Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 3, p 155-167
DOI 10.2478/v10136-012-0006-y
Automated assay of the potency natural antioxidants potency using pipetting robot and spectrophotometry
Miroslav Pohanka, Jiri Sochor, Branislav Ruttkay-Nedecky, Natalia Cernei, Vojtech Adam, Jaromir Hubalek, Marie Stiborova, Tomas Eckschlager, Rene Kizek
Address: Rene Kizek, Department of Chemistry and Biochemistry, Faculty of Agronomy, Mendel University in Brno, Zemedelska 1, 613 00 Brno, Czech Republic
kizek@sci.muni.cz
Received 12th October 2011.
Revised 13th December 2011.
Published online 15th December 2011.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results and discussion
Acknowledgements
References
SUMMARY
In the food industry, in the process of creating new agricultural plant products, and in the testing of anti-cancer drugs there is often a need to assay multiple samples of low molecular weight antioxidants, plant samples and foods rich in antioxidants, with minimal additional costs and low degrees of uncertainty. With these demands in mind, we decided to study the fully automated assay of antioxidants using not only automated sample measurements but also automated processing of samples and application of reagents. The automated pipetting system epMotion 5075 and the automated spectrophotometer BS 400 were chosen for the assay purposes. Five methods were introduced for the automation: 2-diphenyl-1-picrylhydrazyl (DPPH) test, ferric reducing antioxidant power (FRAP) method, 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) based test, N,N-dimethyl-1,4-diaminobenzene (DMPD) based test and the Free Radicals method. Samples containing one of the four antioxidants (standard rutin, quercitrin, ferulic and gallic acid) in a range 1-1000 microg/ml were used throughout. All of the tested methods were found suitable for implementation in an automated assay. However, some of them, such as the ABTS test failed to assay all tested antioxidants. The coefficients of determination were also unequal. From the analytical point of view, FRAP methods provided the most reliable results in the automated assay; because of the capacity of the method, approximately 240 samples per hour (one sample per 15 seconds) can be assayed using the automated protocol. We were encouraged by the data received and we expect further interest in the practical performance of such automation. As a means of testing the robustness of our method, in the next step of our study, oxidative status was assessed in model cell lines derived from prostate cancer (PC-3, PNT1A and 22RV1) that were cultured on ellipticine (0, 0.5, 1, 1.5, 2, 2.5, 5, 7.5, 10, 15 micromol/l) supplemented agar. Antioxidant activity was assessed (DPPH, ABTS, FRAP, DMPD, FR) and calculated on the phenolic antioxidant level (rutin, quercitrin, ferulic and gallic acid), and thus an estimation was formulated of the oxidative stress as a result of the impact of anti-cancer drugs. It can be demonstrated that the new method has wide applicability.
KEY WORDS
DPPH; FRAP, ABTS; DMPD, FR, antioxidant; oxidative stress
Abbreviations
ABTS, 2,2'-azino-bis(3-ethylbenzothiazoline-6- sul-fonic acid;
DMPD, N,N-dimethyl-1,4-diaminobenzene;
DMSO, dimethyl sulfoxide;
DPPH, 2,2-diphenyl-1-picrylhydrazyl;
FeCl3, ferric chloride;
FR, free radical;
FRAP, ferric reducing antioxidant power;
FTB, foetal bovine serum;
TPTZ, 2,4,6-tripyridyl-s-triazine;
UV-VIS, ultraviolet-visible spectrum.
INTRODUCTION
In a body, homeostasis, the maintenance of suitable
inner conditions, is a primary task of many
biochemical pathways. Equilibrium between the
production of reactive oxygen (nitrogen) species and
the ability to be protected from them is a
physiological function of antioxidants. When
antioxidants become depleted, oxidative stress can
develop. Some pathological processes are con-sequences of the uncovered production of reactive
oxygen and nitrogen species. Alzheimer's disease,
Parkinson disease and other age related disorders are
examples of the pathological processes, in which
oxidative stress is suspected of playing a crucial role
(Pohanka 2011). Alimental administration of
antioxidants is considered as a way of preventing the
pathological consequences of oxidative stress related
dysfunctions. Plant extracts and plant enriched
sources especially are considered suitable and easily
available products for the prevention of these
pathologies, as reported by e.g. Kaviarasan et al.
(2008).
Unfortunately, research into low molecular weight
antioxidants is not well standardized and differing
protocols are used for the assay of antioxidants.
Results can be influenced because of methodological
errors as well as faults caused by human factors. The
impact of methodological differences has been well
reported. by e.g Muller et al. (2011), and the
implementation of standard protocols with an
automated assay procedure is not only suitable for
simplification and cost saving but it is also necessary
for the lessening of assay uncertainty and
improvement of data validity. This experiment is
aimed at the performance of a fully automated
procedure suitable for fast and reliable assay of low
molecular weight antioxidants in biological samples.
The procedure uses fully automated manipulation
with samples and reagents in order to receive a value
with minimal cost and uncertainty.
MATERIAL AND METHODS
Device and chemicals
The device used was composed of two basic parts: an
automated pipetting system epMotion 5075
(Eppendorf, Germany) and an automated
spectrophotometer BS 400 (Mindray, China). The
pipetting provides a robotic arm with adapters (TS 50,
TS 300 and TS 1000) and Gripper (TG-T). The empty
microtubes are placed in the position B3 (scheme of
depicted as Fig. 1) in adapter Ep0.5/1.5/2 ml. A
Module Reservoir is located in the position B1, where
stock solutions are available. The device is controlled
by the epMotion control panel. Tips sized 300 and
1000 microl (Eppendorf - Germany) are located in the A4
(ePtips 50), A3 (ePtips 300) and A2 (ePtips 1000)
positions.
The automated spectrophotometer is composed of
a cuvette space tempered to 37±1 °C, reagent space
with a carousel for reagents (tempered to 4±1 °C),
sample space with a carousel for preparation of
samples and an optical detector. The transfer of
samples and reagents is provided by a robotic arm
equipped with a dosing needle (error of dosage up to
5% of volume). Cuvette contents are mixed by an
automatic mixer, including a stirrer, immediately after
the addition of reagents or samples. Contamination is
reduced due to the rinsing system, which includes
rinsing of the dosing needle as well as the stirrer by
MilliQ water.
The chemicals used in this study include
deionized water, rutin, quercitrin, ferulic and gallic
acid, 2.2-diphenyl-1-picrylhydrazyl (DPPH), dimethyl
sulfoxide (DMSO), 2.2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), potassium
peroxodisulphate, 2,4,6-tripyridyl-s-triazine (TPTZ),
hydrochloric acid, ferric chloride hexahydrate,
sodium acetate trihydrate, N,N-dimethyl-
1,4-diaminobenzene (DMPD), acetic acid and were
purchased from Sigma Aldrich (St. Louis, MO, USA).
The reaction buffer, chlorophyllin concentrate and its
catalyst were purchased from Sedium R&D; (Czech
Republic).
Standard
As standards, rutin, quercitrin, ferulic and gallic acid
in a calibration range: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
12.5; 15; 17.5; 20; 25; 30; 40; 50; 60; 70; 80; 90; 100;
125; 150; 175; 200; 250; 300; 350; 400; 450; 500;
750; 1000 g/ml were used. The standard calibration
solutions were achieved by automated dilution of
stock solutions 1000, 100 and 10 microg/ml using
deionized water.

Fig. 1. epMotion 5075 automated pipetting system from frontal part.
Determination of antioxidant activity by the DPPH test
The DPPH test was used in compliance with a paper
by Parejo et al. (2000). The method is based on the
ability of the DPPH reagent to react with hydrogen
donors.
Reagent preparation: 0.95 mmol/ml solution of
radical DPPH was prepared by dissolving DPPH in
50 ml of DMSO followed by mixing with 100 ml of
deionized water. The solution was kept in a dark flask
and could be used for 7 days when stored at 4 °C.
Measurement procedure using the automated
device: A 150 microl volume of reagent is incubated with
15 microl of sample. Absorbance was measured at 505 nm
for 12 minutes, and the output ratio was established
by calculating the difference between absorbance at
the last (12th) minute and the second minute of the
assay procedure.
Determination of antioxidant activity by the ABTS test
The ABTS method is based on neutralization of a
radical-cation arising from the one-electron oxidation
of the chromophore 2,2'-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic) acid. The reaction can be
simply monitored spectrophotometrically (Re et al.
1999).
Reagent preparation: 7 mmol/l ABTS and
4.95 mmol/l potassium were mixed and dissolved in
deionized water. The solution was then diluted with
deionized water in a ratio of 1:9 v/v. The solution was
incubated for 12 hours in the dark and the reagent was
kept suitable in the dark at 4 °C for one week.
Measurement procedure for an automated
analyser: A 150 microl volume of reagent was poured
with 3 microl of sample. Absorbance was measured at
660 nm. Antioxidant activity was calculated as the
difference between absorbance at the last (12th)
minute and at the second minute of the assay
procedure.
Determination of antioxidant activity by the FRAP method
The ferric reducing antioxidant power (FRAP)
method is based on the reduction of ferric to ferrous
salt by antioxidants and the following reaction of
ferrous salt with 2,4,6-tripyridyl-s-triazine (TPTZ)
reagent providing contrast blue (Ou et al. 2002).
Reagent preparation: Solution 1: 10 mmol/l
solution of TPTZ, in 40 mmol/l of hydrochloric acid.
Solution 2: 20 mmol/l solution of ferric chloride
hexahydrate in deionized water. Solution 3: 20
mmol/l acetate buffer, pH 3.6 (weight of sodium
acetate trihydrate was 0.272 g in 100 ml deionized
water, adjusted by HCl). These three solutions
(TPTZ, FeCl3, acetate buffer) are poured in a 1:1:10
ratio. The reagent could be used for seven days if
stored at 4 °C in the dark.
Measurement procedure for an automated
analyser: A 150 microl volume of reagent is injected into
a plastic cuvette with the subsequent addition of a
3 microl sample (gallic acid solution). Absorbance is
measured at 605 nm for 12 minutes. The difference
between absorbance at the last (12th) minute and
second minute of the assay procedure was used for
calculating the antioxidant activity.
Determination of antioxidant activity by the DMPD method
The compound N,N-dimethyl-1,4-diaminobenzene
(DMPD) was converted to a relatively suitable and
coloured radical form in the course of the ferric salt
action. After addition of the antioxidant solution, the
coloured solution of DMPD reagent was decolorized
(Gulcin 2008, Gulcin et al. 2010).
Reagent preparation: Solution 1: acetate buffer
(0.2 mol/l, pH 5.25); 1a) 2.17 g of sodium acetate
trihydrate was dissolved in 80 ml of deionized water;
1b) 300 l of concentrated acetic acid (>99.5 %, v/v)
was diluted to a volume of 20 ml with deionized
water. Shortly after mixing, pH = 5.5 was
spontaneously reached. Solution 2: 0.74 mmol/l of
ferric chloride: 1 mg of ferric chloride hexahydrate
was dissolved with deionized water to a volume of
5 ml. Solution 3: 36.7 mmol/l DMPD: 25 mg of
DMPD was dissolved in 5 ml of deionized water. The
reagents were used immediately and prepared fresh
for each day. Solutions 1,2 and 3 were mixed in a
20:1:1 (v/v/v) ratio.
Measurement procedure for an automated
analyser: A 160 microl volume of reagent was injected
into a plastic cuvette with the subsequent addition of
4 l sample. Absorbance was measured at 505 nm.
The difference between absorbance at the last (12th)
minute and second minute of the assay procedure was
used for calculating the antioxidant activity.
Determination of antioxidant activity by the FR method
This method was based on ability of chlorophyllin
(the sodium-copper salt of chlorophyll) to accept and
donate electrons. This effect was conditioned by an
alkaline environment and the addition of a catalyst
and it was followed by a strong change in the
absorbance maximum (Votruba et al. 1999).
Reagent preparation: 5 ml of reaction buffer (100
mmol/l of hydrochloric acid) was diluted with 45 ml
deionized water. After that, 100 microl of chlorophyllin
was added. Once it became solved, 0.25 ml of catalyst
was added. Reaction buffer was suitable for one
month when stored at 2-8 °C in the dark.
Measurement procedure for an automated
analyser: A 150 microl volume of reagent is injected into
a plastic cuvette with the subsequent addition of a
6 microl sample. Absorbance was measured at 450 nm for
the second minute of assay and the last (12th) minute.
The difference between the two absorbencies was
considered as an outputting value.
Cell line experiments
In our study, we used the following prostatic cell lines
PC-3, PNT1A, and 22RV1. The PC-3 was derived
from the 4th level of prostatic adenocarcinoma. The
PNT1A is a cell line derived from human prostatic
epithelial cells. The last, 22RV1, is a human epithelial
cell line derived from an epithelial graft of the cancer
tissue. Culturing took place in flasks with surface 100
cm2 and lasted 72 hours. Cell growth media HAM's
F12 with 7% FVS for PC-3, RPMI-1640 with 10%
FBS for 22RV1 and PNT1A were used. Ellipticine
was dosed in the cell growth media up to level (0, 0.5,
1, 1.5, 2, 2.5, 5, 7.5, 10 and 15 micromol/l). The cultured
cell lines were washed twice by PBS (6 ml of PBS
and centrifugation on 2,700 RPM at 4 °C for 10
minutes). Supernatant was collected and used for
assay of antioxidants in as described above.
RESULTS AND DISCUSSION
Calibration of the methods for antioxidant activity determination
The five methods described in the experimental
chapter were used for the assay purposes. A summary
of the suitability of the methods for assay of low
molecular weight antioxidants used throughout
experiments is depicted in Tables 1-4. The ABTS
test, the DPPH test and FRAP method were suitable
for assay of all the tested antioxidants. When
regression of calibration curves carried out, good
coefficients of determination were found for the three
methods. The free radicals method was found
unsuitable for assay of ferulic acid. The last method,
DMPD, was found suitable for the assay of ferulic
acid and gallic acid only, but the quercitrin assay by
DMPD test was limited in comparison to the other
methods. The low relevancy of DMPD is quite
surprising as it has been used in previous experiments
without any difficulties for assay of low molecular
weight antioxidants in apricot samples (Sochor et al.
2011). Moreover, no crucial disadvantages were
recognized when the method was tested on a trolox
model (Sochor et al. 2010). The reason for the
ineffectiveness of the DMPD test is not clear, but the
low affinity of quercitrin and rutin toward the DMPD
reagent and the equilibrium shifted to reactants can be
inferred.
Table 1. Summary of the parameters for individual methods related to the standard gallic acid.
| Method |
Wavelength
[nm] |
Measuring range
[microg/ml] |
Calibration equation |
Confidence
coefficient [R2] |
Standard
deviation [%] | | DPPH |
505 |
1-17.5 |
y = -0.1533x - 1.6252 |
0.9945 |
1.96 | | ABTS |
660 |
1-25 |
y = -0.071x - 0.9841 |
0.9977 |
2.26 | | FRAP |
605 |
1-350 |
y = 0.0978x + 0.9774 |
0.9989 |
1.19 | | DMPD |
505 |
1-30 |
y = -0.0509x - 0.2913 |
0.9982 |
2.68 | | FR |
450 |
1-750 |
y = 0.0029x - 0.085 |
0.9991 |
1.11 |
Table 2. Summary of the parameters for individual methods related to the standard ferulic acid.
| Method |
Wavelength
[nm] |
Measuring range
[microg/ml] |
Calibration equation |
Confidence
coefficient [R2] |
Standard
deviation [%] | | DPPH |
505 |
1-100 |
y = -0.0548x - 1.1467 |
0.9927 |
1.53 | | ABTS |
660 |
1-150 |
y = -0.038x - 0.7683 |
0.9915 |
2.28 | | FRAP |
605 |
1-175 |
y = 0.0284x + 0.2702 |
0.9973 |
1.42 | | DMPD |
505 |
17.5-800 |
y = -0.458ln (x) + 0.1955 |
0.9852 |
2.31 | | FR |
450 |
- |
- |
- |
- |
Table 3. Summary of the parameters for individual methods related to the standard quercitrin.
| Method |
Wavelength
[nm] |
Measuring range
[microg/ml] |
Calibration equation |
Confidence
coefficient [R2] |
Standard
deviation [%] | | DPPH |
505 |
7-70 |
y = -0.0484x - 1.3425 |
0.9992 |
1.63 | | ABTS |
660 |
7-70 |
y = -0.0309x - 1.1151 |
0.9959 |
2.03 | | FRAP |
605 |
1-1000 |
y = 0.0342x + 0.4564 |
0.9996 |
1.02 | | DMPD |
505 |
- |
- |
- |
- | | FR |
450 |
1-1000 |
y = 0.0039x + 0.127 |
0.9996 |
1.27 |
We selected the following standards - rutin,
quercitrin, ferulic and gallic acid - to test the automation of methods for determination of
antioxidant activity. All tested molecules can be used
as a standard for calibration of antioxidant assays and
their selection mainly depends on the type of material
analysed and the laboratory equipment used.
However, automated spectrometric assays tested in
this study must be effective with all these substances
to show the versatility of the system. Ferulic acid was
the first compound tested (Fig. 2). The dependencies
of absorbance on the concentration of ferulic acid
measured by the DPPH test, ABTS test, FRAP
method, DMPD method, and the free radicals method
are shown in Figs 2A, B, C, D and E. The structure of
gallic acid is shown in Fig. 2F. The same results were
obtained for ferulic acid (Fig. 3), quercitrin (Fig. 4)
and rutin (Fig. 5).
Table 4. Summary of the parameters for individual methods related to the standard rutin.
| Method |
Wavelength
[nm] |
Measuring range
[g/ml] |
Calibration equation |
Confidence
coefficient [R2] |
Standard
deviation [%] | | DPPH |
505 |
1-25 |
y = -0.0354x - 0.3071 |
0.9966 |
1.15 | | ABTS |
660 |
60-1000 |
y = -0.0053x - 0.5518 |
0.9982 |
2.08 | | FRAP |
605 |
1-175 |
y = 0.0212x + 0.3304 |
0.9983 |
1.28 | | DMPD |
505 |
- |
- |
- |
- | | FR |
450 |
1-1000 |
y = 0.0039x - 0.1429 |
0.9994 |
1.01 |
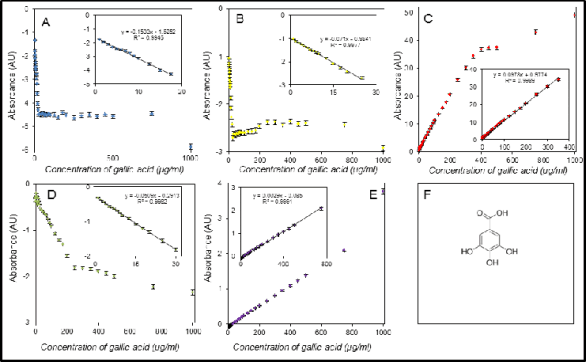
Fig. 2. Assay of gallic acid using the DPPH test (A), the ABTS test (B), the FRAP method (C), the DMPD method (D), and the
FR method (E). The last part of figure is the structure of gallic acid (F).
Calibration plots for the tested methods are shown
in Figs 2-5. We found good coefficients of
determinations in a range 0.9927-0.9992 for the
DPPH test, 0.9915-0.9982 for the ABTS test,
0.9973-0.9996 for the FRAP method, 0.9852 and
0.9982 for the DMPD method and the two successful
calibrations, and 0.9991 - 0.9996 for the free radicals
method and the three successful calibrations. It
clearly follows from the results obtained that the best
correlations were found for the FRAP and free
radicals methods thus favourable adaptation to
automation can be inferred.
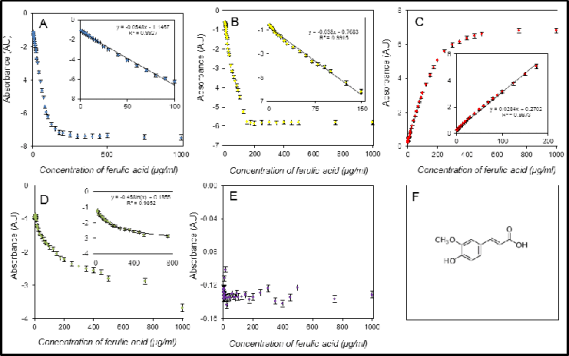
Fig. 3. Assay of ferulic acid using the DPPH test (A), the ABTS test (B), the FRAP method (C), the DMPD method (D), and
the FR method (E). The last part of figure is the structure of ferulic acid (F).
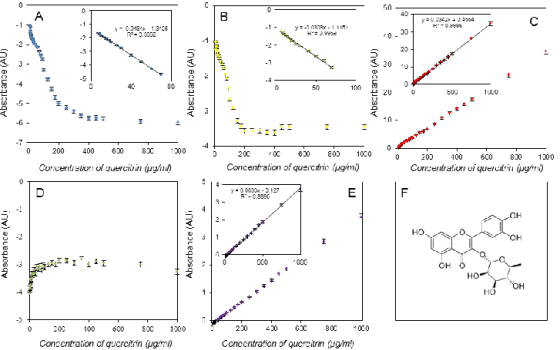
Fig. 4. Assay of quercitrin using the DPPH test (A), the ABTS test (B), the FRAP method (C), the DMPD method (D), and the
FR method (E). The last part of figure is structure of quercitrin (F).
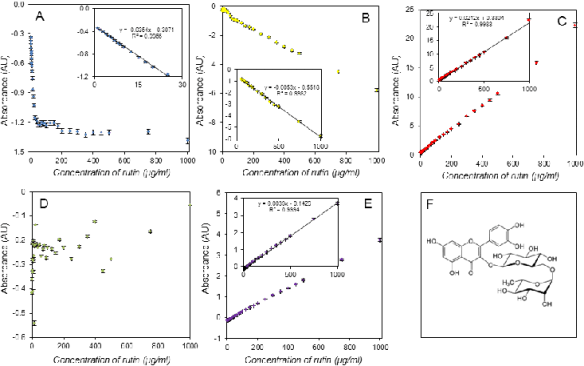
Fig. 5. Assay of rutin using DPPH test (A), ABTS test (B), FRAP method (C), DMPD method (D), FR method (E). The last part
of figure is structure of rutin (F).
The calibrations shown had differing linear
ranges. The FRAP and free radicals methods
providing linear calibration as high as 1,000 g/ml for
some antioxidants, had the longest linear ranges, but in contrast, the DPPH test was limited by
approximately 100 µg/ml for the ferulic acid assay
and even 20 g/ml for gallic acid. The good results
for the FRAP method are not surprising considering
its applicability for low molecular weight antioxidants
assays in tissue samples (Pohanka et al. 2011),
assessment of antioxidants in fruit (Bouayed et al.
2010), and in vitro assessment of antioxidants such as
uric acid assay (Duplancic et al. 2011). Considering
the automation process, the lengths of linear ranges
are not dependent on automation but on the reaction
principle and molecular mechanism of methods
pre-limiting them for applicability in the automation
of assays.
The major advantage of the automated assay is the
short time needed per sample. Though the total time
from inputting the sample to receiving the measured
value is nearly a quarter of an hour, the total time per
sample is less than one minute. When the automated
device is used for an extensive series of samples, the
total time per sample is approximately 15 seconds. It
means that 240 samples can be assayed per hour by
one selected method without any additional effort or
special need for skilled manipulation of the samples.
The performance of an automated pipetting device
with automated spectrophotometer is suitable for a
fast and reliable assay of low molecular weight
antioxidants. The automated pipetting system
epMotion 5075 and the automated spectrophotometer
BS 400 were both suitable for the assay purposes and
all of the selected experimental protocols were
adaptable for analytical purposes. The failure in the
assay of rutin by the DMPD method, and in the assay
of ferulic acid by the free radicals method was not
caused by the inapplicability of the automated assay
but by shortcomings in the applicability of protocols
for some compounds. This was not disclosed in the
databases searched. The easy availability of the
DMPD method has been reported in the work of
Damien Dorman et al. (2011). On the other hand,
samples are commonly assayed by one or two
methods, so discrepancies in natural sample assays
can stay hidden. When the performance of the
automated protocol is considered using all of the
described methods, the FRAP method meets the best
criteria as it was successfully performed for all
antioxidants, it has a long linear range and good
coefficients of determinations. On the other hand, no serious reason arose to discriminate against the other
methods and the last four protocols are applicable for
the construction of a fully automated protocol also.
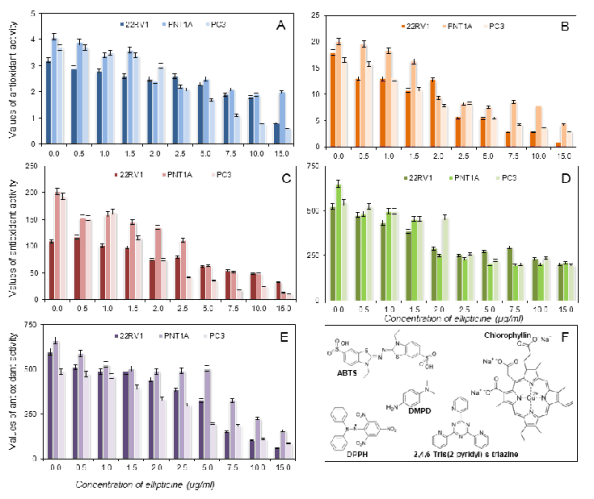
Fig. 6. Assayed antioxidants in cell lines using the DPPH test (A), the ABTS test (B), the FRAP method (C), the DMPD method
(D), and the FR method (E). Calculated on gallic acid.
The significance of low molecular weight
antioxidants in biological samples has been described
by several investigators (Kaviarasan et al. 2008,
Salehi et al. 2011). The methods proposed here can be used for the fast and reliable processing of natural
samples to judge their antioxidant capacity. When the
automated pipetting system and the automated
spectrophotometer are used simultaneously, natural
samples can be assessed with good effectiveness and
low uncertainty.
Application of the optimized methods on real sample analyses
The test for suitability of the automated assay for
evaluation of antioxidants in cell lines was performed
in prostatic cancer cells exposed to ellipticine. The
drug damages the DNA of cancer cells and stops
transcription and replication. The cells are not able to
divide, and as a result, the cells are directed to
apoptosis (Stiborova et al. 2011, Kizek et al. 2012).
However, the drug does not act specifically on cancer
cells and normal cells can be affected, too. The aim of
our experiment was to estimate the scale of oxidative
stress - expressed as the antioxidant activity - in cancer cells and cells affected by a cytostatic drug.
The experiment was aimed at a comparison of the
effect of ellipticine on cancer and normal cell lines.
The results are depicted in Figs 6-9. We used all of
the optimized methods - DPPH, ABTS, FRAP,
DMPD and free radicals- for determination of the
antioxidant activity. Primarily, we re-calculated the
results on the gallic acid, which is the most
commonly accepted standard for calibration of the
assay (Fig. 6). From the methodological point of
view, it clearly follows from the results obtained that
all methods gave similar results, i.e. that increasing
the dose of ellipticine resulted in the decrease of
antioxidant activity. The radicals measured in the real
samples are shown in Fig. 6F. Further, we
re-calculated the results obtained on ferulic acid
(Fig. 7). As was shown above, ferulic acid is not a
suitable standard for calibration of the free radicals
assay. Therefore, we did not use this re-calculation.
The rest of the methods gave similar results to gallic
acid. In the case of re-calculation on quercitrin
(Fig. 8) and rutin (Fig. 9), we did not use the results
obtained from the DMPD assay.
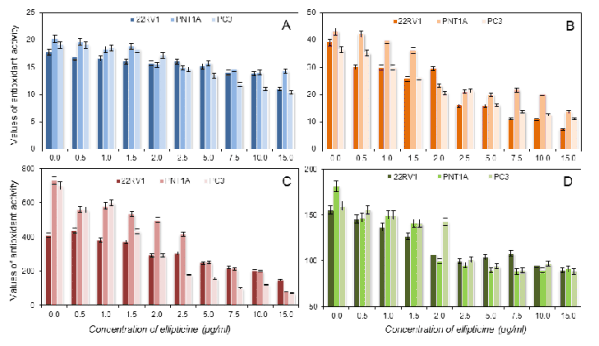
Fig. 7. Assayed antioxidants in cell lines using the DPPH test (A), the ABTS test (B), the FRAP method (C), the DMPD method
(D), and the FR method (E). Calculated on ferulic acid.
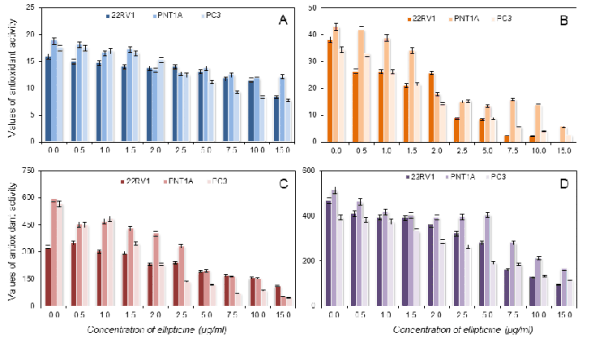
Fig. 8. Assayed antioxidants in cell lines using the DPPH test (A), the ABTS test (B), the FRAP method (C), the DMPD method
(D), and the free radicals method (E). Calculated on quercitrin.
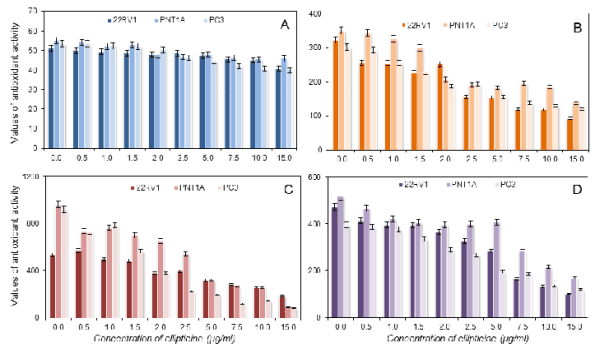
Fig. 9. Assayed antioxidants in cell lines using the DPPH test (A), the ABTS test (B), the FRAP method (C), the DMPD method
(D), and the FR method (E). Calculated on rutin.
From the biological point of view, the depletion of
antioxidants was more striking when the ABTS test
and FRAP were used for assay purposes. The last two
tests were less sensitive. The depletion of antioxidants
scaled by the DMPD and free radical methods proved
less extensive alterations in comparison with the
ABTS test and FRAP; in spite of the fact that
ellipticine is able to generate reactive oxygen species,
the antioxidant capacity decreased. This phenomenon
is related to the fact that the drug used was cytotoxic.
Therefore, the antioxidant capacity clearly correlated
with the viability of the cells. Interestingly, we did
not confirm the differences between the types of cell
line used in our experiment. These results could be
related to the fact that ellipticine is toxic for prostate
cells equally from the point of view of generation of
reactive oxygen species, which has to be considered
prior to use of this drug for the treatment of this
cancer. Besides, the transformation of a healthy
prostate cell line into a tumour did not influence the
sensitivity to antioxidant mechanism in a cell.
Toxicity of anticancer drugs is widely discussed in
scientific works (Zanella et al. 2011, Zong et al.
2011). Oxidative stress, as a consequence of the
impact of the drugs is not adequately investigated, as
elaborative protocols are needed for the assay.
Understanding of the oxidative stress related
processes in isolated conditions is necessary when the
effect of a new drug is researched.
CONCLUSIONS
An automated method for the fast and reliable assay
of antioxidants was developed. The method is suitable
for laboratory estimation of antioxidant potency in
biological samples. Though five methods, ABTS test,
DPPH test, FRAP method, DMPD test and free
radicals method, were successfully performed, their
suitability for automated assay was unequal. From the
methods tested, FRAP provided the most favourable
parameters for implementation into the automated
device. Antioxidant activities in cells exposed to
ellipticine decreased in a dose dependent manner. We
can infer the contribution of the anticancer drug to
oxidative damage in cells and compare the effect on
normal as well as cancer cells. The method performed
is suitable for these purposes.
ACKNOWLEDGEMENTS
Financial supports from the projects CEITEC
CZ.1.05/1.1.00/02.0068, CYTORES GA CR
P301/10/0356 and GA AV NANOSEMED
KAN20813081 are kindly acknowledged.
REFERENCES
Bouayed J, Hoffmann L, Bohn T. Antioxidative mechanisms of whole-apple antioxidants employing different varieties from Luxembourg. J Med Food. 14:
1631-1637, 2011.
[CrossRef]
[PubMed]
Damien Dorman HJ, Shikov AN, Pozharitskaya ON, Hiltunen R. Antioxidant and pro-oxidant evaluation of a Potentilla alba L. rhizome extract. Chem
Biodivers. 8: 1344-1356, 2011.
[CrossRef]
Duplancic D, Kukoc-Modun L, Modun D, Radic N. Simple and rapid method for the determination of uric acid-independent antioxidant capacity. Molecules.
16: 7058-7068, 2011.
[CrossRef]
[PubMed]
Gulcin I. Measurement of antioxidant ability of melatonin and serotonin by the DMPD and CUPRAC methods as trolox equivalent. J Enz Inhib Med Chem.
23: 871-876, 2008.
[CrossRef]
[PubMed]
Gulcin I, Bursal E, Sehitoglu MH, Bilsel M, Goren AC. Polyphenol contents and antioxidant activity of lyophilized aqueous extracts of propolis from
Erzurum, Turkey. Food Chem Toxicol. 48: 2227-2238, 2010.
[CrossRef]
[PubMed]
Kaviarasan K, Kalaiarasi P, Pugalendi V. Antioxidant efficacy of flavonoid-rich fraction from Spermacoce hispida in hyperlipidemic rats. J Appl
Biomed. 6: 165-176, 2008.
[JAB]
Kizek R, Adam V, Hrabeta J, Eckschlager T, Smutny S, Burda JV, Frei E, Stiborova M. Anthracyclines and ellipticines as DNA-damaging anticancer drugs;
Recent advances. Pharmacol Ther. 133: 26-39, 2012.
[CrossRef]
Muller L, Frohlich K, Bohm V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching
assay (alpha TEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 129: 139-148, 2011.
[CrossRef]
Ou B, Huang D, Hampsch-Woodill M, Flanagan JA, Deemer EK. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance
capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: a comparative study. J Agric Food Chem. 50: 3122-3128, 2002.
[CrossRef]
[PubMed]
Parejo I, Codina C, Petrakis C, Kefalas P. Evaluation of scavenging activity assessed by Co(II)/EDTA-induced luminol chemiluminescence and DPPH
(2,2-diphenyl-1-picrylhydrazyl) free radical assay. J Pharmacol Toxicol Meth. 44: 507-512, 2000.
[CrossRef]
Pohanka M. Alzheimer's disease and related neurodegenerative disorders: implication and counteracting of melatonin. J Appl Biomed. 9: 185-196,
2011.
[CrossRef]
[JAB]
Pohanka M, Bandouchova H, Vlckova K, Zdarova Karasova J, Kuca K, Damkova V, Peckova V, Vitula F, Pikula J. Square wave voltammetry on screen printed
electrodes: comparison to ferric reducing antioxidant power in plasma from model laboratory animal (Grey Partridge) and comparison to standard
antioxidants. J Appl Biomed. 9: 103-109, 2011.
[CrossRef]
[JAB]
Re R, Pellegrini N, Preteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improvement ABTS radical cation decolorization
assay. Free Radic Biol Med. 26: 1231-1237, 1999.
[CrossRef]
Salehi P, Sonboli A, Khaligh P, Mirzajani F. Essential oil composition and antioxidant activity of differential extracts of Nepeta
betonicifolia C.A. Meyer and Nepeta saccharata Bunge. Prod Res. 26: 736-743, 2012.
[CrossRef]
[PubMed]
Sochor J, Ryvolova M, Krystofova O, Salas P, Hubalek J, Adam V, Trnkova L, Havel L, Beklova M, Zehnalek J, Provaznik I, Kizek R. Fully automated
spectrometric protocols for determination of antioxidant activity: advantages and disadvantages. Molecules. 15: 8618-8640, 2010.
[CrossRef]
[PubMed]
Sochor J, Skutkova H, Babula P, Zitka O, Cernei N, Rop O, Krska B, Adam V, Provaznik I, Kizek R. Mathematical evaluation of the amino acid and
polyphenol content and antioxidant activities of fruits from different apricot cultivars. Molecules. 16: 7428-7457, 2011.
[CrossRef]
[PubMed]
Stiborova M, Rupertova M, Frei E. Cytochrome P450-and peroxidase-mediated oxidation of anticancer alkaloid ellipticine dictates its anti-tumor
efficiency. Biochim Biophys Acta Proteins Proteomics. 1814: 175-185, 2011.
[CrossRef]
Votruba M, Stopka P, Hroudova J, Vesely K, Nejedlova L. A simple method for quantitative estimation of free radicals in serum. Klin Biochem Metab. 7:
96-101, 1999.
Zanella A, Gandin V, Porchia M, Refosco F, Tisato F, Sorrentino F, Scutari G, Rigobello MP, Marzano C. Cytotoxicity in human cancer cells and
mitochondrial dysfunction induced by a series of new copper(I) complexes containing tris(2-cyanoethyl)phosphines. Invest New Drugs. 29: 1213-1223,
2011.
[CrossRef]
[PubMed]
Zong D, Haag P, Yakymovych I, Lewensohn R, Viktorsson K. Chemosensitization by phenothiazines in human lung cancer cells: impaired resolution of
gamma H2AX and increased oxidative stress elicit apoptosis associated with lysosomal expansion and intense vacuolation. Cell Death Dis. 2: e181.
|
BACK
|










