardium.
Hypercholesterolemia also resulted in increases in the
levels of lipid peroxidation product malondialdehyde
(MDA) and chemiluminescence (M-CL), a measure
of antioxidant reserve, and activities of antioxidant
enzymes (CAT, and GSH-Px) but a decrease in the
activity of SOD in cardiac muscles of rabbits (Prasad
et al. 1997).
Prior to this study the effect of PMFs on
antioxidant enzymes activities in the heart of
hypercholesterolemic rats was never assessed. The
results in this study showed that supplementation of
the diets of hypercholesterolemic rats with PMFort and
niacin resulted in significant reductions in the
activities of catalase, superoxide dismutase,
glutathione reductase and glutathione peroxidase in
the heart compared to the untreated hypercholesterolemic group. No significant differences
were however observed in the activity of glutathione
transferase in the heart in any of the groups studied.
Several studies have shown that feeding rats a
high cholesterol diet results in hepatic steatosis and
elevations in antioxidant enzymes activities in the
liver (Mahfouz and Kummerow 2000, Perlemuter et
al. 2005). Reports on the hepatic activity of
antioxidant enzymes in hypercholesterolemic rats
tend to indicate elevated activities as a result of citrus
consumption. Kim et al. (2004) reported that
superoxide dismutase, catalase, and glutathione
reductase activities were all significantly increased by
supplementation of the citrus flavonoid naringin in
hypercholesterolemic mice compared to control.
Deyhim et al. (2006) also reported elevations in
antioxidant enzymes activities in orchidectomized rats
as a result of citrus juice consumption. In this study,
however, supplementation of the diets of
hypercholesterolemic rats with PMFort resulted in
significant reductions in the hepatic activities of
catalase, glutathione peroxidase, glutathione
transferase and glutathione reductase compared to the
untreated hypercholesterolemic group. Supplementation of the diets of hypercholesterolemic rats
with niacin also resulted in significant reductions in
the hepatic activities of catalase and glutathione
peroxidase but did not significantly alter the activity
of glutathione reductase compared to the untreated
hypercholesterolemic group.
The potential link between dyslipidemia and renal
damage was first postulated by Moorhead et al
(1982). Since then numerous dietary studies showed
that hypercholesterolemia causes oxidative stress in
the kidneys of experimental animal models (Stulak et
al. 2001, Chade et al. 2002, 2007, Montilla et al.
2004). Supplementation of the diets of
hypercholesterolemic rats with PMFort and niacin
resulted in significant reductions in the activities of
catalase and glutathione transferase in the kidney but
had no significant effect on the activities of
glutathione reductase, glutathione peroxidase or
superoxide dismutase compared to the untreated
hypercholesterolemic group.
There is a very high possibility for oxidative
damage to brain tissue because of its high rate of
oxidative metabolic activity, intensive production of
reactive oxygen metabolites, relatively low
antioxidant capacity, low repair mechanism activity,
non-replicating nature of its neuronal cells, and the
high membrane surface to cytoplasm ratio (Evans
1993, Reiter 1995). Brain tissue contains various
types of antioxidants, some of them unique to the
brain (Shohami et al. 1997). The two major types of
antioxidant systems in the brain are enzymes and
low-molecular weight antioxidants (LMWA).
Superoxide dismutase, catalase and peroxidase, as
well as some supporting enzymes are present. The
activities of SOD and glutathione peroxidase, are
quite high, whereas the activity of catalase is
relatively low (Sinet et al. 1980, Reiter 1995).
In this study supplementation of the diets of
hypercholesterolemic rats with PMFort resulted in
significant reductions in the activities of catalase,
glutathione transferase, glutathione peroxidase and
glutathione reductase in the brain compared to the
untreated hypercholesterolemic rats. Supplementation
of the diets of hypercholesterolemic rats with niacin
however, only resulted in significant reductions in the
activities of catalase and glutathione transferase in the
brain compared to the untreated hypercholesterolemic
rats.
Several studies show that oxidative stress is
implicated in brain injury after ischemia and
reperfusion. Lipid peroxidation, protein oxidation,
and DNA damage are induced by the ROS produced
during cerebral ischemia (Traystman et al. 1991,
Chan 2001). PMFort could therefore be used
prophylactically and therapeutically in treating
injuries to the brain caused by hypercholesterolemia.
To our knowledge, there are no published reports
on the effects of PMFs on antioxidant activities in the
spleen of hypercholesterolemic rats. Supplementation
of the diet of hypercholesterolemic rats with PMFort
resulted in significant reductions in the activities of
glutathione peroxidase, glutathione reductase,
glutathione transferase and catalase but resulted in no
significant changes in the activities of superoxide
dismutase compared to the untreated hyper-cholesterolemic group. Supplementation of the diet
with niacin significantly reduced the activity of
glutathione peroxidase but resulted in no significant
changes in the activities of catalase, glutathione
transferase, glutathione reductase or superoxide
dismutase compared to untreated hypercholesterolemic group. PMFort could therefore be beneficial in
the prevention or amelioration of splenic injury by
aiding the endogenous antioxidant system in the
spleen.
Oxidative stress in hypercholesterolemia may
occur by virtue of augmented generation of ROS and
oxidation of LDLs (Lerman and Textor 2001, Chade
et al. 2002). PMFort may be decreasing the activities
of the endogenous antioxidant enzymes by acting as
antioxidants and are being preferentially oxidised by
free radicals. This property of PMFort is important as
both animal and human studies show a positive role
for antioxidants in the prevention of coronary heart
disease - CHD (Kritharides and Stocker 2002).
On evaluation of integrity of organs following
consumption of PMFort, the results suggest that the
integrity of the organs - kidney, spleen, brain, liver
and heart - was preserved in each, as there were no
significant increases in lipid peroxides levels or
antioxidant enzymes activities in the organs of normal
or hypercholesterolemic rats fed PMFort. In the
hypercholesterolemic state, the levels and activities of
lipid peroxidation products and antioxidant enzymes
in rat organs were increased. In fact PMFort
ameliorated oxidative stress caused by hyper-cholesterolemia, as shown in the significant
reductions in lipid peroxide levels as well as
reductions in antioxidant enzyme activities in the
liver, heart, kidney, spleen and brain. PMFort could
therefore be a suitable candidate for further
investigations that involve prophylactic or therapeutic
treatment of hypercholesterolemia.
ACKNOWLEDGEMENTS
The authors of this paper are grateful to the School of
Graduate Studies and Research for funding, Dr.
Dewayne Stennett and Mr. Dennis Bailey for their
assistance.
REFERENCES
Affany A, Salvayre R, Douste-Blazy L. Comparison of the protective effect of various flavonoids against lipid peroxidation of erythrocyte membranes
(induced by cumene hydroperoxide). Fundam Clin Pharmacol. 1: 451-457, 1987.
[CrossRef]
Beers RF, Jr., Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 195: 133-140,
1952.
[PubMed]
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.
Anal Biochem. 72: 248-254, 1976.
[CrossRef]
Carlberg I, Mannervik B. Glutathione reductase assay. In Colowick SP, Kaplan NO (eds.): Methods in Enzymology, vol. 113. Academic Press, Orlando, FL,
pp. 484–495, 1985.
[PubMed]
Chade AR, Krier JD, Galili O, Lerman A, Lerman LO. Role of renal cortical neovascularization in experimental hypercholesterolemia. Hypertension. 50:
729-736, 2007.
[CrossRef]
[PubMed]
Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and
renovascular disease. Circulation. 106: 1165-1171, 2002.
[CrossRef]
[PubMed]
Chait A, Brazg RL, Tribble DL, Krauss RM. Susceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the
atherogenic lipoprotein phenotype, pattern B. Am J Med. 94: 350-356, 1993.
[CrossRef]
Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 21: 2-14, 2001.
[CrossRef]
Davi G, Alessandrini P, Mezzetti A, Minotti G, Bucciarelli T, Costantini F, Cipollone F, Bon GB, Ciabattoni G, Patrono C. In vivo formation of
8-Epi-prostaglandin F(2alpha) is increased in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 17: 3230-3235, 1997.
[CrossRef]
[PubMed]
Deyhim F, Lopez E, Gonzalez J, Garcia M, Patil BS. Citrus juice modulates antioxidant enzymes and lipid profiles in orchidectomized rats. J Med Food.
9: 422-426, 2006.
[CrossRef]
[PubMed]
Domagala B, Hartwich J, Szczeklik A. Incides of lipid peroxidation in patients with hypercholesterolemia and hypertriglyceridemia. Wien Klin
Wochenschr. 101: 425-428, 1989.
[PubMed]
Eddy AA, Lui E, McCulloch L. Interstitial fibrosis in hypercholesterolemic rats: role of oxidation, matrix synthesis, and proteolytic cascades.
Kidney Int. 53: 1182-1189, 1998.
[CrossRef]
[PubMed]
Evans PH. Free radicals in brain metabolism and pathology. Br Med Bull. 49: 577-587, 1993.
[PubMed]
Green CO, Wheatley AO, Osagie AU, Morrison E St. A, Asemota HN. Determination of polymethoxylated flavones in peels of selected Jamaican and Mexican
citrus (Citrus spp.) cultivars by high-performance liquid chromatography. Biomed Chromatogr. 21: 48-54, 2007.
[CrossRef]
[PubMed]
Green CO Wheatley AO, McGrowder DA, Dilworth LL, Asemota HN. Hypolipidemic effects of Ortanique peel polymethoxylated flavones in rats with
diet-induced hypercholesterolemia. J Food Biochem. 35: 1555-1560, 2011a.
[CrossRef]
Green CO, Wheatley AO, Hanchard B, Gibson TN, McGrowder DA, Dilworth LL Asemota HN. Histopathological alterations in organ structures of
hypercholesterolemic rats fed Ortanique peel polymethoxylated flavones. Basic Appl Pathol. 4: 71-77, 2011b.
[CrossRef]
Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 249: 7130-7139,
1974.
[PubMed]
Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 3rd edition. University Press, Oxford, 1999.
Harborne J. Plant Flavonoids in Biology and Medicine. Alan R. Liss, New York 1993.
Hexeberg S, Willumsen N, Rotevatn S, Hexeberg E, Berge RK. Cholesterol induced lipid accumulation in myocardial cells of rats. Cardiovasc Res. 27:
442-446, 1993.
[CrossRef]
[PubMed]
Ibrahim W, Lee US, Yeh CC, Szabo J, Bruckner G, Chow CK. Oxidative stress and antioxidant status in mouse liver: effects of dietary lipid, vitamin E
and iron. J Nutr. 127: 1401-1406, 1997.
[PubMed]
Kannel WB, Castelli WP, Gordon T, McNamara PM. Serum cholesterol, lipoproteins, and the risk of coronary heart disease: The Framingham study. Ann
Intern Med. 74: 1-12, 1971.
[PubMed]
Kim HJ, Oh GT, Park YB, Lee MK, Seo HJ, Choi MS. Naringin alters the cholesterol biosynthesis and antioxidant enzyme activities in LDL
receptor-knockout mice under cholesterol fed condition. Life Sci. 74: 1621-1634, 2004.
[CrossRef]
Kritharides L, Stocker R. The use of antioxidant supplements in coronary heart disease. Atherosclerosis. 164: 211-219, 2002.
[CrossRef]
Lee YJ, Galoforo SS, Berns CM, Chen JC, Davis BH, Sim JE, Corry PM, Spitz DR. Glucose deprivation-induced cytotoxicity and alterations in
mitogen-activated protein kinase activation are mediated by oxidative stress in multidrug-resistant human breast carcinoma cells. J Biol Chem. 273:
5294-5299, 1998.
[CrossRef]
[PubMed]
Lerman L, Textor SC. Pathophysiology of ischemic nephropathy. Urol Clin North Am. 28: 793-803, ix, 2001.
Lledias F, Rangel P, Hansberg W. Oxidation of catalase by singlet oxygen. J Biol Chem. 273: 10630-10637, 1998.
[CrossRef]
[PubMed]
Mahfouz MM, Kummerow FA. Cholesterol-rich diets have different effects on lipid peroxidation, cholesterol oxides, and antioxidant enzymes in rats and
rabbits. J Nutr Biochem. 11: 293-302, 2000.
[CrossRef]
Manthey JA, Grohmann K. Phenols in citrus peel byproducts. Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses.
J Agric Food Chem. 49: 3268-3273, 2001.
[CrossRef]
[PubMed]
Martens S, Mithofer A. Flavones and flavone synthases. Phytochemistry. 66: 2399-2407, 2005.
[CrossRef]
[PubMed]
Mataix J, Quiles JL, Huertas JR, Battino M, Manas M. Tissue specific interactions of exercise, dietary fatty acids, and vitamin E in lipid
peroxidation. Free Radic Biol Med. 24: 511-521, 1998.
[CrossRef]
McMurray JJ, Stewart S. Epidemiology, aetiology, and prognosis of heart failure. Heart. 83: 596-602, 2000.
[CrossRef]
[PubMed]
Melax H, Leeson TS. Comparative electron microscope studies of the myocardium in adult rats fed on normal and cholesterol diets. J Mol Cell Cardiol.
7: 195-202, 1975.
[CrossRef]
Mendez GF, Cowie MR. The epidemiological features of heart failure in developing countries: a review of the literature. Int J Cardiol. 80: 213-219,
2001.
[CrossRef]
Middleton E, Jr., Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and
cancer. Pharmacol Rev. 52: 673-751, 2000.
[PubMed]
Montilla P, Espejo I, Munoz MC, Bujalance I, Munoz-Castaneda JR, Tunez I. Effect of red wine on oxidative stress and hypercholesterolemia induced by
feeding a high-cholesterol diet in rat. J Physiol Biochem. 60: 259-264, 2004.
[CrossRef]
[PubMed]
Moorhead JF, Chan MK, El-Nahas M, Varghese Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 2:
1309-1311, 1982.
[CrossRef]
Napoli C, Postiglione A, Triggiani M, Corso G, Palumbo G, Carbone V, Ruocco A, Ambrosio G, Montefusco S, Malorni A, Condorelli M, Chiariello M.
Oxidative structural modifications of low density lipoprotein in homozygous familial hypercholesterolemia. Atherosclerosis. 118: 259-273, 1995.
[CrossRef]
Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 91: 2546-2551, 1993.
[CrossRef]
[PubMed]
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95: 351-358, 1979.
[CrossRef]
Onody A, Csonka C, Giricz Z, Ferdinandy P. Hyperlipidemia induced by a cholesterol-rich diet leads to enhanced peroxynitrite formation in rat hearts.
Cardiovasc Res. 58: 663-670, 2003.
[CrossRef]
Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 70:
158-169, 1967.
[PubMed]
Parmar HA, Kar A. Protective role of Citrus sinensis, Musa paradisiaca, and Punica granatum peels against diet-induced
atherosclerosis and thyroid dysfunctions in rats. Nutr Res. 27: 710-718, 2007.
[CrossRef]
Perlemuter G, Davit-Spraul A, Cosson C, Conti M, Bigorgne A, Paradis V, Corre MP, Prat L, Kuoch V, Basdevant A, Pelletier G, Oppert JM, Buffet C.
Increase in liver antioxidant enzyme activities in non-alcoholic fatty liver disease. Liver Int. 25: 946-953, 2005.
[CrossRef]
[PubMed]
Pietta PG. Flavonoids as antioxidants. J Nat Prod. 63: 1035-1042, 2000.
[CrossRef]
[PubMed]
Prasad K Mantha SV, Kalra J, Lee P. Hypercholesterolemia-induced oxidative stress in heart and its prevention by vitamin E. Inter J Angiology 6:
13-17, 1997.
[CrossRef]
Reiter RJ. Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J. 9: 526-533, 1995.
[PubMed]
Sanderson JE, Tse TF. Heart failure: a global disease requiring a global response. Heart. 89: 585-586, 2003.
[CrossRef]
[PubMed]
Schulze-Osthoff K, Bauer MK, Vogt M, Wesselborg S. Oxidative stress and signal transduction. Int J Vitam Nutr Res. 67: 336-342, 1997.
[PubMed]
Shohami E, Beit-Yannai E, Horowitz M, Kohen R. Oxidative stress in closed-head injury: brain antioxidant capacity as an indicator of functional
outcome. J Cereb Blood Flow Metab. 17: 1007-1019, 1997.
[CrossRef]
Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 82: 291-295, 1997.
[PubMed]
Sinet PM, Heikkila RE, Cohen G. Hydrogen peroxide production by rat brain in vivo. J Neurochem. 34: 1421-1428, 1980.
[CrossRef]
[PubMed]
Smith GD, Shipley MJ, Marmot MG, Rose G. Plasma cholesterol concentration and mortality. The Whitehall Study. JAMA. 267: 70-76, 1992.
[CrossRef]
Stern K. On the absorbance of catalase. J Biol Chem. 121: 561-567, 1937.
Stulak JM LA, Porcel MR, Caccitolo JA, Romero JC, Schaff HV, Napoli C, Lerman LO. Renal vascular function in hypercholesterolemia is preserved by
chronic antioxidant supplementation. J Am Soc Nephrol. 12: 1882-1891, 2001.
[PubMed]
Sun J, Chen Y, Li M, Ge Z. Role of antioxidant enzymes on ionizing radiation resistance. Free Radic Biol Med. 24: 586-593, 1998.
[CrossRef]
Sung MK, Park MY. Effect of soybean saponins on the growth and antioxidant defense of human hepatocarcinoma cells. Third symposium on the role of the
soy in preventing and treating chronic disease. October 31-November 3, 1999. Omni Shoreham Hotel Washington, DC. USA D-3, 1999.
Traystman RJ, Kirsch JR, Koehler RC. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol. 71: 1185-1195,
1991.
[PubMed]
Vogt M, Bauer MK, Ferrari D, Schulze-Osthoff K. Oxidative stress and hypoxia/reoxygenation trigger CD95 (APO-1/Fas) ligand expression in microglial
cells. FEBS Lett. 429: 67-72, 1998.
[CrossRef]
Wassmann S, Wassmann K, Nickenig G. Modulation of Oxidant and Antioxidant Enzyme Expression and Function in Vascular Cells. Hypertension. 44:
381-386, 2004.
[CrossRef]
[PubMed]
|
BACK
|





