Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 2, p 71-78
DOI 10.2478/v10136-011-0015-2
Pharmacokinetics of acetylcholinesterase reactivator K203 and consequent evaluation of low molecular weight antioxidants/markers of oxidative stress
Jana Zdarova Karasova, Daniela Hnidkova, Miroslav Pohanka, Kamil Musilek, Robert Peter Chilcott, Kamil Kuca
Address: Jana Zdarova Karasova, Department of Public Health, Faculty of Military Health Sciences, Trebesska 1575, 500 01 Hradec Kralove, Czech
Republic
karasova@pmfhk.cz
Received 12th July 2011.
Revised 14th September 2011.
Published online 24th October 2011.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
Acknowledgements
References
SUMMARY
Oxime K203 is a new compound designed to be used as an acetylcholinesterase reactivator for the treatment of intoxication following exposure to tabun
and certain pesticides. After intramuscular administration of a therapeutic (23 mg/kg) dose, the time-course of plasma concentrations of K203 in rats
was determined by HPLC. Maximum concentrations were reached between 40 and 60 min (16.5±2.1 microg/ml in 40 min and 16.6±2.0 in 60 min, respectively)
with the concentration being relatively constant during this period. There was no significant effect on the plasma concentration of thiobarbituric
acid reactive substances (TBARS) during the administration of K203, indicating an absence of oxidative stress. Indeed, administration of K203 led to
a significant increase in low molecular weight antioxidants which could tentatively be interpreted as representing a beneficial effect.
KEY WORDS
K203; nerve agent treatment; HPLC; plasma concentration; absorption
INTRODUCTION
Pharmacokinetic studies of acetylcholinesterase
(AChE; E.C. 3.1.1.7) reactivators began after the
discovery by Wilson and Ginsburg (1955) of their
benefit in the clinical treatment of poisoning caused
by organophosphorus inhibitors (OPI). They also
demonstrated that pralidoxime reactivates
alkylphosphate-inhibited AChE, and so identified a
new class of therapeutic agents (based on pyridinium
aldoximes) for the treatment of OPI intoxication
(Wilson and Ginsburg 1955). This initial finding was
subsequently characterized through ADME
(absorption, distribution, metabolism and excretion)
studies (Vojvodic and Maksimovic 1972, Utley 1987)
prior to the adoption of pralidoxime (mono-
pyridinium-mono-aldoxime) as a standard therapy for
OPI exposure (Jokanovic and Prostran 2009).
The discovery of the efficacy of pralidoxime
subsequently stimulated work to synthesise a range of
related compounds such as the bis-pyridinium-
bis-aldoximes (e.g. obidoxime, methoxime and
trimedoxime) (Kassa et al. 2011). Whilst such
compounds exhibit improved efficacy, they do not
eliminate associated toxicity (Bartosova et al. 2006).
However, further work led to the synthesis of less
toxic compounds such as bis-pyridinium-
mono-aldoxime (HI-6) (Eyer et al. 1998) or other
untypical structures (Guimaraes et al. 2011).
According to clinical experience, HI-6 is able to
shorten the duration of the respiratory muscle
paralysis by reactivation of inhibited AChE.
However, the overall efficacy of oximes is still open
to debate (Eddleston et al. 2002, Kassa and Karasova
2007, Kassa et al. 2009) and it is conceivable that the
mechanism of action of oximes is not mediated solely
through the reactivation of inhibited AChE (Pejchal
et al. 2008, Pohanka et al. 2009, Soukup et al. 2010).
More recent work includes the synthesis and
development of the "K-series" of aldoxime-based
AChE reactivators which have a demonstrable ability
to reverse AChE inhibition following exposure to
certain OPIs (Kalasz et al. 2006, Tekes et al. 2006,
Petroianu et al. 2007, da Silva et al. 2008, Zdarova
Karasova et al. 2009, Kovarik et al. 2009), with
improved survival rates (Nurulain et al. 2009). Based
on in vitro and in vivo studies, oxime K203
(bis-pyridinium- mono-aldoxime; Fig. 1) is a
promising new reactivator with relatively low acute
toxicity which may be of particular benefit in the
treatment of tabun poisoning (Kovarik et al. 2009,
Kassa et al. 2011).
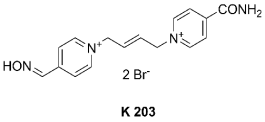
Fig. 1. Structure of tested oxime.
The purpose of this study was to characterize the
pharmacokinetics of oxime K203 following
intramuscular (i.m.) administration in the rat.
Quantification of plasma K203 concentrations was
performed by HPLC. Methods for the HPLC
determination of plasma oxime concentrations have
been previously described (Brown et al. 1978,
Benshop et al. 1981, Guyon et al. 1982, Houze et al.
2005, Zdarova Karasova et al. 2010). We report here
a simplified and sensitive method for the
determination of therapeutic concentrations of K203
in plasma which incorporates an improved method for
sampling with an oxime preparation based on protein
precipitation (Zdarova Karasova et al. 2010). The
secondary objective of this study was to assess related
changes in antioxidants after the application of oxime
K203, by measurement of plasma thiobarbituric acid
reactive substances (TBARS), and ferric reducing
antioxidant power (FRAP).
MATERIAL AND METHODS
Chemicals
K203 [1-(4-hydroxyiminomethylpyridinium)-
4-(4-carbamoylpyridinium)-but-2-ene dibromide]
was synthesized in our laboratory as previously
described. The purity of prepared oximes was
approximately 98-99% (TLC, mp, NMR) (Musilek et
al. 2007). Other chemicals were purchased from
commercial sources (Merck, Darmstadt, Germany and
Sigma-Aldrich, Steinheim, Germany) and were
analytical reagent grade. Solutions were prepared
using double distilled, deionized (HPLC grade) water.
Instrumentation
Samples were analysed by reversed phase HPLC with
UV detection. The HPLC system consisted of a P200
gradient pump (Spectra-Physics Analytical, Fremont,
USA), a 7125 injection valve comprising 10 microl loop
(Rheodyne, Cotati, USA), an UV1000 detector
(Spectra-Physics Analytical, Fremont, USA)
controlled by CSW Clarity 2.6.5.517 software
(DataApex, Prague, Czech Republic). Separation was
achieved using a LiChrospher® 60, 250 × 4.6 (5 microm)
column distal to a standard guard column (4 × 4
RP-select B; Merck, Damstadt, Germany). The
mobile phase was 24% aqueous acetonitrile
containing 5 mmol/l octane sulfonic acid and
5 mmol/l tetramethylammonium chloride. The pH
was adjusted to 2.3 with phosphoric acid (H3PO4).
The flow rate of the mobile phase was 1 ml/min.
Detection was achieved by UV absorption (286 nm)
using an UV1000 detector.
Animal treatment
The use of animals in this study was approved by the
Ethics Committee of the Medical Faculty, Charles
University, Czech Republic. Male Wistar rats (Anlab
Inc., Prague, Czech Republic) were kept in a climate
controlled animal house (temperature 22±2 °C, hu-midity 55±6%) under a 12h:12h light; dark cycle and
were allowed access to standard laboratory food and
water ad libitum.
A total of seven animals were used in this study
(average body weight 320±10 g). After 7 days
acclimatization, each animal was anaesthetized by i.p.
injection of pentobarbital (administered intra-peritoneally; 50 mg/kg body weight) (Novotny et al.
2009). The carotid artery and jugular vein were
cannulated to enable serial removal of arterial blood
samples (300 microl) or venous replacement of 300 microl
saline before administration of the oxime and at
regular intervals thereafter (3, 5, 10, 20, 40, 60, 90,
120 and 180 min) (Zdarova Karasova et al. 2011).
The oxime was administered via i.m. injection (23
mg/kg; prepared in situ using 0.9% saline).
Blood samples were heparinised and the cellular
fraction removed by centrifugation (1600 g, 10 min,
4 °C, Universal 320R, Hettich, Germany). The re-maining plasma samples were stored at -80 °C prior
to analysis.
Sample preparation for HPLC analysis
Samples of plasma (60 l) were mixed with an equal
volume of acetonitrile in order to precipitate proteins.
The samples were then spun at 12,000 g at 4 °C for
15 minutes in a centrifuge (M 240R, Hettich,
Germany). The resulting supernatant was used
immediately for HPLC analysis without any further
processing (Zdarova Karasova et al. 2010).
Calibration
A calibration curve was established using plasma
samples spiked with K203 (1, 6.25, 12.5, 25, 50, 75
and 100 microg/ml samples, in triplicates). These were
stored at 0 °C for up to 24 hours prior to analysis
(which resulted in no detectable decrease in oxime
concentration). The retention time of K203 was ~5.3
min.
Plasma thiobarbituric acid reactive substances
(TBARS)
Samples of plasma (100 microl) were mixed with 200 microl
of 10% (v/v) trichloroacetic acid (TCA) in water. The
mixture was incubated for 15 min and spun at 5,000 g
for 15 min at 4 °C. The resulting supernatant was then
mixed with 200 microl of thiobarbituric acid (0.67% w/v)
and incubated for 10 min at 100 °C prior to
spectrophotometric measurements of absorbance at
532 nm (compared against a blank containing saline).
Ferric reducing antioxidant power (FRAP)
A stock solution of reagent was prepared by the
addition of 2.5 ml 2,4,6-tris(2pyridyl)-s-triazine
(TPTZ; 10 mM, dissolved in 40 mM HCl) to 2.5 ml
aqueous FeCl3 (20 mM), which was then mixed with
25 ml of 0.1 M acetate buffer (pH 3.6). The mixture
was incubated at 37 °C for 10 min. Aliquots (200 microl)
of the TPTZ reagent were then added to 30 microl of
either plasma samples or phosphate buffer prior to
mixing in 1 ml distilled water. The resulting solutions
were incubated for 10 min at 37 °C and then
centrifuged at 10,000 g for 10 min prior to
spectrophotometric measurements of absorbance at
593 nm (compared against a phosphate buffer blank).
Statistics
Pharmacokinetics profile and calibration curve: The
amounts of K203 in each sample were converted to
concentration by interpolation of the calibration curve
using the data analysis and statistical software Prism4
(Graph Pad Software, San Diego, USA). The
pharmacokinetics profile is calculated as mean ±
standard deviation (n = 7; identical time interval).
FRAP and TBARS statistics: Origin 8 (OriginLab
Corporation, Northampton, USA) was used for
significance testing by Bonferroni test at the
significance level 2alpha = 0.05.
RESULTS
The calibration curve for K203 oxime was linear, in
the range of 1-100 microg/ml (Fig. 2). Regression
analysis (least-squares method) yielded the equation
y = 32.03x - 76.58 (R2 = 0.9931).
Following i.m. dosing, a rapid rise in the plasma
concentration of K203 was observed which reached
a plateau of ~16.5 microg/ml between 40 and 60 minutes
post administration (Fig. 3). After 60 minutes, the
decrease in K203 plasma concentration was
confirmed.
There was no significant variation in TBARS
plasma concentration during the 3 hour study (Fig. 4),
with values ranging from 50 to 90 nmol/l. In contrast,
FRAP plasma concentrations steadily increased from
~200 micromol/l after 20 minutes to 490 micromol/l at
180 minutes (Fig. 5), the latter being statistically
significant compared with baseline values.
DISCUSSION
A range of antidotes are available for the treatment of
intoxication caused by exposure to organophosphorus
(OP) compounds that act via inhibition of
cholinesterase. However, no single antidote has
demonstrated universal efficacy against all classes of
cholinesterase inhibitors, despite efforts to expand the
range of cholinesterase reactivators through the
synthesis of novel oximes. Nonetheless, there are a small number of oximes that, with judicial selection,
can be used to treat a wide variety of OP intoxication.
These include K027 for treating exposure to a range
of pesticides (Petroianu et al. 2007) and 2-PAM,
HI-6, HLo-7, MMB-4 and K203 as a medical
countermeasure for many types of nerve agent.
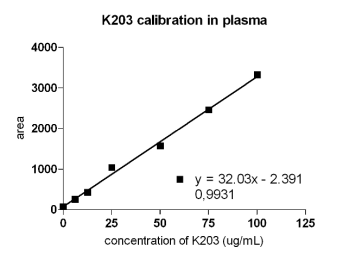
Fig. 2. Calibration plot of plasma samples spiked with K203. The correlation coefficient is 0.9931.
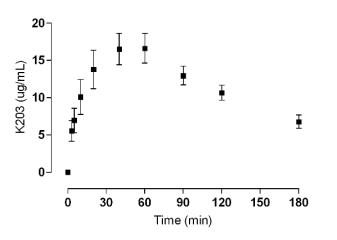
Fig. 3. Plasma level of K203 following intramuscular (i.m.) administration, dose 23.00 mg/kg.
The oximes HI-6, HLo-7 and MMB-4 are of
particular interest due to their relatively wide
spectrum of therapeutic activity against nerve agents.
Athough they are effective against sarin (GB),
cyclosarin (GF), soman (GD) and VX, these oximes
are relatively ineffective against tabun (GA) (Berend
et al. 2008, Kovarik et al. 2008), possibly due to
reduced access to the active site through steric
hindrance resulting from conformational changes
induced by the tabun-cholinesterase complex.
Consequently, not all reactivators are able to attack
the tabun-AChE complex (Ekstrom et al. 2006).
Previous studies have demonstrated that some
bis-pyridinium-bis-aldoximes (obidoxime, trime-doxime) are generally more effective for the treatment
of tabun poisoning (Zdarova Karasova et al. 2009).
However, obidoxime and trimedoxime exhibit
increased toxicity compared to K203 (Musilek et al.
2007). Among the new oximes developed, K203 is
presently regarded as being highly effective against poisoning by tabun and the pesticide paraoxon
(Petroianu et al. 2007). The formerly synthesized
oximes (e.g. K027, K048, K074 and K075) were
developed to increase the effectiveness of treatment
in case of tabun poisoning. However, these
compounds only attained partial success against
tabun. The oxime K203 was prepared by combining
the structural features of two relatively effective
precursors; K048 and K075 (Musilek et al. 2007),
resulting in a bis-pyridinium-mono-aldoxime with a
markedly lower inherent toxicity than classical
oximes developed against tabun (Kovarik et al. 2008,
Kassa et al. 2011).
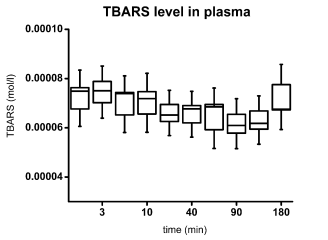
Fig. 4. TBAR level in rat plasma. The box indicates standard error of mean, the error bars indicate standard deviation.
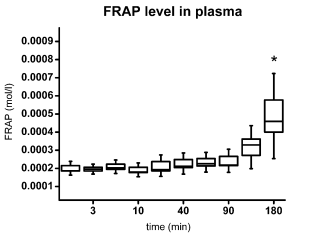
Fig. 5. FRAP level in rat plasma. The box represents standard error of mean; the error bars indicate standard deviation. Asterisk
depicts significance as compared with the start time point (ANOVA with Bonferroni test).
Determination of the pharmacokinetics of newly
synthesized compounds is an essential step before
further (preclinical) investigation. Maximal
concentrations after dosage, time to achieve maximal
(therapeutic) oxime concentrations and their
subsequent elimination kinetics are clearly critical
data to acquire in order to ensure candidate
compounds are likely to be of clinical relevance. If
AChE reactivators are applied i.m., the entire dose is
potentially available for systemic absorption and thus
this route of administration offers the highest
bioavailability. Oximes are widely distributed in the
body and rapidly eliminated by the kidney through
secretion by renal tubules (Spohrer et al. 1994). This
study has demonstrated that i.m. administration of
K203 results in rapid systemic absorption, with 30%
of the maximum plasma concentration (Cmax)
achieved within 3 minutes with a subsequent plateau
between 40 and 60 minutes, the calculated Cmax
corresponding to 49 minute after i.m. application.
Similar absorption kinetics were found in a previous
study (Kalasz et al. 2008) using a higher dose of
K203, although elimination at the higher dose was
more rapid, being 15% of Cmax at 120 min compared
to 60% of Cmax in this study.
Although the animal treatment, HPLC method and
doses (equimolar) were the same, absorption curves
of obidoxime and oxime HI-6 were quite different.
Oxime HI-6 gave single Cmax 31 and obidoxime 9
minutes after i.m. injection. The HI-6 Cmax was
similar, only ~16.0 microg/ml and obidoxime Cmax was
slightly higher ~26.1 microg/ml. Both oximes were
eliminated at faster rates (Zdarova Karasova et al.
2010). The other oximes that were studied were
trimedoxime and oxime K027. The chemical
structures of trimedoxime and K027 are similar.
Therefore, it is not surprising that equimolar doses of
both oximes resulted in near identical plasma profiles.
Trimedoxime gave single Cmax 33 (~18.6 microg/ml) and
trimedoxime 38 minutes (~20.1 microg/ml) after i.m.
injection (Zdarova Karasova et al. 2011).
Based on this information, oxime K203 has a
better pharmacokinetics profile; the minimal
therapeutical concentration was reached 3 min after
application and relatively stable plasma concentration
was maintained for a long interval of time.
Measurements of low molecular weight
antioxidants (FRAP assay) or oxidative stress
(TBARS) provide a useful indication of the effect of
K203 on the redox state (Pohanka et al. 2011). The
apparent lack of effect on TBARS values indicated no
significant oxidative stress and the significant
increase in plasma antioxidants may be of potential
benefit during nerve agent intoxication, although
further work should be undertaken to quantify any
therapeutic benefits of enhanced antioxidant status
during OP-induced cholinergic crises.
ACKNOWLEDGEMENTS
This work was supported by the projects:
FVZ0000604 and OVUOFVZ200811. Thanks are due
to Mrs. M. Hrabinova and Mr. P. Stodulka for skilled
technical assistance.
REFERENCES
Bartosova L, Kuca K, Kunesova G, Jun D. The acute toxicity of the acetylcholinesterase reactivators in mice in relation to their structure.
Neurotoxicity Res. 9: 291-296, 2006.
[CrossRef]
[PubMed]
Benschop HP, Konings KA, Kossen SP, Ligtenstein DA. Determination of some pyridinium aldoxime compounds by means of ion-pair reversed-phase
high-performance liquid chromatography: application in biological material. J Chromatogr. 225: 107-114, 1981.
Berend S, Vrdoljak AL, Radic B, Kuca K. New bispyridinium oximes: in vitro and in vivo evaluation of their biological efficiency in
soman and tabun poisoning. Chem Biol Interact. 25: 413-416, 2008.
[CrossRef]
[PubMed]
Brown ND, Hall LL, Steeman HK, Doctor BP, Dewaree DE. Ion-pair high-performance liquid chromatographic separation of a multicomponent anticholinergic
drug formulation. J Chromatogr. 148: 453-457, 1978.
[CrossRef]
da Silva AP, Farina M, Franco JL, Dafre AL, Kassa J, Kuca K. Temporal effects of newly developed oximes (K027, K048) on malathion-induced
acetylcholinesterase inhibition and lipid peroxidation in mouse prefrontal cortex. Neurotoxicology. 29: 184-189, 2008.
[CrossRef]
[PubMed]
Eddleston M, Szinicz L, Eyer P, Buckley N. Oximes in acute organophosphorus pesticide poisoning: a systematic review of clinical trials. Q J Med. 95:
275-283, 2002.
Ekstrom F, Akfur C, Tunemalm AK, Lundberg S. Structural changes of phynylalanine 338 and histidine 447 revealated by the crystal structures of
tabun-inhibited murine acetylcholinesterase. Biochemistry. 45: 74-81, 2006.
[CrossRef]
[PubMed]
Eyer P, Hagedorn I, Ladstetter B. Study on the stability of the oxime HI 6 in aqueous solution. Arch Toxicol. 62: 224-226, 1998.
[CrossRef]
[PubMed]
Guimaraes AP, Franca TCC, Ramalho TC, Renno MN, Ferreira da Cunha EF, Matos KS, Mancini DT, Kuca K. Docking studies and effects of syn-anti
isomery of oximes derived from pyridine imidazol bicycled systems as potential human acetylcholinesterase reactivators. J Appl Biomed. 9: 163-171,
2011.
[CrossRef]
[JAB]
Guyon F, Tambute A, Caude M, Rosset R. Determination of N-methylpyridinium 2-aldoxime methylsulfate (Contrathion) in rat plasma and urine by
high-performance copper(II) - silica ligand-exchange chromatography. J Chromatogr. 229: 475-480, 1982.
Houze P, Borron SW, Scherninski F, Bousquet B, Gourmel B, Baud F. Measurement of serum pralidoxime methylsulfate (Contrathion) by high-performance
liquid chromatography with electrochemical detection. J Chromatogr B Analyt Technol Biomed Life Sci. 814: 149-154, 2005.
[CrossRef]
[PubMed]
Jokanovic M, Prostran M. Pyridinium oximes as cholinesterase reactivators. Structure-activity relationship and efficacy in the treatment of poisoning
with organophosphorus compounds. Curr Med Chem. 16: 2177-2188, 2009.
[CrossRef]
[PubMed]
Kalasz H, Hasan MY, Sheen R, Kuca K, Petroianu G, Ludanyi K, Gergely A, Tekes K. HPLC analysis of K-48 concentration in plasma. Anal Bioanal Chem.
385: 1062-1067, 2006.
[CrossRef]
[PubMed]
Kalasz H, Laufer R, Szegi P, Kuca K, Musilek K, Tekesz K. HPLC study of the pharmacokinetics of K203. Acta Chromatogr. 20: 575-584, 2008.
[CrossRef]
Kassa J, Karasova J. The evaluation of the neuroprotective effects of bispyridinium oximes in tabun-poisoned rats. J Toxicol Environ Health A. 70:
1556-1567, 2007.
[CrossRef]
[PubMed]
Kassa J, Karasova J, Musilek K, Kuca K, Bajgar J. An evaluation of reactivating and therapeutic efficacy of newly developed oximes (K206, K269) and
commonly used oximes (obidoxime, HI-6) in cyclosarin-poisoned rats and mice. Clin Toxicol (Phila). 47: 72-76, 2009.
[CrossRef]
[PubMed]
Kassa J, Karasova JZ, Sepsova V, Bajgar J. A comparison of the reactivating and therapeutic efficacy of the newly developed bispyridinium oxime K203
with currently available oximes, in sarin poisoned rats and mice. J Appl Biomed. 9: 225-230, 2011.
[CrossRef]
[JAB]
Kovarik Z, Calic M, Sinko G, Bosak A, Berend S, Vrdoljak AL, Radic B. Oximes: Reactivators of phosphorylated acetylcholinesterase and antidotes in
therapy against tabun poisoning. Chem Biol Interact. 175: 173-179, 2008.
[CrossRef]
Kovarik Z, Vrdoljak AL, Berend S, Katalinic M, Kuca K, Musilek K, Radic B. Evaluation of oxime K203 as antidote in tabun poisoning. Arh Hig Rada
Toksikol. 1: 19-26, 2009.
[CrossRef]
[PubMed]
Musilek K, Jun D, Cabal J, Kassa J, Gunn-Moore F, Kuca K. Design of a potent reactivator of tabun-inhibited acetylcholinesterase - synthesis and
evaluation of (E)-1-(4-carbamoylpyridinium)-4-(4-hydroxyiminomethylpyridinium)-but-2-ene dibromide (K203). J Med Chem. 50: 5514-5518, 2007.
[CrossRef]
[PubMed]
Novotny L, Misik J, Karasova J, Kuca K, Bajgar J. Influence of different ways of euthanasia on the activity of cholinesterases in the rat. J Appl
Biomed. 7: 133-136, 2009.
[JAB]
Nurulain SM, Lorke DE, Hasan YM, Shafiullah M, Kuca K, Musilek K, Petroianu GA. Efficacy of eight experimental bispyridinium oximes against
paraoxon-induced mortality: comparison with the conventional oximes pralidoxime and obidoxime. Neurotox Res. 16: 60-67, 2009.
[CrossRef]
[PubMed]
Pejchal J, Osterreicher J, Kuca K, Jun D, Bajgar J, Kassa J. The influence of acetylcholinesterase reactivators on selected hepatic functions in
rats. Basic Clin Pharmacol Toxicol. 103: 119-123, 2008.
[CrossRef]
Petroianu GA, Nurulain SM, Nagelkerke N, Shafiullah M, Kassa J, Kuca K. Five oximes (K-27, K-48, obidoxime, HI-6 and trimedoxime) in comparison with
pralidoxime: survival in rats exposed to methyl-paraoxon. J Appl Toxicol. 27: 453-457, 2007.
[CrossRef]
[PubMed]
Pohanka M, Karasova JZ, Musilek K, Kuca K, Kassa J. Effect of five acetylcholinesterase reactivators on tabun intoxicated rats: induction of
oxidative stress versus reactivation efficacy. J Appl Toxicol. 29: 483-488, 2009.
[CrossRef]
[PubMed]
Pohanka M, Bandouchova H, Vlckova K, Karasova JZ, Kuca K, Damkova V, Peckova L, Vitula F, Pikula J. Square wave voltammetry on screen printed
electrodes: comparison to ferric reducing antioxidant power in plasma from model laboratory animal (Grey Partridge) and comparison to standard
antioxidants. J Appl Biomed. 9: 103-109, 2011.
[CrossRef]
[JAB]
Soukup O, Holas O, Binder J, Killy K, Tobin G, Jun D, Fusek J, Kuca K. The effect of trimedoxime on acetylcholinesterase and on the cholinergic
system of the rat bladder. J Appl Biomed. 8: 87-92, 2010.
[CrossRef]
[JAB]
Spohrer U, Thiermann H, Klimmek R, Eyer P. Pharmacokinetics of the oximes HI-6 and Hlo-7 in dogs after i.m. injection with newly developed dry/wet
autoinjectors. Arch Toxicol. 68: 480-489, 1994.
[CrossRef]
Tekes K, Hasan YM, Sheen R, Kuca K, Petroianu G, Ludanyi K. High-performance liquid chromatographic determination of the plasma concentration of
K-27, a novel oxime-type cholinesterase reactivator. J Chromatogr A. 1122: 84-87, 2006.
[CrossRef]
[PubMed]
Utley D. Analysis of formulations containing pralidoxime mesylate by liquid chromatography. J Chromatogr. 396: 237-250, 1987.
[CrossRef]
Vojvodic VB, Maksimovic M. Absorption and excretion of pralidoxime in man after intramuscular injection of PAM-2Cl and various cholinolytics. Eur J
Clin Pharmacol. 5: 58-61, 1972.
[CrossRef]
Wilson IB, Ginsburg S. Reactivation of acetylcholinesterase inhibited by alkylphosphates. Biochim Biophys Acta. 18: 169-171, 1955.
[CrossRef]
Zdarova Karasova J, Kassa J, Musilek K, Pohanka M, Novotny L, Kuca K. Effect of seven newly synthesized and currently available oxime cholinesterase
reactivators on cyclosarin-intoxicated rats. Int J Mol Sci. 10: 3065-3075, 2009.
[CrossRef]
[PubMed]
Zdarova Karasova J, Novotny L, Antos K, Zivna H, Kuca K. Time-dependent changes in concentration of two clinically used acetylcholinesterase
reactivators (HI-6 and obidoxime) in rat plasma determined by HPLC techniques after in vivo administration. Anal Sci. 26: 63-67, 2010.
[CrossRef]
[PubMed]
Zdarova Karasova J, Chladek J, Hroch M, Fusek J, Hnidkova D, Kuca K. Pharmacokinetics study of two acetylcholinesterase reactivators, trimedoxime and
newly synthesized oxime K027, in rat plasma. J Appl Toxicol. 2011 (in press).
[CrossRef]
[PubMed]
|
BACK
|






