Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 2, p 109-117
DOI 10.2478/v10136-011-0016-1
A cherry nutraceutical modulates melatonin, serotonin, corticosterone, and total antioxidant capacity levels: effect on ageing and chronotype
Jonathan Delgado*, Maria Pilar Terron*, Maria Garrido, Jose Antonio Pariente, Carmen Barriga, Ana Beatriz Rodriguez, Sergio Damian Paredes
Address: Ana Beatriz Rodriguez Moratinos, Department of Physiology (Neuroimmunophysiology and Chrononutrition Research Group), Faculty of Science, University of Extremadura, Avda. de Elvas, s/n, 06006, Badajoz, Spain
moratino@unex.es
Received 20th July 2011.
Revised 5th September 2011.
Published online 31st October 2011.
Full text article (pdf)
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
Acknowledgements
References
SUMMARY
Impaired daily rhythms in vertebrate physiology occur with age. Particularly, age-related changes in melatonin and serotonin rhythms and hypercortisolemia have been reported to be linked to age-related disorders. This study was aimed at assessing the effect of a Jerte Valley cherry-based nutraceutical product (patent no ES 2342141 B1), which contains high levels of tryptophan, serotonin, and melatonin, on the serum melatonin, serotonin, corticosterone, and total antioxidant capacity (TAC) levels in young and old ring doves (Streptopelia risoria) and rats (Rattus norvegicus) as representatives of animals with diurnal and nocturnal habits, respectively. The animals consumed the cherry product for 10 days. Serum melatonin, serotonin, corticosterone, and TAC were measured with commercial ELISA kits. The consumption of the cherry product induced a significant increase in the circulating levels of melatonin and serotonin, as well as in the serum TAC and a significant decrease in the circulating levels of corticosterone in both species and groups of age as compared to their respective values in the control groups. The consumption of a Jerte Valley cherry-based nutraceutical product may help to counteract the decrease in melatonin and serotonin and the increase in oxidative stress, suggesting a potential health benefit especially in aged populations where these parameters have been found to be altered.
KEY WORDS
cherry; corticosterone; melatonin; serotonin; total antioxidant capacity
* Both authors contributed equally to this work.
INTRODUCTION
Serotonin is a neurotransmitter involved in many
functions throughout the brain. It participates in the
synchronization of the circadian clock located in the
suprachiasmatic nucleus, and in the regulation of the
sleep/wake cycle, as well as exerting a fundamental
role in the biosynthetic pathway of melatonin, a
pineal indole that also carries out regulatory functions
on the sleep-wake rhythm (Zhdanova et al. 2001,
Berger 2008, Paredes et al. 2009a). In addition,
melatonin is a potent free radical scavenger and
antioxidant (Paredes et al. 2007a, Reiter et al. 2008a,
2010) that not only scavenges especially highly toxic
hydroxyl radicals, but also performs indirect
antioxidant actions via its ability to stimulate
antioxidant enzymes (Gitto et al. 2001, Paredes et al.
2009b), diminishing free radical formation at the
mitochondrial level by reducing the leakage of
electrons from the electron transport chain (Reiter et
al. 2008b). Moreover, melatonin is a lipophilic-hydrophilic molecule that diffuses widely into cellular
compartments, thus providing on-site protection
against free radical-mediated damage to biomolecules
(Reiter et al. 2008b, Paredes and Reiter 2010).
The production of melatonin wanes with
increasing age leading some to speculate that its loss
contributes to the ageing process (Reiter et al. 2002,
2008a). This phenomenon seems to be universal and
includes birds and mammals. It has been suggested
that the age-related melatonin decrease could be
produced by serotonin deficiencies. In fact, the
synthesis, metabolism, and circulating levels of this
neurotransmitter are strongly reduced in old ring
doves (Garau et al. 2006, Paredes et al. 2006, 2007b).
In mammals, age related reductions in the binding of
serotonin receptors have been found in the brain of
humans (Wang et al. 1995, Rosier et al. 1996) and,
importantly, in the suprachiasmatic nucleus of rodents
(Duncan et al. 2000).
The loss of melatonin in advanced age leads to
disturbances in the circadian pacemarker, which
causes internal temporal desynchronization, inducing
a variety of chronopathologies, and leads to a
generalized deterioration of health (Paredes and
Reiter 2010, Reiter et al. 2010). The intake of
tryptophan, the precursor of both serotonin and
melatonin, appears to reverse, at least in part, some of
the effects of ageing on the circadian impairment, as
it increases the availability of brain tryptophan and
consequently the brain and blood serotonin and
melatonin levels in mammals and birds, as it is the
case of ringdoves and rats (Fernstrom and Wurtman
1971, Esteban et al. 2004, Garau et al. 2006, Paredes
et al. 2009a, b).
We recently reported high levels of tryptophan
(Cubero et al. 2010), serotonin, and/or melatonin
(Gonzalez-Gomez et al. 2009) in Jerte Valley
cherries, and that the consumption of fresh cherries
had positive effects on nocturnal rest as well as
elevating the levels of 6-sulfatoxymelatonin and
antioxidants in the urine of middle-aged and elderly
subjects (Garrido et al. 2010a). Hence, the aim of the
present work was to evaluate whether the
consumption of a Jerte Valley cherry-based
nutraceutical product (patent no ES 2342141 B1)
which contains high levels of tryptophan, serotonin,
and melatonin may restore the age-related changes in
melatonin and serotonin rhythms and improve the
serum antioxidant status in ring doves (Streptopelia
risoria) and rats (Rattus norvegicus) as
representatives of animals with diurnal and nocturnal
habits, respectively. Since the basal secretion of
corticosterone (and cortisol) increases with ageing,
the effect of the Jerte cherry-based nutraceutical on
circulating corticosterone levels was also evaluated.
MATERIALS AND METHODS
Animals
Male Wistar rats (Rattus norvegicus) aged 6-7
months (young) and 18-20 months (old) (n=16 per
age group), and male and female ring doves
(Streptopelia risoria) of 4-5 years of age (young) and
12-14 years of age (old) (n=16 per age group) were
individually housed under controlled environmental
conditions (20±2 °C; 70% humidity), maintained
under a 12/12 h light/dark photoperiod (darkness from
20:00 to 08:00 h) and fed ad libitum. All handling
during lights-off was done under dim red light (<2
lux).
The study was approved by the Ethical Committee
of the University of Extremadura (Badajoz, Spain) in
accordance with the National Institute of Health
Guide for the Care and Use of Laboratory Animals,
and the European Community's Council Directives
(86/609/EEC).
Animal treatment
Each age group was divided into two subgroups:
Control and treated. Control animals consumed tap
water ad libitum. In the treated animals, tap water was
replaced with a 27.85 g powdered freeze-dried
nutraceutical product mix (patent no ES 2342141 B1)
diluted in 250 ml of water. The product mix was
freshly prepared every day and consisted of 18.85 g
pitted freeze-dried cherries (equivalent to 141 g fresh
cherries) in equal parts of 4 Jerte Valley cherry
cultivars (Bourlat, Navalinda, Pico Negro, and Pico
Colorado), plus 7.5 g maltodextrin, and 1.5 g ascorbic
acid (Garrido et al. 2009). The treatment with the
nutraceutical product mix was administered for 10
consecutive days.
Serum collection
Blood samples were drawn from all animals (control
and treated) at the end of the treatment (day 10). The
extractions (1 ml) were done by syringe from the
lateral tail vein (rats) or the brachial vein (birds) and
then transferred unheparinized to a pre-prepared tube
containing serum-separating gel. The samples were
centrifuged at room temperature for 30 min at 300xg.
The serum was then divided into aliquots in
Eppendorf vials, and kept frozen at -30 °C until the
time of assay. The extractions were performed one
hour after lights on for the determination of serum
corticosterone and total antioxidant capacity, one
hour before lights off for the determination of serum
serotonin, and at the acrophases of the melatonin
rhythm in each species and group of age, as
previously reported (Mateos et al. 2009, Paredes et al.
2007c, 2009a). At least one week was allowed
between consecutive extractions until all selected
points of serum collection had been covered for each
animal.
Measurement of corticosterone, melatonin, and
serotonin in serum
Serum corticosterone, melatonin, and serotonin levels
were determined by means of commercial ELISA kits
(IBL, Hamburg, Germany), according to the
manufacturer's instructions. Determinations were
made in duplicate. Results are expressed in ng/ml for
corticosterone and serotonin, and in pg/ml in the case
of melatonin.
Measurement of antioxidant capacity in serum
Total antioxidant capacity was evaluated by means of
a colorimetric assay kit (Cayman, MI, USA),
according to the manufacturer's instructions. This
assay relies on the ability of antioxidants in the
sample to inhibit the oxidation of ABTS®
(2,2'-azino-di-[ethylbenzthiazoline sulfonate]) to
ABTS®+ by metmyoglobin. The capacity of the
antioxidants in the sample to prevent ABTS®
oxidation was compared with that of Trolox, a
water-soluble tocopherol analogue, and quantified as
millimolar Trolox equivalents.
Statistical analysis
Each value represents the mean ± S.E.M. (Standard
Error of the Mean) of the number of determinations.
The results were analysed by using a non-parametric
one-way ANOVA followed by Tukey's multiple
comparison test. A significance level of 2alpha=0.05 was
used. All analyses were performed using GraphPad
Prism (version 5.0, 2007; GraphPad Software, Inc;
San Diego, CA).
RESULTS
The serum melatonin levels at acrophases in young
and old rats and ringdoves in control conditions and
after the administration of a 10-day cherry-based
nutraceutical product treatment are shown in Figs 1A
and 1B, respectively. In both species, the melatonin
levels in the control young animals were significantly
higher than in the control old animals. Cherry product
consumption increased significantly the circulating
levels of melatonin in both species and age groups, as
compared with the results obtained in their respective
control groups. However, the levels of the
indoleamine reached in both old rodents and birds
after the intake of the product were still significantly
lower than those obtained in the young treated
animals.
The serum serotonin concentrations reached 1-hr
before lights off in control conditions and after the
administration of a 10-day cherry-based nutraceutical
product treatment are shown in Fig. 2 for both rats
(Fig. 2A) and ringdoves (Fig. 2B). Again in both
species, the serotonin levels in the control young
animals were significantly higher than in the control
old animals. Cherry product administration
significantly increased the circulating levels of
serotonin in both young and old animals of both
species, with the young values being still greater than
those quantified in their respective counterparts from
the old group.
The serum corticosterone levels measured 1-hr
after lights on in young and old rats and ringdoves in
control conditions and after the administration of a
10-day cherry-based nutraceutical product treatment
are shown in Figs 3A and 3B, respectively. Here, in
contrast with the case of melatonin and serotonin, the
values of this hormone in the control conditions were
higher in old animals than in young individuals,
although the difference was not significant for rats. A
10-day treatment with the nutraceutical product from
Jerte Valley cherries induced a significant decrease in
the circulating corticosterone levels in both young
and old rats and ringdoves as compared to their
respective values in the control conditions, with the
levels of the old animals being, however, significantly
greater than in the young individuals.
The variations in the total antioxidant capacity
(quantified as millimolar Trolox equivalents) from the
serum collected 1-hr after lights on are shown in
Fig. 4. In the control conditions, no significant
changes were observed in total antioxidant capacity
levels between the age groups in both rats and
ringdoves. However, in relation to the values obtained
after cherry product consumption, a significant rise
was found in both species and age groups with
respect to the control values, with the levels of the
young animals being higher than those measured in
the old groups (Figs 4A and 4B).
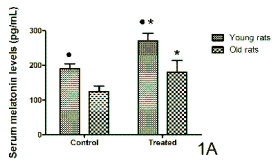 
Fig. 1. Serum levels of melatonin at their corresponding hours of acrophase in control conditions and after 10 days of
treatment with a nutraceutical product based on Jerte Valley cherries to young and old rats (Fig. 1A) and ringdoves
(Fig. 1B). Each value represents the mean ± S.E.M. (Standard Error of the Mean) of 10 determinations performed in duplicate.
* Statistically significant as compared with their corresponding values in the control group.
* Statistically significant as compared with their corresponding values in the old animals.
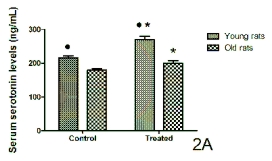 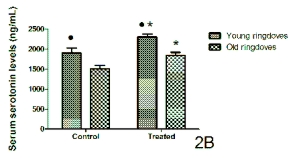
Fig 2. Serum levels of serotonin obtained 1-hr before lights off in control conditions and after a 10-day administration with
a nutraceutical product based on Jerte Valley cherries to young and old rats (Fig. 2A) and ringdoves (Fig. 2B). Each value
represents the mean ± S.E.M. (Standard Error of the Mean) of 10 determinations performed in duplicate.
Symbols as in Fig. 1.
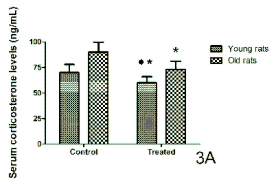 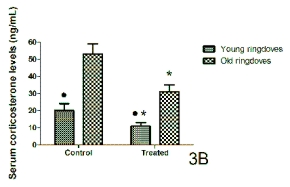
Fig. 3. Serum levels of corticosterone obtained 1-hr after lights on in control conditions and after 10 days of treatment
with a nutraceutical product based on Jerte Valley cherries to young and old rats (Fig. 3A) and ringdoves (Fig. 3B). Each
value represents the mean ± S.E.M. (Standard Error of the Mean) of 10 determinations performed in duplicate.
Symbols as in Fig. 1.
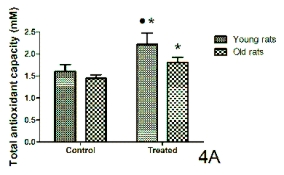 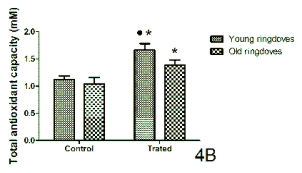
Fig. 4. Serum levels of total antioxidant capacity (mM) measured 1-hr after lights on in control conditions and after a
10-day administration of a nutraceutical product based on Jerte Valley cherries to young and old rats (Fig. 4A) and
ringdoves (Fig. 4B). Each value represents the mean ± S.E.M. (Standard Error of the Mean) of 10 determinations performed in
duplicate.
Symbols as in Fig. 1.
DISCUSSION
In the present work, it was observed that the
consumption of the cherry product induced a
significant increase in the circulating levels of
melatonin and serotonin, as well as in the serum TAC,
and a significant decrease in the circulating levels of
corticosterone in both species and age groups as
compared to their respective values in the control
groups.
Older individuals appear to be more prone to
internal desynchronization than younger subjects,
suggesting a weakening of the internal coupling
among various rhythms. Among the rhythms that
appear to change considerably with age are the
rhythms in the production and secretion of serotonin,
melatonin and cortisol (Zisapel et al. 2005). In
previous studies we have noted a significant decline
in circulating melatonin levels in old ringdoves
compared with the concentration observed in both
mature and young animals (Terron et al. 2002, 2004)
as well as a significant decline in the amplitude and
mean levels of melatonin (Paredes et al. 2006). The
amplitude of the rhythm of serum serotonin was also
much reduced in the old relative to the young birds
(Paredes et al. 2006). Other workers have reported the
absence of a circadian rhythm in brain serotonin
synthesis and metabolism in old ringdoves (Garau et
al. 2006). Age-related decreases in melatonin levels
are also evident in gerbils, hamsters, and rats (Myers
and Badia 1995). Here, the age-related decrease in the
circulating levels of both melatonin and serotonin in
the two animal chronotypes analysed, diurnal and
nocturnal was confirmed. Also, old rats presented
higher but not significant corticosterone circulating
levels than their respective values found in the young
group. Previous studies have shown no significant
differences between the plasma corticosterone levels
of young and aged rats (Bodnoff et al. 1995, Bowman
et al. 2006, Garrido et al. 2010b), although basal
increased brain levels of the hormone due to ageing
have been reported (Garrido et al. 2010b). In humans,
a basal secretion of cortisol seems to occur (Van
Cauter et al. 2000). This may be related to the
increased basal corticosterone levels found in control
old ringdoves.
The relationship between diet and health has led
to intense research into bioactive compounds in
foods. As for cherries, several studies indicate that the
consumption of these fruits is health promoting,
particularly in reducing the effects of some diseases
(Kang et al. 2003, Kim et al. 2005, Kelley et al.
2006). Previously, we reported that the intake of the
cherry-based nutraceutical product tested in the
present work exerted beneficial physiological effects
in humans (Garrido et al. 2009). Here, we showed
that the intake of this nutraceutical increased
significantly the circulating levels of melatonin and
serotonin in rats and ringdoves, species of nocturnal
and diurnal habits respectively. A significant rise was
observed in both young and old animals as compared
to the values obtained in the control groups. Although
there is only a modicum of evidence indicating what
ingredients may be responsible for the alleged
beneficial properties, the recent discovery of
melatonin (Gonzalez-Gomez et al. 2009, Paredes et
al. 2009c), serotonin, or tryptophan (Gonzalez-Gomez
et al. 2009, Cubero et al. 2010) in Jerte Valley
cherries points to the fact that these molecules may be
involved in the afore-mentioned restoration of the
circulating levels of serotonin and melatonin. In fact,
the consumption of this fruit has been shown to
improve sleep as well as increasing TAC and
6-sulfatoxymelatonin levels, a metabolite that is
considered to reflect the nocturnal melatonin
concentration, in first-void urines of middle-aged and
elderly humans (Garrido et al. 2010a).
Similar findings have been reported by other
workers, where associations have been established
between the consumption of vegetables that are high
in melatonin content and elevated melatonin in both
blood and urine (Nagata et al. 2005, Reiter et al.
2005). In particular, Reiter et al. (2005) showed that
the consumption of walnuts, which are rich in
melatonin, provoked a threefold increase in
circulating melatonin levels and also improved serum
antioxidant capacity measured in trolox equivalents.
This is consistent with the results shown in the
present study. In fact, both species and age groups
experienced a rise in the levels of serum TAC after
cherry treatment, the effect being greater in the young
individuals. Heretofore it has been shown that in birds
and mammals, including humans, fluctuations in
blood melatonin concentrations strongly correlate
with the ability of the blood to detoxify toxic free
radicals and related reactants (Paredes et al. 2007a, b,
Terron et al. 2005, 2009). In addition, orally
administered tryptophan (and melatonin) also
enhances the phagocytic response and detoxification
of superoxide anion radicals derived from this
immune function in both species, as has been
documented elsewhere (Paredes et al. 2007d, 2009a,
b, Sanchez et al. 2004, 2008a). This has also been
linked to the rise in the circulating levels of melatonin
induced by both the indoleamine and its precursor
(Paredes et al. 2007c, e, Sanchez et al. 2008b).
However, cherries contain other important
antioxidants, e.g., anthocyanins and polyphenols that
are also absorbed from the gut and influence the total
antioxidant capacity of serum (Garrido et al. 2009,
2010a).
That tryptophan, serotonin, and melatonin may be
involved in the observed increase in the circulating
levels of serotonin, melatonin and serum TAC
observed in both rats and ringdoves, is reinforced by
previous reports showing that dietary-rich tryptophan
supplementation increases the brain and blood levels
of serotonin, as well as modulating the circulating
levels of melatonin (Garau et al. 2006, Paredes et al.
2007e). The increase of serum serotonin observed
after the 10-day consumption of the cherry-based
nutraceutical product would indicate a higher
availability of tryptophan which, after passing
through the blood-brain barrier, would be converted
into serotonin, thus increasing the production of this
neurotransmitter in the brain as has been observed in
the ringdove (Garau et al. 2006) and other animal
species ( Fernstrom and Wurtman 1971, Huether et al.
1993, Esteban et al. 2004). This serotonin in the brain
would be the substrate for melatonin synthesis,
increasing the circulating levels of this
neurohormone. It has been shown, using in vivo
microdialysis and voltammetry, that an elevated
dietary intake of tryptophan results in an increased
functional release of serotonin also in mammals
(Boadle-Biber 1993).
Treatment with the cherry product also provoked
a decrease in the circulating levels of corticosterone
in young and old rats and ringdoves. Again,
melatonin contained in the product or its precursors,
once converted into the indoleamine after being
assimilated by the organism, may be involved in this
effect. In fact, melatonin treatment has been
repeatedly reported to decrease blood corticosterone
levels in mammals and birds (Saito et al. 2005,
Zisapel et al. 2005, Detanico et al. 2009).
In summary, the consumption of the Jerte Valley
cherry-based nutraceutical product induced a
restorative effect in the circulating levels of melatonin
and serotonin in old rats and ringdoves, animals with
nocturnal and diurnal habits, respectively. It also
enhanced the blood levels of the neuroindole and the
neurotransmitter in the young individuals as well as
decreasing the circulating levels of corticosterone in
both species and age groups as compared to their
respective values in the control groups. The increase
in melatonin levels correlated with an increased blood
antioxidative capacity as reflected by augmentation of
the trolox equivalent antioxidant capacity of serum
values. These results suggest that the consumption of
the cherry nutraceutical may help to counteract the
decrease in melatonin and serotonin and the increase
in oxidative stress that normally occurs in aged
animals. In fact, the close relationship between
age-related disorders, circadian disruption as well as
oxidative stress suggests that the intake of the cherry
nutraceutical may be viewed as a beneficial tool to
improve human and animal physiology.
ACKNOWLEDGEMENTS
This research was supported by a grant from
University of Extremadura (Plan de Iniciacion a la
Investigacion, Accion VII - 18L202 -). S. D. Paredes
was the beneficiary of a grant by Consejeria de
Economia, Comercio e Innovacion-Fondo Social
Europeo (Junta de Extremadura, REI09009).
REFERENCES
Berger J. A two-clock model of circadian timing in the immune system of mammals. Pathol Biol (Paris). 56: 286-291, 2008.
[CrossRef]
[PubMed]
Boadle-Biber MC. Regulation of serotonin synthesis. Prog Biophys Mol Biol. 60: 1-15, 1993.
[CrossRef]
Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning,
synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 15: 61-69, 1995.
[PubMed]
Bowman RE, Maclusky NJ, Diaz SE, Zrull MC, Luine VN. Aged rats: sex differences and responses to chronic stress. Brain Res. 1126: 156-166, 2006.
[CrossRef]
Cubero J, Toribio F, Garrido M, Hernandez MT, Maynar J, Barriga C, Rodriguez AB. Assays of the amino acid tryptophan in cherries by
HPLC-fluorescence. Food Anal Methods. 3: 36-39, 2010.
[CrossRef]
Detanico BC, Piato AL, Freitas JJ, Lhullier FL, Hidalgo MP, Caumo W, Elisabetsky E. Antidepressant-like effects of melatonin in the mouse chronic
mild stress model. Eur J Pharmacol. 607: 121-125, 2009.
[CrossRef]
[PubMed]
Duncan MJ, Crafton CJ, Wheeler DL. Ageing regulates 5-HT(1B) receptors and serotonin reuptake sites in the SCN. Brain Res. 856: 213-219, 2000.
[CrossRef]
Esteban S, Nicolaus C, Garmundi A, Rial RV, Rodriguez AB, Ortega E, Ibars CB. Effect of orally administered L-tryptophan on serotonin, melatonin, and
the innate immune response in the rat. Mol Cell Biochem. 267: 39-46, 2004.
[CrossRef]
[PubMed]
Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science. 173: 149-152, 1971.
[CrossRef]
[PubMed]
Garau C, Aparicio S, Ruben VR, Nicolau MC, Esteban S. Age-related changes in circadian rhythm of serotonin synthesis in ring doves: Effects of
increased tryptophan ingestion. Exp Gerontol. 41: 40-48, 2006.
[CrossRef]
[PubMed]
Garrido M, Espino J, Gonzalez-Gomez D, Lozano M, Cubero J, Toribio-Delgado AF, Maynar-Marino JI, Terrón MP, Munoz JL, Pariente JA, Barriga C, Paredes
SD, Rodriguez AB. A nutraceutical product based on Jerte Valley cherries improves sleep and augments the antioxidant status in humans. e-SPEN, the
European e-Journal of Clinical Nutrition and Metabolism. 4: e321-e323, 2009.
Garrido M, Paredes SD, Cubero J, Lozano M, Toribio-Delgado AF, Munoz JL, Reiter RJ, Barriga C, Rodriguez AB. Jerte Valley cherry-enriched diets
improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J Gerontol A
Biol Sci Med Sci. 65: 909-914, 2010a.
[CrossRef]
[PubMed]
Garrido P, de Blas M, Del Arco A, Segovia G, Mora F. Aging increases basal but not stress-induced levels of corticosterone in the brain of the awake
rat. Neurobiol Aging. 33: 375-382, 2010b.
[CrossRef]
[PubMed]
Gitto E, Tan DX, Reiter RJ, Karbownik M, Manchester LC, Cuzzocrea S, Fulia F, Barberi I. Individual and synergistic antioxidative actions of
melatonin: studies with vitamin E, vitamin C, glutathione and desferrioxamine (desferoxamine) in rat liver homogenates. J Pharm Pharmacol. 53:
1393-1401, 2001.
[CrossRef]
[PubMed]
Gonzalez-Gomez D, Lozano M, Fernandez-Leon MF, Ayuso MC, Bernalte MJ, Rodriguez AB. Detection and quantification of melatonin and serotonin in eight
sweet cherry cultivars (Prunus avium L.). Eur Food Res Technol. 229: 223-229, 2009.
[CrossRef]
Huether G, Poeggeler B, Adler L, Ruther E. Effects of indirectly acting 5-HT receptor agonists on circulating melatonin levels in rats. Eur J
Pharmacol. 238: 249-254, 1993.
[CrossRef]
Kang SY, Seeram NP, Nair MG, Bourquin LD. Tart cherry anthocyanins inhibit tumor development in Apc(Min) mice and reduce proliferation of human colon
cancer cells. Cancer Lett. 194: 13-19, 2003.
[CrossRef]
Kelley DS, Rasooly R, Jacob RA, Kader AA, Mackey BE. Consumption of Bing sweet cherries lowers circulating concentrations of inflammation markers in
healthy men and women. J Nutr. 136: 981-986, 2006.
[PubMed]
Kim DO, Heo HJ, Kim YJ, Yang HS, Lee CY. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J Agric Food Chem. 53:
9921-9927, 2005.
[CrossRef]
[PubMed]
Mateos SS, Sanchez CL, Paredes SD, Barriga C, Rodriguez AB. Circadian levels of serotonin in plasma and brain after oral administration of tryptophan
in rats. Basic Clin Pharmacol Toxicol. 104: 52-59, 2009.
[CrossRef]
Myers BL, Badia P. Changes in circadian rhythms and sleep quality with aging: mechanisms and interventions. Neurosci Biobehav Rev. 19: 553-571,
1995.
[CrossRef]
Nagata C, Nagao Y, Shibuya C, Kashiki Y, Shimizu H. Association of vegetable intake with urinary 6-sulfatoxymelatonin level. Cancer Epidemiol
Biomarkers Prev. 14: 1333-1335, 2005.
[CrossRef]
Paredes SD, Reiter RJ. Melatonin: Helping cells cope with oxidative disaster. Cell Membr Free Radic Res. 2: 99-111, 2010.
Paredes SD, Terron MP, Cubero J, Valero V, Barriga C, Reiter RJ, Rodriguez AB. Comparative study of the activity/rest rhythms in young and old
ringdove (Streptopelia risoria): correlation with serum levels of melatonin and serotonin. Chronobiol Int. 23: 779-793, 2006.
[CrossRef]
[PubMed]
Paredes SD, Terron MP, Marchena AM, Barriga C, Pariente JA, Reiter RJ, Rodriguez AB. Effect of exogenous melatonin on viability, ingestion capacity,
and free-radical scavenging in heterophils from young and old ringdoves (Streptopelia risoria). Mol Cell Biochem. 304: 305-314, 2007a.
[CrossRef]
[PubMed]
Paredes SD, Barriga C, Rodriguez AB. Melatonin and tryptophan as therapeutic agents against the impairment of the sleep-wake cycle and
immunosenescence due to aging in Streptopelia risoria. Neuro Endocrinol Lett. 28: 757-760, 2007b.
[PubMed]
Paredes SD, Terron MP, Valero V, Barriga C, Reiter RJ, Rodriguez AB. Orally administered melatonin improves nocturnal rest in young and old ringdoves
(Streptopelia risoria). Basic Clin Pharmacol Toxicol. 100: 258-268, 2007c.
[CrossRef]
Paredes SD, Terron MP, Marchena AM, Barriga C, Pariente JA, Reiter RJ, Rodriguez AB. Tryptophan modulates cell viability, phagocytosis and oxidative
metabolism in old ringdoves. Basic Clin Pharmacol Toxicol. 101: 56-62, 2007d.
[CrossRef]
Paredes SD, Terron MP, Cubero J, Valero V, Barriga C, Reiter RJ, Rodriguez AB. Tryptophan increases nocturnal rest and affects melatonin and
serotonin serum levels in old ringdove. Physiol Behav. 90: 576-582, 2007e.
[CrossRef]
Paredes SD, Marchena AM, Bejarano I, Espino J, Barriga C, Rial RV, Reiter RJ, Rodriguez AB. Melatonin and tryptophan affect the activity-rest rhythm,
core and peripheral temperatures, and interleukin levels in the ringdove: changes with age. J Gerontol A Biol Sci Med Sci. 64: 340-350, 2009a.
[CrossRef]
[PubMed]
Paredes SD, Bejarano I, Terron MP, Barriga C, Reiter RJ, Rodriguez AB. Melatonin and tryptophan counteract lipid peroxidation and modulate superoxide
dismutase activity in ringdove heterophils in vivo. Effect of antigen-induced activation and age. Age (Dordr). 31: 179-188, 2009b.
[CrossRef]
[PubMed]
Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ. Phytomelatonin: a review. J Exp Bot. 60: 57-69, 2009c.
[CrossRef]
[PubMed]
Reiter RJ, Tan DX, Mayo JC, Sainz RM, Lopez-Burillo S. Melatonin, longevity and health in the aged: an assessment. Free Radic Res. 36: 1323-1329,
2002.
[CrossRef]
[PubMed]
Reiter RJ, Manchester LC, Tan DX. Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood. Nutrition. 21:
920-924, 2005.
[CrossRef]
[PubMed]
Reiter RJ, Paredes SD, Korkmaz A, Manchester LC, Tan DX. Melatonin in relation to the "strong" and "weak" versions of the free radical theory of
aging. Adv Med Sci. 53: 119-129, 2008a.
[CrossRef]
[PubMed]
Reiter RJ, Paredes SD, Korkmaz A, Jou MJ, Tan DX. Melatonin combats molecular terrorism at the mitochondrial level. Interdiscip Toxicol. 1: 137-149,
2008b.
[CrossRef]
[PubMed]
Reiter RJ, Tan DX, Paredes SD, Fuentes-Broto L. Beneficial effects of melatonin in cardiovascular disease. Ann Med. 42: 276-285, 2010.
[CrossRef]
[PubMed]
Rosier A, Dupont P, Peuskens J, Bormans G, Vandenberghe R, Maes M, de Groot T, Schiepers C, Verbruggen A, Mortelmans L. Visualisation of loss of
5-HT2A receptors with age in healthy volunteers using [18F]altanserin and positron emission tomographic imaging. Psychiatry Res. 68: 11-22, 1996.
[CrossRef]
Saito S, Tachibana T, Choi YH, Denbow DM, Furuse M. ICV melatonin reduces acute stress responses in neonatal chicks. Behav Brain Res. 165: 197-203,
2005.
[CrossRef]
[PubMed]
Sanchez S, Paredes SD, Martin MI, Barriga C, Rodriguez AB. Effect of tryptophan administration on circulating levels of melatonin and phagocytic
activity. J Appl Biomed. 2: 169-177, 2004.
[JAB]
Sanchez S, Paredes SD, Sanchez CL, Barriga C, Reiter RJ, Rodriguez AB. Tryptophan administration in rats enhances phagocytic function and reduces
oxidative metabolism. Neuro Endocrinol Lett. 29: 1026-1032, 2008a.
[PubMed]
Sanchez S, Sanchez CL, Paredes SD, Rodriguez AB, Barriga C. The effect of tryptophan administration on the circadian rhythms of melatonin in plasma
and the pineal gland of rats. J Appl Biomed. 6: 177-186, 2008b.
[JAB]
Terron MP, Cubero J, Marchena JM, Barriga C, Rodriguez AB. Melatonin and aging: in vitro effect of young and mature ring dove physiological
concentrations of melatonin on the phagocytic function of heterophils from old ring dove. Exp Gerontol. 37: 421-426, 2002.
[CrossRef]
Terron MP, Paredes SD, Barriga C, Ortega E, Rodriguez AB. Comparative study of the heterophil phagocytic function in young and old ring doves
(Streptopelia risoria) and its relationship with melatonin levels. J Comp Physiol B. 174: 421-427, 2004.
[CrossRef]
[PubMed]
Terron MP, Paredes SD, Barriga C, Ortega E, Reiter RJ, Rodriguez AB. Melatonin, lipid peroxidation, and age in heterophils from the ring dove
(Streptopelia risoria). Free Radic Res. 39: 613-619, 2005.
[CrossRef]
[PubMed]
Terron MP, Delgado J, Paredes SD, Barriga C, Reiter RJ, Rodriguez AB. Effect of melatonin and tryptophan on humoral immunity in young and old
ringdoves (Streptopelia risoria). Exp Gerontol. 44: 653-658, 2009.
[CrossRef]
[PubMed]
Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in
healthy men. JAMA. 284: 861-868, 2000.
[CrossRef]
Wang GJ, Volkow ND, Logan J, Fowler JS, Schlyer D, MacGregor RR, Hitzemann RJ, Gur RC, Wolf AP. Evaluation of age-related changes in serotonin 5-HT2
and dopamine D2 receptor availability in healthy human subjects. Life Sci. 56: PL249-253, 1995.
[CrossRef]
Zhdanova IV, Wurtman RJ, Regan MM, Taylor JA, Shi JP, Leclair OU. Melatonin treatment for age-related insomnia. J Clin Endocrinol Metab. 86:
4727-4730, 2001.
[CrossRef]
Zisapel N, Tarrasch R, Laudon M. The relationship between melatonin and cortisol rhythms: Clinical implications of melatonin therapy. Drug Dev Res.
65: 119-125, 2005.
[CrossRef]
|
BACK
|









