Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 3, p 119-136
DOI 2478/v10136-012-0008-9
Non-alcoholic steatohepatitis: an overview including treatments with herbals as alternative therapeutics
Menaka Chanu Thounaojam, Ravirajsinh Navalsinh Jadeja, Ranjisinh Vijaysinh Devkar, Ayalur Vadathala Ramachandran
Address: Ranjitsinh Vijaysinh Devkar, Division of Phytotherapeutics and Metabolic Endocrinology, Faculty of Science, The M. S. University of Baroda, Vadodara-390002, Gujarat, India
phyto_met@yahoo.com
Received 4th August 2011.
Revised 25th October 2011.
Published online 25th October 2011.
Full text article (pdf)
Summary
Key words
Introduction
Prevalence and risk factors
Ethnicity, genetics and environment
Clinical features and diagnostics
Role of liver biopsy
Etiology
Pathogenesis
Treatment of NASH
Herbal medicines and NASH
Current scenario and future perspectives
Acknowledgement
References
SUMMARY
Non-alcoholic steatohepatitis (NASH), an under- recognized hepatic ailment with increasing prevalence, is fast emerging as the dark horse of hepatic related morbidity and mortality. Though introduced as a hepatic condition as early as 1980, detailed exploration of its causes, underlying mechanisms and therapeutics has largely remained an ignored or neglected field. Only recently, the focus of attention has gravitated towards an understanding of NASH as a pathological manifestation of significance and a search for possible therapeutic interventions. Treatment schedules as of now involve life style management and usage of anti-diabetic or anti-obesity drugs and antioxidants. In the present review, we have focused on the available treatment schedules including herbal agents, plant extracts, polyherbal formulations and isolated phytocompounds. We present here a review of the available literature on pre-clinical and clinical evaluations of herbals including our recent findings on two plant extracts which can be used in mitigating NASH. This review attempts to provide a comprehensive account of NASH and the future of therapeutics and remediation by herbal principles, an aspect of the urgent need to target this important medical condition that contributes to many cases of silent morbidity and mortality.
KEY WORDS
non-alcoholic steatohepatitis; herbal medicine; phytocompounds; polyherbal formulation
INTRODUCTION
Ludwig et al. (1980) were the first to introduce this
condition in 1980 to describe a series of patients with
hepatic cirrhosis similar to alcoholic cirrhosis but who
did not have a history of alcohol consumption.
Non-alcoholic fatty liver disease (NAFLD) is a
broader category of hepatic disorder, manifesting
despite non-alcoholism, and encompassing a spectrum
of alterations ranging from simple fatty liver/hepatic
steatosis (the accumulation of fat in liver to upward of
5-10% of the liver mass) to non-alcoholic
steatohepatitis (NASH) and increasing fibrosis
leading to cirrhosis, liver failure and hepatocellular
carcinoma. Non-alcoholic steatohepatitis (NASH) is
rapidly becoming a worldwide public health problem.
Despite the high prevalence of NASH, the underlying
etiological factors that determine disease progression
through fibrosis to cirrhosis remain poorly
understood. Moreover, the available non-invasive
techniques to study hepatic metabolism in humans are
limited, and liver biopsies are required to identify
individuals with NASH (Erickson 2009). According
to popula-tion-based studies on NASH, it is
histologically similar to alcohol-induced
steatohepatitis. Moreover, many of the factors
implicated in the development of alcoholic
steatohepatitis are also associated with NASH
(Matteoni et al. 1999).
PREVALENCE AND RISK FACTORS
Estimates of the global prevalence among the general
populace of NAFLD/NASH, a leading cause of
hepatic dysfunction and cirrhosis, suggest a
prevalence rate of 20-30% in the western and U.S
population but with a higher incidence of 75-100% in
obese (BMI30) and morbidly obese (BMI40)
subjects respectively (Fan et al. 2011). In United
States, NASH is the third most common liver disease
after hepatitis C and alcoholic fatty liver (Patel and
Lee 2001) with a current estimate of 20%. Recent
estimates suggest 6 million individuals of the general
populace in U.S to have progressed to NASH and
0.6 million to NAFLD related cirrhosis (Erickson
2009). The overall prevalence of NASH in the
Asia-Pacific region is at present broadly similar to
that in the west (Harish and Thomas 2008). However,
there are some differences between the demographic
and epidemiologic features of NASH in developing
and developed countries. The prevalence amongst the
obese population ranges as high as 75-92% and a
serious cause of concern is the prevalence of NASH
even amongst the pediatric population, to the tune of
about 10% in the age group 2-19 years (Manco et al.
2008). Of still greater concern, and with implications
for future disease burden, is the higher rate of
prevalence of NAFLD amongst obese children
(Erickson 2009). Available data on national
occurrence shows that NAFLD reportedly affects
20% of obese children and adolescents from the US
(Strauss et al. 2000), 44% from Italy (Sartorio et al.
2007) and 74% from China (Chan et al. 2004). The
disease associates significantly with type 2 diabetes
mellitus (T2D) and all features of the metabolic
syndrome (MetS) (Erickson 2009). A similar increase
in the prevalence of obesity and metabolic syndrome
in Asia with increasing rates of NASH suggests the
likely possibility of a progressive increase in the
prevalence of NASH in the next decade (Fan 2006).
The most commonly identified risk factors for NASH
are a high-fat diet, a high-calorific diet, a sedentary
lifestyle, insulin resistance, the metabolic syndrome
and its components such as obesity, hypertension,
dyslipidemia and T2D (Adams et al. 2010).
ETHNICITY, GENETICS AND ENVIRONMENT
Though NASH affects all racial groups, Hispanic
populations show a higher prevalence compared to
White and non-White populations. Interestingly,
NASH and cryptogenic cirrhosis are of low
prevalence in African-American (AA) populations
compared to White populations (Caldwell et al. 2002,
Browning et al. 2004). An evaluation of hepatic
triglyceride content by magnetic resonance
spectroscopy by Browning et al. (2004) suggested
that AA subjects are less likely to develop steatosis
compared to White and Hispanic subjects. They
considered the inherent biological differences in lipid
metabolism [as suggested by a lesser degree of
hypertriglyceridemia and a lower level of
high-density lipoprotein (HDL) cholesterol] among
AA subjects to be responsible for the lower incidence
of steatosis in AA. In this context, genetic differences
in hepatic gene expression between populations of
Caucasian and AA patients with NASH have been
reported (Stepanova et al. 2008). The phenotypic
variation in NASH between racial and ethnic groups
observed by Lewis and Mohanty (2010) lends support
to the above contention. Of late, several Asian
countries like Korea, China, Japan and India have
witnessed an increasing incidence of NASH
(Amarapurkar et al. 2007, Fan et al. 2007). New body
mass index (BMI) criteria have been generated to
adjust for the anthropometric difference of higher
levels of visceral adiposity at a lower BMI in Asian
patients with NASH compared to the Caucasian
population (Fan et al. 2007).
A genetic predisposition for NASH has been
suggested based on the differential prevalence of
NASH among different ethnic groups and variable
rates of progression in individuals with identical risk
factors (Willner et al. 2001). Osterreicher and
Brenner (2007) have reviewed the available literature
on genetics and Edmison and McCullough (2007)
have listed the identified potential genes based on the
full spectrum of genes reported for NASH. The higher
incidence of NASH in American populations of
Hispanic origin relative to Whites and lower
incidence in AA despite greater obesity provide
substantiation for ethnic/racial implication in
susceptibility to progressive NASH (Caldwell et al.
2002, Browning et al. 2004). The possibility of gene
variants impacting shared molecular pathways
between metabolic syndrome (MetS), type 2 diabetes
(T2D) and cardiovascular disorder (CVD) and NASH
is indicated by the higher risk for these disorders
among South Asian Indian and other Asia-Pacific
populations (Amarapurkar et al. 2007, Fan et al.
2007). Similarly, NASH is of general occurrence
throughout Latin America in keeping with the greater
propensity for MetS in Americus-Indian populations
(Lazo and Clark 2008). Although obesity and IR,
identified as risk factors, can precipitate hepatic
steatosis, only a minority of patients progress to
NASH and cirrhosis, suggesting an apparent interplay
between a genetic predisposition and environmental
factors (Petta et al. 2009). Screening of NASH
patients for gene variants, using genome scans, is
yielding dividends as PNPLA3/adiponutrin rs738409
CG genotype, encoding for I148M, appears related
to the severity of steatosis and fibrosis and the
presence of nonalcoholic steatohepatitis (Valenti et al.
2010), and ethnic differences in this variant have been
associated with differing propensities to NASH
(Romeo et al. 2000). Heightened susceptibility to the
development of NASH is co-relatable with
polymorphisms in genes implicated in lipid
metabolism, IR, oxidative stress, cytokines/
adipokines and fibrognesis (Wilfred de Alwis and
Day 2007). Studies on NASH individuals have
demonstrated an association between advanced
hepatic fibrosis in obese patients and polymorphisms
in the angiotensinogen and TGF-beta1 genes (Dixon et
al. 2003).
Recently, two common SNP variants in the
adiponectin gene (previously associated with
cardio-metabolic risk) have been found to be
associated with dietary fat in NASH patients (Musso
et al. 2008). Further, Dongiovanni et al. (2010) have
shown that ectoenzyme nucleotide pyrophosphate
phosphodiesterase 1/ plasma cell antigen-1 (ENPP1/
PC-1) 121Gln and IRS-1 972Arg polymorphisms
affecting insulin receptor activity predispose to liver
damage and decrease hepatic insulin signalling in
patients with NASH. Apparently, the genetic effect of
defective insulin signalling may play a causal role in
the progression of liver damage in NASH. Yoneda et
al. (2009) have shown an association between SNPs
in the angiotensin II type 1 receptor and increased
risk of NASH and NASH -related fibrosis. Further
studies to identify more candidate genes that may not
only provide information on pathogenesis and
prognosis of the disease but also serve as novel
treatment targets, have been suggested (Dowman et
al. 2010).
Also, screening for chromosomal regions
harbouring gene variants that could influence the
onset and progression of NASH in mouse models is
being undertaken currently with a view to translating
the information obtained to the human genome to
identify areas with greater probability of NASH
progression (Erickson 2009).
CLINICAL FEATURES AND DIAGNOSTICS
Many patients with NASH (30-40%) have
complained of prior non-specific symptoms like
weakness, fatigue and malaise (Bacon et al. 1994).
Despite the many similarities between NASH and
ASH, the former is mostly asymptomatic while the
latter is symptomatic. Patients with drug induced
NASH (due to nucleoside analogs, anti-mitotic agents
or tetracyclines) (Diehl 1999) generally develop
dramatic and rapid onset of fulminate hepatic failure.
The most common symptoms of NASH patients are
hepatomegaly in most of the cases and splenomegaly
in some of the cases (Leevy 1962), and very often, the
presence of ascites and spider angiomata indicates
cirrhotic development (Itoh et al. 1987).
Despite the increased understanding of
non-alcoholic fatty liver conditions, the diagnosis and
staging of NASH remain to-date a difficult
proposition. The only acceptable mode of diagnosis
of NASH valid so far has been a consideration of
medical history along with a liver biopsy. Though
liver biopsy alone represents an unequivocal way of
assessing the stage pf NASH, it is nevertheless beset
with limitations, either over-estimating or
under-estimating the degree of disease progression
(Ratziu et al. 2005). Due to the many caveats
associated with this technique apart from its
invasiveness, reliable alternate non-invasive methods
need to be pursued for effective diagnosis and staging
of NASH. Though many techniques had been
proposed, none have found clinical acceptance
(Erickson 2009). Nevertheless, some non-invasive
techniques involving a combination of radiological
and laboratory techniques for staging NAFLD have
found differing degrees of acceptance. A listing and
description of these non-invasive techniques can be
found in recent publications (Dowman et al. 2011,
McPherson et al. 2010). Diagnosis of NASH usually
requires testing for liver biochemistry, as most such
cases stand diagnosed subsequent to an evaluation of
abnormal liver function tests and/or ultrasound or
computed tomography scans indicating a fatty liver
status. Ultrasonography, computed tomography,
magnetic resonance imaging, and radionucleotide
techniques, are routine techniques employed to
characterize hepatic steatosis. Ironically, none of
these techniques helps distinguish between simple
steatosis and steatohepatitis with progressive fibrosis.
NASH being essentially a clinicohistologic entity,
histology is of prime importance in confirming the
diagnosis (Lewis and Mohanty 2010).
The level of aminotransferase activity seems to be
typically increased by four times compared to
alcoholic liver disease, with alanin transaminase
activity being higher than aspartate transaminase.
Though the bilirubin level remains in the normal
range, a doubled level of alkaline phosphatase activity
appears to be a feature (Charlton 2004). A detailed
history of patients with abnormal liver biochemistry
is necessary to exclude the possibilities of excessive
alcohol consumption, steatohepatitis inducing
pharmacotherapy, surgical procedures, and occu-pational exposure to hepatotoxins, along with a
nutritional history, particularly of rapid weight gain or
loss, essentially to over-rule clinical conditions
associated with steatohepatitis. Some other associated
clinical conditions such as Wilson's, disease, viral
hepatitis, and autoimmune liver disease, whose
exclusion is impossible by the simple consideration of
histories require serologic/biochemical exclusion.
Very often, the majority of NASH patients show one
or more features of metabolic syndrome such as
increased waist circumference, hypertriglyceridemia,
low high-density lipoprotein cholesterol, hyper-tension, and a fasting glucose of 110 mg/dl or higher
(Charlton 2004). It is however not clear as to how far
these symptoms find specific association with NASH.
A caveat however is that, aminotransferase
elevations, though used to diagnose NASH, lack
adequate sensitivity to detect patients with NASH and
are entirely nonspecific in predicting liver injury.
Though characterized by focal areas of fat in the liver,
NASH can be difficult to diagnose because of
difficulties in distinguishing the disease from primary
malignancies or metastasis and fine needle aspiration
may be required to exclude malignancy. It is also
worth noting the possibility of the presence of NASH,
especially the chronic progressive form, even in the
backdrop of apparently normal values of liver
function tests and mild fatty liver. Further complexity
in the diagnosis of NASH is its non-obligatory
association with obesity, MetS or T2D. Apparently,
individuals free of these conditions can, and in fact,
do develop NASH. Moreover, not all individuals who
are obese or have MetS or T2D develop progressive
NASH.
ROLE OF LIVER BIOPSY
From the above it is clear that, suspected diagnosis of
NASH is essentially based on a symptomatic
elevation of aminotransferases, radiological features
of fatty liver and hepatomegaly while diagnostic
establishment is possible in the context of clinical
history. A significant feature of NASH associated
hepatic triglyceride accumulation seems to be a
decreased production of apolipoprotein B (Apo-B).
Increased output of reactive oxygen species (ROS)
from mitochondria seems to trigger steatohepatitis
and fibrosis by three main mechanisms: lipid
peroxidation, cytokine induction and induction of fas
ligand. A deficiency in the enzymes of peroxisomal
oxidation that leads to the accumulation of significant
amounts of dicarboxylic acids is apparently another
major cause of micro-vesicular steatosis and
steatohepatitis; an added consequence of the
deficiency of peroxisomal enzymes is the sustained
hyper-activation of peroxisome proliferator-activated
receptor = alpha (PPAR-alpha) regulated genes (Angulo
2002). The induction of cytokines (TNF-alpha, TGF-beta
and IL-8) by reactive oxygen species seems to be
triggered by lipid peroxidation and the release of
malon-dialdehyde (MDA) and -hydroxy noneal
(HNE). Moreover, mitochondrial ROS induced
expression of fas-ligand on hepatocytes and
interaction between fas-ligands of neighbouring
hepatocytes may lead to fractional killing (Angulo
2002) and MDA and HNE may promote further cell
death, Mallory hyaline formation and collagen
synthesis.
ETIOLOGY
Currently, there exists a significant gap in our
understanding of the complex etiology of NASH and
its progression. No doubt it is multi-factorial and
many cases appear more related to a 'Western
lifestyle' (i.e. nutrient abundance coupled with a
sedentary lifestyle); however, an increased risk seems
more related to genetic predisposition (Erickson
2009). The first recognizable stage of NASH,
"simple" benign steatosis, is an indication of an
exceeded fat storage capacity of visceral adipose
tissue, considered a major risk factor for NASH and
its progression. Increased visceral adiposity in turn
can result in a higher output of adipocyte hormones
and pro- and anti-inflammatory cytokines and
chemokines (Kershaw and Flier 2004), which could
fan the progression of NASH to its less benign stages.
Molecular pathways identified in NASH and its
progression seem more similar to those activated in
injured organs and tissues. The innate immune
system, apart from dysregulation in lipid metabolism,
also stands implicated in the initial response of the
liver to insult/injury; in fact, similar mechanisms also
seem related to a fibrotic response (Jou et al. 2008).
Both host factors and liver specific regulators are
likely to be involved in the overall development and
progression of NASH.
PATHOGENESIS
First hit
Formulation of an hypothesis on the pathogenesis of
NASH occurred almost two decades after the first
description of the disease in 1980; an hypothesis that
was revised immediately (Day and James 1998, Day
2002) and is known as a 'two hit' hypothesis. The
first hit, marked by triglyceride loading within
hepatocytes known as steatosis or fatty liver
(NAFLD) is due to an overflow of free fatty acids
(FFA) into the liver and consequent esterification
(Fig. 1). The hepatic steatosis marked by high
triglyceride accumulation is reflective of an excessive
inflow of FFA and this, rather than triglyceride per
se, seems to be the factor responsible for the first hit
development of NAFLD (Shiota and Tsuchiya 2006)
and the subsequent vulnerability of the liver for
second hits leading to NASH and/or fibrosis (Fig. 1).
As already mentioned, insulin resistance of all the
causes, is the only metabolic syndrome that appears
to depict a consistent association with NASH, and
which can precipitate hepatic steatosis, lipolysis and
hyperinsulinemia. Both lipolysis and hyper-insulinemia lead to higher FFA levels in the
circulation (adipose tissue lipolysis) and in the liver
(glycolytic synthesis) respectively and, the greater
hepatic FFA load results in a mitochondrial oxidation
overload and consequent steatosis due to decreased
Apo-B production.
Second hit
This background of hepatic steatosis sets the stage of
vulnerability for a second hit which, as it appears
now, may represent a set of factors (multi hits) that
may involve complex interactions between
hepatocytes, stellate cells, adipose cells, Kupffer
cells, inflammatory mediators, and reactive oxygen
species driving NAFLD state to NASH (Fig. 1).
Though the cause of progression from NAFLD to
NASH/fibrosis remains unclear, animal studies tend
to suggest that the driving force is the formation of
harmful adducts as by-products of fatty acid oxidation
by mitochondria, peroxisomes or microsomes.
Fibrosis could be a consequence of hepatic injury
inflicted by oxidized by-products (Edmison and
McCullough 2007). Hepatic fibrosis is also a likely
effect of increased production of hydroxynonenal
(HNE) and malondialdehyde (MDA) by way of lipid
peroxidation and oxidative stress, acting through
stellate cells and increased production of transforming
growth factor-beta (TGF-beta) (Browning and Horton
2004). As mentioned below, an under expression of
uncoupling proteins leading to increased generation
of reactive oxygen species and kupffer cell activation,
might aggravate injury in NASH. Additionally, leptin
mediated insulin resistance could also be a factor of
significance in fibrogenesis as seen from animal
models of NASH (Honda et al. 2002).
Involvement of inflammatory mediators
The role of inflammatory mediators in the progression
of NAFLD that could form the focus for future
development of therapeutics, is also gaining attention.
Two of the inflammatory proteins, adiponectin and
tumor necrosis factor-alpha (TNFalpha), implicated in the
pathogenesis of NAFLD seem to play pivotal roles
(Fig. 1). Adiponectin, an adipose tissue hormone,
when lowered, is likely to increase fatty acid
oxidation and hepatic gluconeogenesis contributing to
the increased severity of hepatic inflammation (Xu et
al. 2003, Targher et al. 2006). TNF, an inflammatory
cytokine elaborated by macrophages, adipocytes and
hepatocytes has been reportedly elevated in obese
patients with insulin resistance and NASH, which can
mediate hepatic injury by the inhibition of
mitochondrial electron transport , the release of ROS
and the promotion of lipid peroxidation (Pessayre et
al. 2004). Moreover, nuclear factor kappa beta
(NF-kappaB), a proinflammatory transcription factor is
also often found elevated in patients with NASH
(Lewis and Mohanty 2010). Recently, the inactivation
of kupffer cells, the resident macrophages of the liver
that function in both innate and adaptive immunity as
active phagocytosing agents and antigen-presenting
cells (via toll-like receptors, among others) to T-cells,
has been found to be associated with the pathogenesis
of NASH and impaired hepatic regenerative capacity.
Moreover, the elimination of kupffer cells seems to
improve NASH, implicating the over-activation of
kupffer-cell-mediated immune response to be the
underlying cause of liver injury in NAFLD. An
increased hepatic lipid load seems to alter the kupffer
cell physiology due to overcrowding of liver
sinusoids resulting in prolonged exposure of kupffer
cells to antigens, reduced kupffer cell outflow, and an
attendant sustained inflammatory response. While
NAFLD stands histologically defined by hepatic
loading of fat, evidence also points to it being a
consequence of heightened catabolic events and
suboptimal hepatic defenses (Lewis and Mohanty
2010). Interestingly, patients diagnosed with both
NASH and alcoholic liver disease also provide
evidence of up regulated expression of the
pro-apoptotic bax gene along with increased caspase
activity, and a marker of cellular apoptosis (Ramalho
et al. 2006). NASH patients also appear to reveal
compromised antioxidant potential as marked by
reduced glutathione levels (Vendemiale et al. 2001).
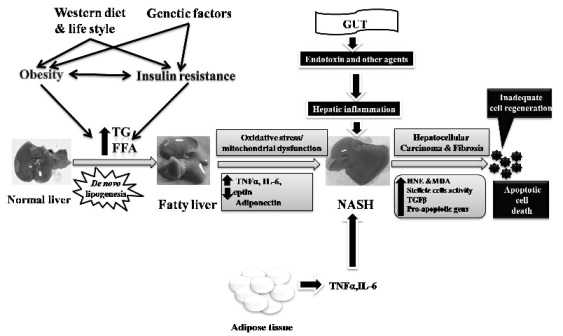
Fig. 1. Pathophysiology of nonalcoholic steatohepatitis depicting multiple hits as contributing factors.
Third and multiple hit
An additional component representing a 'third hit'
was later identified reflecting inadequate hepatocyte
proliferation (Jou et al. 2008). Recently, additional
components representing parallel onslaughts
contributing to hepatic inflammation and consequent
steatosis and NASH have gained recognition. Such
onslaughts or parallel hits are identified as
gut-derived (endotoxin) and adipose tissue derived
(tumour promoting cytokines, IL-6 and TNFalpha) factors
that promote liver inflammation and steatosis, which
can then progress into NASH (Tilg and Moschen
2010). These recent inputs have given rise to the
concept of 'multiple hits' contributing to the
development of NASH as depicted in Fig. 1.
The earlier publication of Jou et al. (2008) clearly
highlights the competence of healthy liver to
undertake compensative replication of mature
hepatocytes to replace dead cells and restore and
reconstitute normal hepatic function. Ironically,
oxidative stress implicated in NAFLD pathogenesis
as a principal feature effectively inhibits the
replication of mature hepatocytes and therefore goads
the system instead to expansion of the hepatic
progenitor cell (oval cell) population (Roskams et al.
2003). This last work highlights the capacity of these
progenitor cells to differentiate into hepatocyte-like
cells and draws a strong correlation between the
presence of a greater number of both oval and
intermediate hepatocyte-like cells with fibrosis
(Roskams et al. 2003). Apparently, as suggested by
the above authors, cumulative hepatocyte loss triggers
the formation and accumulation of progenitor cells
and their differentiation towards hepatocytes; an
implicated consequence of which is hepatocellular
carcinogenesis. In short, the above works reveal the
compromised efficacy of hepatocyte regeneration
under conditions of chronic liver injury to be the
prime mover of the system towards fibrosis/cirrhosis
and therefore, cell death with impaired proliferation
of hepatocyte progenitors represents the proposed
'third hit' in NASH pathogenesis.
An interaction between genes and environment,
that begins as early as the intrauterine period of life,
is also likely to promote development of NASH. The
diagnosis of the condition even in children of 3 years
of age lends adequate support to this hypothesis
(Manco et al. 2008). Moreover, the chances of
developing NASH in children born with small
gestation weight (SGA) seem much greater (Nobili et
al. 2007). The concept of early life programming and
developmental plasticity changes as a major
conspirator for childhood or adult onset of metabolic
diseases (thrifty phenotype) is well recognized and
adequately documented in recent times (Hales and
Barker 2001). The genetic-environment conspiracy
concept as a major basis for the progression of
metabolic diseases can also find application to
NASH, as it is usually associated with obesity, insulin
resistance (IR) and all the clustering features related
to metabolic syndrome (Erickson 2009). Some recent
works from our laboratory have strongly
demonstrated a link between early neonatal
programming by corticosterone and an adult
propensity for metabolic dyshomeostasis with regard
to carbohydrates and lipids and a predisposition
towards type 2 diabetes and/or metabolic syndrome
(Baxi et al. 2011). It is likely that such individuals
may be more vulnerable to a 'double' or a 'triple hit'
leading to NASH and fibrosis. Apart from a
predisposition towards the development of insulin
resistance/metabolic syndrome during adult life, such
early life experiences may also affect liver
development and functioning and as such,
under/malnutrition during mid and late gestational
ages have been shown to maintain brain growth at the
expense of trunk growth, especially growth of the
liver and the establishment of liver functions (Barker
2002).
TREATMENT OF NASH
Dietary and lifestyle modifications
The first and foremost treatment for NASH is induced
weight loss, along with other lifestyle modifications.
It stands well documented that rapid weight loss (very
low calorie diet or starvation) has a negative impact
on NASH contributing to an increased risk of
cirrhosis of the liver (Neuschwander-Tetri and
Caldwell 2003). Therefore, instead of inducing rapid
weight loss, one should aim at a controlled weight
loss of less than 10% body weight over a period of
6-12 months (Okita et al. 2001). It is advisable to
consume more vegetables and fruits rich in fiber and
complex carbohydrates with a low glycaemic index
and, avoid meat, saturated fat and products with less
complex carbohydrates. Apart from lifestyle modifications, one also needs to avoid consumption of
alcohol. Liver biopsy is highly recommended in
patients with diabetes, dyslipidemia or glucose
intolerance also diagnosed with NASH. The general
diet recommendations individualized to achieve
energy deficit of 500 to 1000 kcal per day depending
on the patient's BMI, involve consumption of reduced
saturated fat and a total fat intake less than 30% of the
total energy intake, reduced refined sugars and
increased soluble fibre intake. Physical activities for
60 minutes per day for at least 3 days a week and
progressive increase of an exercise regimen to five
times a week form part of an ameliorative program. In
the present scenario, life style modifications and
dietary restrictions are the only available therapeutic
approaches as no specific drugs are available to-date
for treatment of NASH.
Pharmacological agents
At present, no FDA or EMEA approved drug exists
against NASH, as there is no report on any agent with
proven benefit. Though drugs that reduce insulin
resistance such as metformin and thiazolidinediones
(rosiglitazone, pioglitazone) were considered to be of
some promise (Tahan et al. 2007, Sanyal et al. 2010),
and some studies had shown some improvement in
histological manifestations of NASH (Belfort et al.
2006), they are now being discontinued due to their
adverse effects. In general, all drugs that induce
weight loss might be beneficial against NASH, in
particular when diet and life-style modifications do
not work. Both sibutramine and orlistat have shown
improvement in some characteristics of NASH such
as the sonographically visible degree of liver steatosis
and histologically observable degree of steatosis and
fibrosis (Harrison et al. 2004, Hussein et al. 2007).
Though some preliminary data tend to suggest the
efficacy of metformin and glitazones in improving
liver histology in patients with non-diabetic NASH,
their routine use cannot be recommended at present.
Some preliminary studies have also tested probiotics
and various cytoprotective drugs like ursodesoxycholic acid, antioxidants like vitamin E, anti
TNF agents like pentoxiphylene and antifibrotic
drugs like losartan but with no success in terms of
clinical application (Lirussi et al. 2007, Velayudham
et al. 2009, Leuschner et al. 2010). There were also
some proposals for the use of antioxidants and
cytoprotective substances like vitamin E, vitamin C,
glutathione, betaine, acetylcysteine, S-adenosyl-L-
methionine and ursodesoxycholic acid in treating
cases of NAFLD and NASH. However, none of these
substances have shown any promise after a recent
cochrane analysis of validated randomized studies
(Lirussi et al. 2007).
HERBAL MEDICINES AND NASH
Historical development
Herbal medication has a traditional history stretching
back to ancient times as a part of the harmonious
co-existence with nature by early human civilizations.
Either by a divine gift or by close observation and
understanding of nature, ancient humans, including
seers in India, had a clear perception of the
availability of remedial measures in nature. Of
particular importance was the development of the
Indian Ayurvedic system starting from Charak
Sauhita, somewhere between 400-200 B.C. The
earliest literatures on Indian medicinal practice appear
during the Vedic period. These, along with traditional
folklore practices in other parts of the world have
played a major role in the development of modern
civilization. Primitive humans had great appreciation
for the great diversity of plants in their immediate
environment and understanding of medicinal uses of
plants as medicine/curatives came not only by trial
and errors methods but also by close observations of
wild animals. With the passage of time more and
more new herbs were added to the knowledge base of
tribes and their methodological collection of
information led to a well defined herbal
pharmacopeia.
Recent scenario
These have essentially served as the basis for much of
the modern pharmacopeia of scientific medicine.
Potentially, many of the modern drugs in common use
have a herbal origin and indeed at least 25% of the
prescribed drugs in the world contain at least one
active ingredient of herbal origin. It is of interest that
about 11% of essential medicines in the list of WHO
are exclusively of plant origin (Rates 2001). Primary
health care of more than 80% of African and Asian
populations is essentially based on traditional
medicines and more than 80% of the rural population
of India uses medicinal herbs as a part of the
indigenous system of medicine (WHO 2008). Nearly
1000 plants species find application in Indian herbal
industries, of which nearly 180 are of high volume
exceeding 100 metric tonnes a year (Sahoo et al.
2010). Based on the nature of usage herbal
preparations seem to fall into three categories 1) those
used in the crude form as is the practice of tribal and
traditional healers, 2) isolated and purified active
compounds from plant extracts and 3) use after
scientific validation through animal experimentation
(Iwu et al. 1999). According to a WHO
categorization, four groups of herbal drugs such as
indigenous herbal medicine, herbal medicine in the
system, modified herbal medicines and imported
product with a herbal medicine base have been
recognized (WHO 2003). Secondary metabolites
seem to constitute the active principle chemicals of
herbal preparation that affect physiological function
and hence could exhibit better compatibility with the
human body. The World Health Organization has
recommended screening and evaluation of potential
herbs for application as effective therapeutants,
especially in remote areas that lack advanced health
care and availability of safe modern drugs (WHO
1996). In this context, the turn of the century has
witnessed an up-spring in the use of herbals in the
developed world (Kamboj 2000). Though various
synthetic drugs are available for the treatment of
hyperlipidemia/ hypercholesterolemia and related
disorders such as obesity, steatohepatitis and
atherosclerosis, ironically, manifestation of side
effects limit their usage to a great extent. Further,
synthetic anti-diabetic and hypolipidemic drugs are
also unable to alleviate the plurotropic effects of these
metabolic disorders. The pharmaceutical industries
therefore face a serious challenge to develop herbal
alternatives or even a combination therapy against the
development of NASH in diabetic, obese and IR
individuals. Because of their minimal side effects and
their multiple modes of action, herbal medicines are
gaining increasing popularity and recognition in the
management of hyperlipidemia, obesity and IR.
Phytocompounds as therapeutic agents against NASH
Antioxidant phytocompounds are being increasingly
used in combating/preventing NASH and from a
literature survey, four amongst the vast array of
compounds merit scrutiny for their potential against
experimental NASH. Resveratrol, a type of
ubiquitous polyphenol present in a variety of plants
and reported to possess antioxidant and
hypolipidemic (Zhu et al. 2008) potentials, has shown
competence in preventing NASH in experimental
models of feeding and fasting cycles (Bujanda et al.
2008). Epigallocatechin gallate (EGCG), a well-
known antioxidant phytocompound present in green
tea, has demonstrated potential to ameliorate NASH
in HFD fed C57BL6/J mice (Bose et al. 2008, Kuzu
et al. 2008). Gallic acid and lycopene, two other
popular antioxidants, have also shown ameliorative
potential against NASH; the former at a dose range of
50-100 mg/kg for 10 weeks; the latter, at a dose of
2 mg/kg for 6 weeks against HFD induced NASH in
rats and NASH induced hepatocarcinogenosis (Hsu
and Yen 2007, Bahcecioglu et al. 2010).
Polyherbal formulations against NASH: pre-clinical and clinical studies
Yo jyo hen shi ko (YHK), derived from Henshiko
(Kyotsujigyo Inc., Japan), has four major ingredients
(Panax pseudoginseng, Eucommia ulmoides,
Polygonati rhizoma, and licorice root), which have
found popular application against various forms of
hepatic injury. De Lima et al. (2007) and Stefano et
al. (2007) have shown improvement in pathophysiological manifestations of NASH while, Chande et
al. (2006) have successfully demonstrated the
protective role of the same substance in human
subjects of NASH. In another report, Lou et al. (2008)
showed the therapeutic effect of Yiqi Sanju Formula
(YQSJF) [traditional Chinese herbal medicine used in
the treatment of non-alcoholic fatty liver disease
(NAFLD)] in patients with NASH. Keishibukuryogan
(KBG, TJ-25), a kempho medicine, prepared from a
combination of Cinnamomum cassia, Paeonia
lactiflora, Prunus persica, Poria cocos and Paeonia
suffruticosa, has been shown to attenuate liver injury
and inflammation in patients with nonalcoholic fatty
liver disease (Fujimoto et al. 2010). Further, Fujimoto
et al. (2008) have demonstrated the ameliorative
effects of Orengedokuto (OGT,TJ-15), a medicinal
preparation from a combination of Scutellaria
baicalensis, Coptis japonica, Gardenia jasminoides,
and Phellodendron amurense, as well as of
Shosaikoto (SST, TJ-9), another medicinal
preparation from Bupleurum falcatum, Pinellia
ternata, Scutellaria baicalensis, Zizyphus jujuba,
Panax ginseng, Glycyrrhiza uralensis and Zingiber
officinale Roscoe against experimental NASH.
Clinical trials with plant extract/phytocompounds
Compared to clinical trials on compounds/extracts of
herbal origin for other diseases, there exist only a few
trials for NASH. Randomized single-blind controlled
clinical trials lasting for 3-4 months with extracts of
grape seed and Gynostemma pentaphylum have
shown potent beneficial effects in patients with
NASH (Chou et al. 2006, Khoshbaten et al. 2010).
Sylimarin, a flavonolignan isolated from Silybum
marianum, is the most widely used hepatoprotective
phytocompound against various hepatic ailments
(Pradhan and Girish 2006). Studies on various
preclinical models of liver disease have substantiated
the hepatoprotective potential of sylimarin and, this
compound alone or in combination with vitamin E
could improve lipid profile and serum markers of
hepatic function in patients with NASH
(Hajaghamohammadi et al. 2008, Hajiani and
Hashemi 2009, Hashemi et al. 2009). Vitamin E, a
lipophilic antioxidant and vitamin C, a hydrophilic
antioxidant, have both found wide application as
effective antioxidant agents against various oxidative
stress related disorders and, in this context, Oliveira
et al. (2003) evaluated the potential of a combination
of these two in the preclinical management of NASH.
The encouraging findings of this pre-clinical study led
to its successful clinical trial by different research
groups (Harrison et al. 2003, Vajro et al. 2004, Ersöz
et al. 2005, Nobili et al. 2006). Very recently, Foster
et al. (2011) have also reported the beneficial effect of
a combination of Atrovastatin (20 mg), Vitamin C
(1 g) and Vitamin E (1000 IU) in a clinical trial.
CURRENT SCENARIO AND FUTURE PERSPECTIVES
Having witnessed the credentials of herbal
preparations against various metabolic disorders, we
initiated studies with two folklore plants from
Manipur, India (Sida rhomboidea Roxb. and
Clerodendron glandulosum Coleb) to provide
scientific validity for their proclaimed efficacies
(Jadeja et al. 2009). Our sequential studies
demonstrated a marked potential of both these plants
against a variety of conditions like hyperlipidemia
and hypercholesterolemia (Thounaojam et al. 2009,
Jadeja et al. 2010a), insulin resistance (Jadeja et al.
2010c, Thounaojam et al. 2010a), obesity (Jadeja et
al. 2011a, Thounaojam et al. 2011a), and
atherosclerosis (Jadeja et al. 2011b, Thounaojam et al.
2011b). Moreover, toxicity evaluations have also
indicated relative safety for long term application
(Jadeja et al. 2011c, Thounaojam et al. 2010b).
Buoyed with these findings, we decided to test the
possible utility of both these herbs in diet induced
NASH in C57BL/6J mice. Co-supplementation of
high fat diet with the extracts of both plants greatly
prevented the manifestations of hepatic steatosis,
oxidative stress and fibrosis (Jadeja et al. 2010b;
Thounaojam et al. 2010c). Substantiation for the
observed effect against steatosis came from in vitro
studies using HepG2 cells (Jadeja et al. 2010b,
Thounaojam et al. 2010b). Recorded observations
from the above studies also indicated the efficacy of
the plants against conditions that predispose to NASH
like hyperlipidemia, obesity and insulin resistance.
Apart from our own studies, many others have also
demonstrated the potential of many other plants
belonging to different families on different animal
models (Table 1) of NAFLD/NASH. An overview of
these studies is provided in Table 2. Obviously, these
encouraging results provide an impetus for more
detailed studies on these plants either singly or in
combination for their potential in preventing/-ameliorating NASH. Such pre-clinical studies need to
be taken to their logical conclusion by appropriate
and controlled clinical trials.
Table 1. In vivo and in vitro experimental models for non-alcoholic steatohepatitis.
|
Model |
Mode of induction |
References | | Genetic |
ob/ob mice |
Mutation prevents synthesis
of leptin |
Li et al. 2003 | | db/db mice |
Mutation in leptin receptor |
Larter and Yeh 2008 | | fa/fa mice |
Mutation in leptin receptor |
Larter and Yeh 2008 | | Nutritional |
C57BL/6J mice |
45-60% fat diet |
Jadeja et al. 2010d | | Wistar and Sprague-Dawley
rats |
20-40% fat diet |
Shetty et al. 2010, Xu et al.
2010 | | Albino rats |
60% fructose rich diet |
Chidambaram and Carani
Venkatraman 2010 | | Albino rats |
Methionine-choline deficient
diet |
Oz et al. 2008 | | Nutritional +
physical stress |
Male Wistar rats |
fat- and sugar-enriched diet
and chronic stress |
Fu et al. 2010 | | Genetic + nutritional |
ob/ob mice + MCD |
Mutation prevents synthesis
of leptin + MCD |
de Oliveira et al. 2008 | | db/db mice + MCD |
Mutation in leptin receptor +
MCD |
Rinella et al. 2008 | | ob/ob mice + MCD+HFD |
Mutation prevents synthesis
of leptin + hugh calorie
+ MCD |
de Oliveira et al. 2008 | | fa/fa rats + HFD |
Mutation in leptin receptor +
high calorie |
Carmiel-Haggai et al. 2005 | | Cell line |
HepG2 |
Combination of palmitic acid
and oleic acid for 24 hr |
Yao et al. 2011 | | 0.5 mM oleic acid for 48 hr |
Cui et al. 2010 | | 0.5 mm Na-palmitate-BSA
complex for 24 hr |
Wang et al. 2009 | | 2 mM oleic acid for 24 hr |
Thounaojam et al. 2010d | | 0.7 mM palmitate, oleate or
linoleate-BSA complex for
24 hr |
Li et al. 2007 | | 0.1% v/v (oleic acid, linoleic
acid, and alpha-linolenic acid) or
0.1% w/v (stearic acid)-BSA
complex for 24 hr
|
Kohjima et al. 2009 |
Table 2. Herbal extract used for treatment of NASH.
| Plan extract |
Fraction |
Experimental model |
References | | Acanthopanax senticosus |
50% ethanol |
HFD fed C57BL/6J mice |
Park et al. 2006 | | Alisma orientalis |
Methanolic |
rats fed with HFD |
Hong et al. 2006 | | Avena sativa L. |
Ethanol |
oleic acid treated HepG2 cells |
Cai et al. 2011 | | Camellia sinensis |
Theaflavins |
rats fed the HFD |
Lin et al. 2007 | | Powder |
ob/ob mice |
Park et al. 2011 | | Fermented tea |
rats fed with MCD |
Mori et al. 2009 | | Powder |
ob/ob mice |
Bruno et al. 2008 | | Cissus quadrangularis |
Methanol |
rats fed with HFD |
Chidambaram and Carani
Venkatraman 2010 | | Clerodenron glandulosum |
Aqueous |
C57BL/6J mice fed with HFD |
Jadeja et al. 2010 | | Eriobotrya japonica |
70% ethanol |
rats fed with MCD |
Yoshioka et al. 2010 | | Linum usitatissimum |
Seed Oil |
hamster fed with HFD |
Yang et al. 2009 | | Nelumbo nucifera Leaf |
Flavonoids |
hamsters fed with HFD |
Lin et al. 2009 | | Olea europaea |
Ethanol |
SHR/NDmcr-cp rats |
Omagari et al. 2010 | | Origanum majorana and
Cichorium intybus |
Aqueous |
rats fed with HFD |
Ahmed et al. 2009 | | Phyllanthus urinaria |
Powder |
C57BL/6 and db/db mice fed
with MCD |
Shen et al. 2008 | | Picrorhiza kurroa Royle |
Hydro-ethanol |
Rats fed with HFD |
Shetty et al. 2010 | | Platycodon grandiflorum |
Saponins |
C57BL/6 mice fed with HFD |
Noh et al. 2010 | | Punica granatum L. |
Methanol |
Zucker diabetic fatty rats |
Xu et al. 2009 | | Salacia oblonga root |
Water extract |
Zucker diabetic fatty rats |
Huang et al. 2006 | | Sida rhomboidea Roxb. |
Aqueous |
C57BL/6J mice fed with HFD |
Thounaojam et al. 2010c | | Silibum marianum |
Silibinin |
Rats fed with HFD |
Haddad et al. 2011 | | Teucrium polium |
Ethyl acetate |
Rats fed with MCD |
Amini et al. 2009, 2010,
Nosrati et al. 2010 | | Trigonella foenum |
Powder |
Zucker obese rats |
Raju and Bird 2006 | | Vitis coignetiae |
Water extract |
Rats fed with MCD |
Takayama et al. 2009b |
HFD; high fat diet and MCD; methionine choline deficient diet
One discordant note however regarding
pre-clinical trials is the non-availability of a true
mimic of human NASH as most of the animal models,
genetic, dietary or stress induced seem to have one or
more drawbacks (Fan and Qiao 2009, Takayama et al.
2009a). It is our considered opinion that genetic and stress induced models do not truly represent the
modes of development of NASH in humans as the
major causative factors are life style alterations and
fat rich diet that push individuals with metabolic
syndrome towards NASH. So, an appropriate strain of
animals like mice, as they are genetically more alike
to humans than rats, with dietary induction would be
more appropriate and in this context, C57BL/6J mice
is a relevant model for a metabolic syndrome, which
can proceed towards NASH with a fat rich diet.
We expect this review to generate more interest
and studies on NASH, as this silent fast developing
debilitating disorder needs serious and immediate
attention, especially in the context of modern life
style.
ACKNOWLEDGEMENT
Authors Menaka Chanu Thounaojam and Ravirajsinh
Navalsinh Jadeja are thankful to the University
Grants Commission and Council of scientific and
Industrial Research, New Delhi India for providing
financial assistance in the form of RFSMS and
CSIR-SRF fellowships respectively.
REFERENCES
Adams LA, Feldstein AE. Nonalcoholic steatohepatitis: risk factors and diagnosis: metabolic risk factors for NASH. Expert Rev Gastroenterol
Hepatol. 4: 623-635, 2010.
[CrossRef]
Ahmed LA, Ramadan RS, Mohamed RA. Biochemical and histopathological studies on the water extracts of marjoram and chicory herbs and their mixture in
obese rats. Pak J Nutr. 8: 1581-1587, 2009.
[CrossRef]
Amarapurkar DN, Hashimoto E, Lesmana LA, Sollano JD, Chen P, Goh KL. How common is non-alcoholic fatty liver disease in the Asia-Pacific region, and
are there local differences? J Gastroenterol Hepatol. 22: 788-793, 2007.
[CrossRef]
[PubMed]
Amini R, Nosrati N, Yazdanparast R, Molaei M. Teucrium polium in prevention of steatohepatitis in rats. Liver Int. 29: 1216-1221, 2009.
[CrossRef]
[PubMed]
Amini R, Yazdanparast R, Aghazadeh S, Ghaffari SH. Teucrium polium reversed the MCD diet-induced liver injury in rats. Hum Exp Toxi. Doi:
0960327110388961, 2010. 2011.
Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 346: 1221-1231, 2002.
[CrossRef]
[PubMed]
Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 107:
1103-1109, 1994.
[PubMed]
Bahcecioglu IH, Kuzu N, Metin K, Ozercan IH, Ustundag B, Sahin K, Kucuk O. Lycopene prevents development of steatohepatitis in experimental
nonalcoholic steatohepatitis model induced by high-fat diet. Vet Med Int. 2010. Doi: 10.4061/2010/262179, 2010.
[CrossRef]
[PubMed]
Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 13: 364-368, 2002.
[CrossRef]
Baxi DB, Singh PK, Vachhrajani KD, Ramachandran AV. Diabetic glucose dyshomeostasis and dyslipidemia in estrogen deficient rats: melatonin
Supplementation more potent than estrogen replacement therapy in alleviating the symptoms. Diabetologia Croatica. 40: 1-13, 2011.
Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ et al. A placebo-controlled
trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 355: 2297-2307, 2006.
[CrossRef]
[PubMed]
Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic
syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 138: 1677-1683, 2008.
[PubMed]
Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 114: 147-152, 2004.
[PubMed]
Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population
in the United States: impact of ethnicity. Hepatology. 40: 1387-1395, 2004.
[CrossRef]
[PubMed]
Bruno RS, Dugan CE, Smyth JA, DiNatale DA, Koo SI. Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and
injury. J Nutr. 138: 323-331, 2008.
[PubMed]
Bujanda L, Hijona E, Larzabal M, Beraza M, Aldazabal P, García-Urkia N, Sarasqueta C, Cosme A, Irastorza B, Gonzalez A, Arenas JI, Jr. Resveratrol
inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 8: 40, 2008.
[CrossRef]
[PubMed]
Cai S, Huang C, Ji B, Zhou F, Wise ML, Zhang D, Yang P. In vitro antioxidant activity and inhibitory effect, on oleic acid-induced hepatic
steatosis, of fractions and subfractions from oat (Avena sativa L.) ethanol extract. Food Chem. 124: 900-905, 2011.
[CrossRef]
Caldwell SH, Harris DM, Patrie JT, Hespenheide EE. Is NASH underdiagnosed among African Americans? Am J Gastroenterol. 97: 1496-1500, 2002.
[CrossRef]
[PubMed]
Carmiel-Haggai M, Cederbaum AI, Nieto N. A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. FASEB J. 19:
136-138, 2005.
[PubMed]
Chan DF, Li AM, Chu WC, Chan MH, Wong EM, Liu EK, Chan IH, Yin J, Lam CW, Fok TF, Nelson EA. Hepatic steatosis in obese Chinese children. Int J Obes
Relat Metab Disord. 28: 1257-1263, 2004.
[CrossRef]
[PubMed]
Chande N, Laidlaw M, Adams P, Marotta P. Yo Jyo Hen Shi Ko (YHK) improves transaminases in nonalcoholic steatohepatitis (NASH): a randomized pilot
study. Dig Dis Sci. 51: 1183-1189, 2006.
[CrossRef]
[PubMed]
Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2: 1048-1058,
2004.
[CrossRef]
Chidambaram J, Carani Venkatraman A. Cissus quadrangularis stem alleviates insulin resistance, oxidative injury and fatty liver disease in
rats fed high fat plus fructose diet. Food Chem Toxicol. 48: 2021-2029, 2010.
[CrossRef]
[PubMed]
Chou SC, Chen KW, Hwang JS, Lu WT, Chu YY, Lin JD, Chang HJ, See LC. The add-on effects of Gynostemma pentaphyllum on nonalcoholic fatty liver
disease. Altern Ther Health Med. 12: 34-39, 2006.
[PubMed]
Cui W, Chen SL, Hu KQ. Quantification and mechanisms of oleic acid-induced steatosis in HepG2 cells. Am J Transl Res. 2: 95-104, 2010.
Day CP. Non-alcoholic steatohepatitis (NASH): where are we now and where are we going? Gut. 50: 585-588, 2002.
[CrossRef]
[PubMed]
Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 114: 842-845, 1998.
[CrossRef]
de Lima VM, de Oliveira CP, Sawada LY, Barbeiro HV, de Mello ES, Soriano FG, Alves VA, Caldwell SH, Carrilho FJ. Yo jyo hen shi ko, a novel Chinese
herbal, prevents nonalcoholic steatohepatitis in ob/ob mice fed a high fat or methionine-choline-deficient diet. Liver Int. 27: 227-234,
2007.
[CrossRef]
[PubMed]
de Oliveira CPMS, de Lima VMR, Simplicio FI, Soriano FG, de Mello ES, de Souza HP, Alves VAF, Laurindo FRM, Carrilho FJ, de Oliveira MG. Prevention
and reversion of nonalcoholic steatohepatitis in OB/OB mice by S-nitroso-N-acetylcysteine treatment. J Am Coll Nutr. 27: 299-305, 2008.
[PubMed]
Diehl AM. Nonalcoholic steatosis. Sem Liv Dis. 19: 221-229, 1999.
[CrossRef]
[PubMed]
Dixon JB, Bhathal PS, Jonsson JR, Dixon AF, Powell EE, O'Brien PE. Pro-fibrotic polymorphisms predictive of advanced liver fibrosis in the severely
obese. J Hepatol. 39: 967-971, 2003.
[CrossRef]
Dongiovanni P, Valenti L, Rametta R, Daly AK, Nobili V, Mozzi E, Leathart JB, Pietrobattista A, Burt AD, Maggioni M, Fracanzani AL, Lattuada E et al.
Genetic variants regulating insulin receptor signalling are associated with the severity of liver damage in patients with non-alcoholic fatty liver
disease. Gut. 59: 267-273, 2010.
[CrossRef]
[PubMed]
Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 103: 71-83, 2010.
[CrossRef]
[PubMed]
Dowman JK, Tomlinson JW, Newsome PN. Systematic review: the diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic
steatohepatitis. Aliment Pharmacol Ther. 33: 525-540, 2011.
[CrossRef]
Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis. 11: 75-104, 2007.
[CrossRef]
[PubMed]
Erickson SK. Nonalcoholic fatty liver disease (NAFLD). J Lipid Res. 50: S412-S416, 2009.
[CrossRef]
[PubMed]
Ersoz G, Gunşar F, Karasu Z, Akay S, Batur Y, Akarca US. Management of fatty liver disease with vitamin E and C compared to ursodeoxycholic acid
treatment. Turk J Gastroenterol. 16: 124-128, 2005.
[PubMed]
Fan J. What are the risk factors and settings for NAFLD in Asia Pacific? The Consensus on NAFLD/NASH. APDW, Cebu 2006.
Fan JG, Qiao L. Commonly used animal models of non-alcoholic steatohepatitis. Hepatobiliary Pancreat Dis Int. 8: 233-240, 2009.
[PubMed]
Fan JG, Saibara T, Chitturi S, Sung JJY, Kim BI, Chutaputti A. The Asia-Pacific working party for NAFLD. What are the risk factors and settings for
non-alcoholic fatty liver disease in Asia-Pacific? J Gastroenterol Hepatol. 22: 794-800, 2007.
[CrossRef]
[PubMed]
Fan JG, Jia J, Li YM, Wang BY, Lu LG, Shi JP, Chan LY. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: Update 2010.
J Dig Dis. 12: 38-44, 2011.
[CrossRef]
[PubMed]
Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the
St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 106: 71-77, 2011.
[CrossRef]
[PubMed]
Fu JH, Sun HS, Wang Y, Zheng WQ, Shi ZY, Wang QJ. The effects of a fat- and sugar-enriched diet and chronic stress on nonalcoholic fatty liver
disease in male Wistar rats. Dig Dis Sci. 55: 2227-2236, 2010.
[CrossRef]
[PubMed]
Fujimoto M, Tsuneyama K, Kainuma M, Sekiya N, Goto H, Takano Y, Terasawa K, Selmi C, Gershwin ME, Shimada Y. Evidence-based efficacy of Kampo
formulas in a model of non alcoholic fatty liver. Exp Biol Med. 233: 328-337, 2008.
[CrossRef]
[PubMed]
Fujimoto M, Tsuneyama K, Kinoshita H, Goto H, Takano Y, Selmi C, Keen CL, Gershwin ME, Shimada Y. The traditional Japanese formula keishibukuryogan
reduces liver injury and inflammation in patients with nonalcoholic fatty liver disease. Ann N Y Acad Sci. 1190: 151-158, 2010.
[CrossRef]
[PubMed]
Haddad Y, Vallerand D, Brault A, Pierre S. Antioxidant and hepatoprotective effects of silibinin in a rat model of nonalcoholic steatohepatitis.
eCAM, doi:10.1093/ecam/nep164, 2011.
Hajaghamohammadi AA, Ziaee A, Rafiei R. The efficacy of silymarin in decreasing transaminase activities in non-alcoholic fatty liver disease: A
randomized controlled clinical trial. Hepat Mon. 8: 191-195, 2008.
Hajiani E, Hashemi SJ. Comparison of therapeutic effects of silymarin and vitamin e in nonalcoholic fatty liver disease: results of an open-labele,
prospective, randomized study. Jundishapur J Nat Pharm Prod. 40: 8-14, 2009.
Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 60: 5-20, 2001.
[CrossRef]
[PubMed]
Harish K, Thomas V. Non-alcoholic fatty liver disease in Asians - An emerging problem. Calicutta Med J. 6(3): e6, 2008.
Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic
steatohepatitis. Am J Gastroenterol. 98: 2485-2490, 2003.
[CrossRef]
[PubMed]
Harrison SA, Fincke C, Helinski D, Torgerson S, Hayashi P.A pilot study of orlistat treatment in obese, nonalcoholic steatohepatitis patients.
Aliment Pharmacol Ther. 20: 623-628, 2004.
[CrossRef]
[PubMed]
Hashemi SJ, Hajiani E, Sardabi EH. A placebo-controlled trial of silymarin in patients with nonalcoholic fatty liver disease. Hepat Mon. 9: 265-270,
2009.
Honda H, Ikejima K, Hirose M, Yoshikawa M, Lang T, Enomoto N, Kitamura T, Takei Y, Sato N. Leptin is required for fibrogenic responses induced by
thioacetamide in the murine liver. Hepatology. 36: 12-21, 2002.
[CrossRef]
[PubMed]
Hong X, Tang H, Wu L, Li L. Protective effects of the Alisma orientalis extract on the experimental nonalcoholic fatty liver disease. J Pharm
Pharmacol. 58: 1391-1398, 2006.
[CrossRef]
Hsu CL, Yen GC. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br J Nutr. 98: 727-735,
2007.
[CrossRef]
Huang TH, Peng G, Li GQ, Yamahara J, Roufogalis BD, Li Y. Salacia oblonga root improves postprandial hyperlipidemia and hepatic steatosis in
Zucker diabetic fatty rats: Activation of PPAR-alpha. Toxicol Appl Pharmacol. 210: 225-235, 2006.
[CrossRef]
[PubMed]
Hussein O, Grosovski M, Schlesinger S, Szvalb S, Assy N. Orlistat reverse fatty infiltration and improves hepatic fibrosis in obese patients with
nonalcoholic steatohepatitis (NASH). Dig Dis Sci. 52: 2512-2519, 2007.
[CrossRef]
[PubMed]
Itoh S, Yougel T, Kawagoe K. Comparison between alcoholic and nonalcoholic hepatitis. Am J Gastroenterol. 82: 650, 1987.
[PubMed]
Iwu MM, Duncan AR, Okunji CO. New antimicrobials of plant origin. In: Janick J (ed.). Perspectives on New Crops and New Uses. ASHS Press, Alexandria
1999, p. 457.
Jadeja RN, Thounaojam MC, Ansarullah, Devkar RV, Ramachandran AV. A preliminary study on hypolipidemic effect of aqueous leaf extract of
Clerodendron glandulosum Coleb. Int J Green Pharm. 3: 285-289, 2009.
[CrossRef]
Jadeja RN, Thounaojam MC, Ansarullah, Devkar RV, Ramchandran AV. Clerodendron glandulosum Coleb., Verbenaceae, ameliorates high fat
diet-induced alteration in lipid and cholesterol metabolism in rats. Rev Bras Farmacogn. 20: 117-123, 2010a.
[CrossRef]
Jadeja RN, Thounaojam MC, Ansarullah, Patel VB, Devkar RV, Ramachandran AV. Protective effect of Clerodendron glandulosum extract against
experimentally induced metabolic syndrome in rats. Pharm Biol. 48: 1312-1319, 2010b.
[CrossRef]
[PubMed]
Jadeja RN, Thounaojam MC, Dandekar DS, Devkar RV, Ramachandran AV. Clerodendron glandulosum. Coleb extract ameliorates high fat diet/fatty
acid induced lipotoxicity in experimental models of non alcoholic steatohepatitis. Food Chem Toxicol. 48: 3424-3431, 2010c.
[CrossRef]
[PubMed]
Jadeja RN, Thounaojam MC, Devkar RV, Ramachandran AV. Clerodendron glandulosum. Coleb extract prevents in vitro human LDL oxidation and
oxidized LDL induced Apoptosis in human monocyte derived macrophages. Food Chem Toxicol. 49: 1195-1202, 2011a.
[CrossRef]
[PubMed]
Jadeja RN, Thounaojam MC, Ramani UV, Devkar RV, Ramachandran AV. Anti-obesity potential of Clerodendron glandulosum. Coleb leaf aqueous
extract. J Ethnopharmacol. 135: 338-343, 2011b.
[CrossRef]
[PubMed]
Jadeja RN, Thounaojam MC, Ansarullah, Jadav SV, Patel MD, Patel DK, Salunke SP, Padate GS, Devkar RV, Ramachandran AV. Toxicological evaluation and
hepatoprotective potential of Clerodendron glandulosum. Coleb leaf extract. Hum Exp Toxicol. 30: 63-70, 2011c.
[CrossRef]
Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 28: 370-379, 2008.
[CrossRef]
[PubMed]
Kamboj VP. Herbal medicine. Curr Sci. 78: 1, 2000.
Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 89: 2548-2556, 2004.
[CrossRef]
Khoshbaten M, Aliasgarzadeh A, Masnadi K, Farhang S, Tarzamani MK, Babaei H, Kiani J, Zaare M, Najafipoor F. Grape seed extract to improve liver
function in patients with nonalcoholic fatty liver change. Saudi J Gastroenterol. 16: 194-197, 2010.
[CrossRef]
[PubMed]
Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Nakashima M, Nakamuta M. The effects of unsaturated fatty acids on lipid metabolism in HepG2 cells.
In Vitro Cell Dev Biol Anim. 45: 6-9, 2009.
[CrossRef]
Kuzu N, Bahcecioglu IH, Dagli AF, Ozercan IH, Ustündag B, Sahin K. Epigallocatechin gallate attenuates experimental non-alcoholic steatohepatitis
induced by high fat diet. J Gastroenterol Hepatol. 23: e465-470, 2008.
[CrossRef]
[PubMed]
Larter CZ, Yeh MM. Animal models of NASH: getting both pathology and metabolic context right. J Gastroenterol Hepatol. 23: 1635-1648, 2008.
[CrossRef]
[PubMed]
Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 28: 339-350, 2008.
[CrossRef]
[PubMed]
Leevy CM. Fatty liver: A study of 270 patients with biopsy proven fatty liver and a review of the literature. Medicine. 41: 249, 1962.
[CrossRef]
[PubMed]
Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rössle M, Cordes HJ, Zeuzem S, Hein J, Berg T et al. High-dose ursodeoxycholic acid therapy for
nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology. 52: 472-479, 2010.
[CrossRef]
[PubMed]
Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 55: 560-578, 2010.
[CrossRef]
[PubMed]
Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and
improve nonalcoholic fatty liver disease. Hepatology. 37: 343-350, 2003.
[CrossRef]
[PubMed]
Li Z, Srivastava S, Yang X, Mittal S, Norton P, Resau J, Haab B, Chan C. A hierarchical approach employing metabolic and gene expression profiles to
identify the pathways that confer cytotoxicity in HepG2 cells. BMC Syst Biol. 11: 1-21, 2007.
[CrossRef]
Lin CL, Huang HC, Lin JK. Theaflavins attenuate hepatic lipid accumulation through activating AMPK in human HepG2 cells. J Lipid Res. 48: 2334-2343,
2007.
[CrossRef]
[PubMed]
Lin MC, Kao SH, Chung PJ, Chan KC, Yang MY, Wang CJ. Improvement for high fat diet-induced hepatic injuries and oxidative stress by
flavonoid-enriched extract from Nelumbo nucifera leaf. J Agric Food Chem. 57: 5925-5932, 2009.
[CrossRef]
[PubMed]
Lirussi F, Azzalini L, Orando S, Orlando R, Angelico F. Antioxidant supplements for non-alcoholic fatty liver disease and/or steatohepatitis.
Cochrane Database Syst Rev. 24: CD004996, 2007.
Lou SY, Liu Y, Ma YY, Chen HY, Chen WH, Ying J, He YM, Wang WJ. Effects of Yiqi Sanju Formula on non-alcoholic fatty liver disease: a randomized
controlled trial. Zhong Xi Yi Jie He Xue Bao. 6: 793-798, 2008.
[CrossRef]
[PubMed]
Ludwig J, TR Viggiano, DB McGill, BJ OH. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 55:
434-438, 1980.
[PubMed]
Manco M, Marcellini M, DeVito R, Comparcola D, Sartorelli MR, Nobili V. Metabolic syndrome and liver histology in paediatric nonalcoholic
steatohepatitis. Int J Obes. 32: 381-387, 2008.
[CrossRef]
[PubMed]
Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, Mccullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological
severity. Gastroenterol. 116: 1413-1419, 1999.
[CrossRef]
McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in
patients with non-alcoholic fatty liver disease. Gut. 59: 1265-1269, 2010.
[CrossRef]
[PubMed]
Mori A, Takayama F, Kawasaki H, Nakamoto K, Mankura M, Ogino T, Egashira T, Hidaka Y. Beneficial effects of fermented green tea extract in a rat
model of non-alcoholic steatohepatitis. J Clin Biochem Nutr. 44: 239-246, 2009.
[CrossRef]
[PubMed]
Musso G, Gambino R, De Michieli F, Durazzo M, Pagano G, Cassader M. Adiponectin gene polymorphisms modulate acute adiponectin response to dietary
fat: possible pathogenetic role in NASH. Hepatology. 47: 1167-1177, 2008.
[CrossRef]
[PubMed]
Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 37: 1202-1219, 2003.
[CrossRef]
[PubMed]
Nobili V, Manco M, Devito R, Ciampalini P, Piemonte F, Marcellini M. Effect of vitamin E on aminotransferase levels and insulin resistance in
children with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 24: 1553-1561, 2006.
[CrossRef]
[PubMed]
Nobili V, Marcellini M, Marchesini G, Vanni E, Manco M, Villani A, Bugianesi E: Intrauterine growth retardation, insulin resistance, and nonalcoholic
fatty liver disease in children. Diabetes Care. 30: 2638-2640, 2007.
[CrossRef]
[PubMed]
Noh JR, Kim YH, Gang GT, Yang KJ, Kim SK, Ryu SY, Kim YS, Lee CH, Lee HS. Preventative effects of Platycodon grandiflorum treatment on hepatic
steatosis in high fat diet-fed C57BL/6 mice. Biol Pharm Bull. 33: 450-454, 2010.
[CrossRef]
Nosrati N, Aghazadeh S, Yazdanparast R. Effects of Teucrium polium on insulin resistance in nonalcoholic steatohepatitis. J Acupunc Merid
Stud. 3: 104-110, 2010.
[CrossRef]
Okita M, Hayashi M, Sasagawa T, Takagi K, Suzuki K, Kinoyama S, Ito T, Yamada G. Effect of a moderately energy-restricted diet on obese patients with
fatty liver. Nutrition. 17: 542-547, 2001.
[CrossRef]
Oliveira CP, Gayotto LC, Tatai C, Della Nina BI, Lima ES, Abdalla DS, Lopasso FP, Laurindo FR, Carrilho FJ. Vitamin C and vitamin E in prevention of
nonalcoholic fatty liver disease (NAFLD) in choline deficient diet fed rats. Nutr J. 7: 2-9, 2003.
[CrossRef]
[PubMed]
Omagari K, Kato S, Tsuneyama K, Hatta H, Sato M, Hamasaki M, Sadakane Y, Tashiro T, Fukuhata M, Miyata Y, Tamaru S, Tanaka K, Mune M. Olive leaf
extract prevents spontaneous occurrence of non-alcoholic steatohepatitis in SHR/NDmcr-cp rats. Pathology. 42: 66-72, 2010.
[CrossRef]
[PubMed]
Osterreicher CH, Brenner DA. The genetics of nonalcoholic fatty liver disease. Ann Hepatol. 6: 83-88, 2007.
[PubMed]
Oz HS, Chen TS, Neuman M. Methionine deficiency and hepatic injury in a dietary steatohepatitis model. Dig Dis Sci. 53: 767-776, 2008.
[CrossRef]
[PubMed]
Park HJ, DiNatale DA, Chung MY, Park YK, Lee JY, Koo SI, O'Connor M. Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis
and enhancing hepatic antioxidant defenses in ob/ob mice. J Nutr Biochem. 22: 393-400, 2011.
[CrossRef]
[PubMed]
Park SH, Lee SG, Kang SK, Chung SH. Acanthopanax senticosus reverses fatty liver disease and hyperglycemia in ob/ob mice. Arch Pharm Res. 29:
768-776, 2006.
[CrossRef]
[PubMed]
Patel T, Lee JG. Fatty liver. eMed J. 2: 8, 2001.
Pessayre D, Fromenty B, Mansouri A. Mitochondrial injury in steatohepatitis. Eur J Gastroenterol Hepatol. 16: 1095-1105, 2004.
[CrossRef]
Petta S, Muratore C, Craxi A. Non-alcoholic fatty liver disease pathogenesis: The present and the future. Dig Liver Dis. 41: 615-625, 2009.
[CrossRef]
[PubMed]
Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 124: 491-504,
2006.
[PubMed]
Raju J, Bird RP. Alleviation of hepatic steatosis accompanied by modulation of plasma and liver TNF-alpha levels by Trigonella foenum graecum
(fenugreek) seeds in Zucker obese (fa/fa) rats. Int J Obes. 30: 1298-1307, 2006.
[CrossRef]
[PubMed]
Ramalho RM, Cortez-Pinto H, Castro RE, Sola S, Costa A, Moura MC, Camilo ME, Rodrigues CM. Apoptosis and Bcl-2 expression in the livers of patients
with steatohepatitis. Eur J Gastroenterol Hepatol. 18: 21-29, 2006.
[CrossRef]
Rates SMK. Plants as source of drugs. Toxicon. 39: 603-613, 2001.
[CrossRef]
Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T, LIDO Study Group. Sampling variability of liver
biopsy in nonalcoholic fatty liver disease. Gastroenterology. 128: 1898-1906, 2005.
[CrossRef]
[PubMed]
Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient
diet. J Lipid Res. 49: 1068-1076, 2008.
[CrossRef]
[PubMed]
Romeo SJ. Kozlitina C, Xing A, Pertsemlidis D, Cox LA, Pennacchio E, Boerwinkle J, Cohen C, Hobbs HH. Genetic variation in PNPLA3 confers
susceptibility to nonalcoholic fatty liver disease. Nat Genet. 40: 1461-1465, 2000.
[CrossRef]
[PubMed]
Roskams T, Yang SQ, Koteish A, Durnez A, DeVos R, Huang X, Achten R, Verslype C, Diehl AM. Oxidative stress and oval cell accumulation in mice and
humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 163: 1301-1311, 2003.
[CrossRef]
Sahoo N, Manchikanti P, Dey S. Herbal drugs: standards and regulation. Fitoterapia. 81: 462-471, 2010.
[CrossRef]
[PubMed]
Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J et
al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 362: 1675-1685. 2010.
[CrossRef]
[PubMed]
Sartorio A, Del Col A, Agosti F, Mazzilli G, Bellentani S, Tiribelli C, Bedogni G. Predictors of non-alcoholic fatty liver disease in obese children.
Eur J Clin Nutr. 61: 877-883, 2007.
[CrossRef]
[PubMed]
Shen B, Yu J, Wang S, Chu ES, Wong VW, Zhou X, Lin G, Sung JJ, Chan HL. Phyllanthus urinaria ameliorates the severity of nutritional steatohepatitis
both in vitro and in vivo. Hepatology. 47: 473-483, 2008.
[CrossRef]
[PubMed]
Shetty SN, Mengi S, Vaidya R, Vaidya AD. A study of standardized extracts of Picrorhiza kurroa Royle ex Benth. in experimental nonalcoholic
fatty liver disease. J Ayurveda Integr Med. 1: 203-210, 2010.
[CrossRef]
[PubMed]
Shiota G, Tsuchiya. Pathphysiology of NASH: Insulin resistance, free fatty acids and oxidative stress. J Clin Biochem Nutr. 38: 127-132, 2006.
[CrossRef]
Stefano JT, de Oliveira CP, Correa-Giannella ML, de Lima VM, de Sa SV, de Oliveira EP, de Mello ES, Giannella-Neto D, Alves VA, Carrilho FJ.
Nonalcoholic steatohepatitis (NASH) in ob/ob mice treated with yo jyo hen shi ko (YHK): effects on peroxisome proliferator-activated receptors
(PPARs) and microsomal triglyceride transfer protein (MTP). Dig Dis Sci. 52: 3448-3454, 2007.
[CrossRef]
[PubMed]
Stepanova M, Elariny H, Rafiq N, Afendy A, Srishord M, Younossi ZM, Baranova A, Srishord M, Chandhoke V. Differences in hepatic gene expression of
Caucasians and African American patients with non-alcoholic fatty liver disease (NAFLD)The 59th Annual Meeting of the American Association for the
Study of Liver Diseases. San Francisco, 2008.
Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum amino transferase values in overweight and obese adolescents. J Pediatr. 136: 727-733,
2000.
[PubMed]
Tahan V, Eren F, Avsar E, Yavuz D, Yuksel M, Emekli E, Imeryuz N, Celikel C, Uzun H, Haklar G, Tozun N. Rosiglitazone attenuates liver inflammation
in a rat model of nonalcoholic steatohepatitis. Dig Dis Sci. 52: 3465-3472, 2007.
[CrossRef]
[PubMed]
Takayama F, Egashira T, Kawasaki H, Mankura M, Nakamoto K, Okada S, Mori A. A Novel Animal model of nonalcoholic steatohepatitis (NASH): hypoxemia
enhances the development of NASH. J Clin Biochem Nutr. 45: 335-340, 2009a.
[CrossRef]
[PubMed]
Takayama F, Nakamoto K, Kawasaki H, Mankura M, Egashira T, Ueki K, Hasegawa A, Okada S, Mori A. Beneficial effects of Vitis coignetiae Pulliat
leaves on nonalcoholic steatohepatitis in a rat model. Acta Med Okayama. 63: 105-111, 2009b.
[PubMed]
Targher G, Bertolini L, Rodella S, Zoppini G, Scala L, Zenari L, Falezza G. Associations between plasma adiponectin concentrations and liver
histology in patients with nonalcoholic fatty liver disease. Clin Endocrinol. 64:679–683, 2006.
[CrossRef]
[PubMed]
Thounaojam M, Jadeja R, Ansarullah, Devkar R, Ramachandran AV. Dysregulation of lipid and cholesterol metabolism in high fat diet fed hyperlipidemic
rats: Protective effect of Sida rhomboidea Roxb. leaf extract. J Health Sci. 55: 413-420, 2009.
[CrossRef]
Thounaojam MC, Jadeja RN, Ansarullah, Devkar RV, Ramachandran AV. Prevention of high fat diet induced insulin resistance in C57/BL/6J mice by Sida
rhomboidea Roxb. extract. J Health Sci. 56: 92-98, 2010a.
[CrossRef]
Thounaojam MC, Jadeja RN, Patel DK, Devkar RV, Ramchandran AV. Acute and sub chronic oral toxicity of Sida rhomboidea Roxb. leaf extract. J
Complement Integ Med. 7: 1, 2010b.
Thounaojam MC, Jadeja RN, Devkar RV, Ramachandran AV. Sida rhomboidea Roxb. extract alleviates pathophysiological changes in experimental
in vivo and in vitro models of high fat diet/fatty acid induced non-alcoholic steatohepatitis. Exp Toxicol Pathol. In press, 2010c.
[CrossRef]
[PubMed]
Thounaojam MC, Jadeja RN, Ramani UV, Devkar RV, Ramachandran AV. Sida rhomboidea Roxb. leaf extract down-regulates expression of
PPARgamma2 and leptin genes in high fat diet fed C57BL/6J mice and retards in vitro 3T3L1 pre-adipocyte differentiation. Int J Mol Sci.
12: 4661-4677, 2011a.
[CrossRef]
[PubMed]
Thounaojam MC, Jadeja RN, Devkar RV, Ramachandran AV. In Vitro evidence for the protective role of Sida rhomboidea Roxb. extract
against LDL oxidation and oxidized LDL induced apoptosis in human monocyte derived macrophages. Cardiovasc Toxicol. 11: 168-179, 2011b.
[CrossRef]
[PubMed]
Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 52: 1836-1846,
2010.
[CrossRef]
[PubMed]
Vajro P, Mandato C, Franzese A, Ciccimarra E, Lucariello S, Savoia M, Capuano G, Migliaro F. Vitamin E treatment in pediatric obesity-related liver
disease: a randomized study. J Pediatr Gastroenterol Nutr. 38: 48-55, 2004.
[CrossRef]
[PubMed]
Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, Dongiovanni P, Nobili V, Mozzi E, Roviaro G, Vanni E, Bugianesi E, Maggioni M et al.
Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver
disease. Hepatology. 51: 1209-1217, 2010.
[CrossRef]
[PubMed]
Velayudham A, Dolganiuc A, Ellis M, Petrasek J, Kodys K, Mandrekar P, Szabo G. VSL#3 probiotic treatment attenuates fibrosis without changes in
steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 49: 989-997, 2009.
[CrossRef]
[PubMed]
Vendemiale G, Grattagliano I, Caraceni P, Caraccio G, Domenicali M, Dall'Agata M, Trevisani F, Guerrieri F, Bernardi M, Altomare E. Mitochondrial
oxidative injury and energy metabolism alteration in rat fatty liver: effect of the nutritional status. Hepatology. 33: 808-815, 2001.
[CrossRef]
[PubMed]
Wang GL, Fu YC, Xu WC, Feng YQ, Fang SR, Zhou XH. Resveratrol inhibits the expression of SREBP1 in cell model of steatosis via Sirt1-FOXO1 signaling
pathway. Biochem Biophys Res Commun. 380: 644-649, 2009.
[CrossRef]
[PubMed]
WHO 1996. Guidelines for the Assessment of Herbal Medicines. WHO expert committee on specification for pharmaceutical preparations. Technical report
series No. 863. Geneva.
WHO. Guidelines for the Regulation of Herbal Medicines in the South-East Asia Region: Developed at the Regional Workshop on the Regulation of Herbal
Medicines, Bangkok, and 24-26 June 2003. New Delhi: Regional office for South East Asia, World Health Organization, 2003.
WHO. Traditional medicine; 2008. http://www. who.int/mediacentre/factsheets/fs134/en/ (accessed on 22nd March 2011).
Wilfred de Alwis NM, Day CP. Genetics of alcoholic liver disease and nonalcoholic fatty liver disease. Semin Liver Dis. 27: 44-54, 2007.
[CrossRef]
[PubMed]
Willner IR, Waters B, Patil SR, Reuben A, Morelli J, Riely CA. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial
tendency and severity of disease. Am J Gastroenterol. 96: 2957-2961, 2001.
[CrossRef]
[PubMed]
Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in
mice. J Clin Invest. 112: 91-100, 2003.
[PubMed]
Xu KZ, Zhu C, Kim MS, Yamahara J, Li Y. Pomegranate flower ameliorates fatty liver in an animal model of type 2 diabetes and obesity. J
Ethnopharmacol. 123: 280-287, 2009.
[CrossRef]
[PubMed]
Xu ZI, Fan JG, Ding XD, Qiao L, Wang GL. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis
Sci. 55: 931-940, 2010.
[CrossRef]
[PubMed]
Yang SF, Tseng JK, Chang YY, Chen YC. Flaxseed oil attenuates nonalcoholic fatty liver of hyperlipidemic hamsters. J Agric Food Chem. 57: 5078-5083,
2009.
[CrossRef]
[PubMed]
Yao HR, Liu J, Plumeri D, Cao YB, He T, Lin L, Li Y, Jiang YY, Li J, Shang J. Lipotoxicity in HepG2 cells triggered by free fatty acids. Am J Transl
Res. 3: 284-291, 2011.
[PubMed]
Yoneda M, Hotta K, Nozaki Y, Endo H, Uchiyama T, Mawatari H, Iida H, Kato S, Fujita K, Takahashi H, Kirikoshi H, Kobayashi N et al. Association
between angiotensin II type 1 receptor polymorphisms and the occurrence of nonalcoholic fatty liver disease. Liver Int. 29: 1078-1085, 2009.
[CrossRef]
[PubMed]
Yoshioka S, Hamada A, Jobu K, Yokota J, Onogawa M, Kyotani S, Miyamura M, Saibara T, Onishi S, Nishioka Y. Effects of Eriobotrya japonica seed
extract on oxidative stress in rats with non-alcoholic steatohepatitis. J Pharm Pharmacol. 62: 241-246, 2010.
[CrossRef]
[PubMed]
Zhu L, Luo X, Jin Z. Effect of resveratrol on serum and liver lipid profile and antioxidant activity in hyperlipidemia rats. Asian Australas J Anim
Sci. 21: 890-895, 2008.
|
BACK
|


