Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 4, p 195-209
DOI 10.2478/v10136-012-0009-8
Antioxidative/oxidative effects of strontium-doped bioactive glass as bone graft. In vivo assays in ovariectomised rats
Samira Jebahi, Hassane Oudadesse, Hafed el Feki, Tarek Rebai, Hassib Keskes, Pascal Pellen, Abdelfattah el Feki
Address: Hassane Oudadesse, University of Rennes 1, UMR CNRS 6226, Campus de Beaulieu, 263 av. du General Leclerc, 35042 Rennes, France
hassane.oudadesse@univ-rennes1.fr
Received 9th November 2011.
Revised 5th January 2012.
Published online 18th January 2012.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
References
SUMMARY
Recently, oxidative stress has been identified as a pivotal pathological factor inducing bone osteoporosis. This phenomenon is responsible for low bone
density. It alters bone quality and generates bone fractures. Strontium is found to induce osteoblast activity by stimulating bone formation and
reducing bone resorption by restraining osteoclasts. Bioglass (BG) has been used to repair bone defects, and, in combination with strontium (BG-Sr),
offers an opportunity to treat this disease. This study investigated the potential role of BG-Sr in improving antioxidant activity and regenerative
bone capacity, The effects of both BG-Sr and BG were tested on osteoblast SaOS2 and endothelial EAhy926 cell proliferation in vitro. In
vivo, BG-Sr and BG were implanted in the femoral condyles of Wistar rats and compared to that of control groups. Cell proliferation increased
significantly by 120% at SaOS2 and 127% at EAhy926. Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPx) were significantly
enhanced in BG-Sr treated rats compared to other groups. Moreover, a significant decrease of thiobarbituric acid-reactive substances (TBARs) was
observed. The Ca/P ratio increase improved progressive bone mineralization. According to these results, BG-Sr ameliorated cell proliferation and
developed an antioxidative defense against ROS. The histological findings highlight the BG-Sr implications in the osteoporosis treatment confirmed by
bone construction. The development of BG-Sr as a therapeutic biomaterial protecting against oxidative stress might make an effective choice for
application in tissue engineering.
KEY WORDS
oxidative stress; osteoporosis; strontium-substituted bioactive glasses; free radical; bone regeneration
INTRODUCTION
When bone defects occur, a remarkable yield of Reactive Oxygen Species (ROS) is generated by the damaged tissues
and implicates oxidative stress. This mechanism is defined as an imbalance between excessive ROS generation and
insufficient antioxidant defense mechanisms (Sanchez-Rodriguez et al. 2007). The enhanced osteoclastic activity
observed in bone disorders may be responsible for increased ROS production, and severe metabolic malfunctions
(Hensley et al. 2000) especially in post-menopausal women.
In rodents, ovariectomy induces the post- menopausal period and serves as an osteoporotic model (Chesnut et
al. 2000, Saito et al. 2002). For the treatment and prevention of osteoporosis, strontium ranelate (SrR) has been
shown to reduce the number of hip and vertebral fractures in post-menopausal women (Meunier et al. 2004). The
drug combines two atoms of stable Sr with the organic moiety ranelic acid. The cellular mode of SrR action appears
to be through the Sr cations themselves, which have been shown to work by stimulating osteoblasts to make new
bone and preventing osteoclasts from resorbing bone (Gentleman et al. 2010).
Because of its chemical and physical similarities to calcium (Ca), Sr is also a natural bone-seeking element and
about 98% of the total body Sr content is localised in bone tissues (Effah Kaufmann et al. 2000, Jell et al. 2008). Sr
has been shown to promote osteoblast replication, differentiation and survival (Hamdy 2009), and is regarded as a
bone-forming agent due to its stimulation of the osteoprogenitor cells replication and collagen synthesis. Moreover,
Sr can not only stimulate non-collagenic protein synthesis in osteoblasts but in vitro also can inhibit the release of
Ca from pre-labelled bone (Caverzasio and Thouverey 2011). Nevertheless, the action of the molecular mechanisms
of Sr on bone forming cells are still under investigation, although in fact, a limited amount of research has indicated
the dual effects of Sr delivery by BG in the promotion of bone formation and reduction of bone resorption.
BG forms a strong bond with living tissues via the formation of an hydroxyapatite layer on their surface (Hench
et al.1971) and has been used to repair hard tissues in a variety of craniofacial, maxillofacial, and periodontal
applications (Hojjatie and Anusavice 1990). New potential bioactive glass possessing a composition similar to that
of Hench's 45S5 - called 46S6 in the SiO2-CaO-Na2O-P2O5 system - has been investigated in vivo. and, in view
of its potential clinical application, we investigated whether it could stimulate cultured cells to proliferate.
The aim of this work was to clarify whether 46S6 glass actually exerts toxic effects, and whether Sr, which it
releases, might play a role in raising such cytotoxicity. In order to assess the possibility that an in vitro model can
mimic what may happen in vivo, indices of oxidative stress are followed in an osteoporotic rat model. While the
response of bone to oxidative stress in ovariectomised rats has been well studied, the effects of implanted Sr doped
bioactive glass on the ROS balance have not yet been addressed in an osteoporotic model. Therefore, the present
study aims to investigate the antioxidant activity of novel Sr-doped bioactive glass against free radicals induced by
estrogen deficiency.
MATERIAL AND METHODS
Bioactive glass synthesis
The first material studied was pure 46S6 possessing a composition close to that of Hench's 45S5 (Hench 1998)
whichwas used as a reference to validate our experimental procedure. Then, 0.1% wt of Sr was introduced into the
46S6 bioglass; a similar content of Sr to that of bone. Appropriate amounts of calcium metasilicate, sodium
metasilicate, sodium metaphos-phate, and magnesium oxide were weighed and mixed for 45 min using a planetary
mixer. The powdered mixture was heated in a platinum crucible at 1300 °C for 3 h. The molten material was then
poured into preheated brass molds to form cylinders of 13 mm in diameter and 10 mm in height. The prepared
samples were annealed for 4 h at the appropriate temperature, corresponding to the phase transition temperature of
the glass composition (about 560 °C), in a regulated muffle furnace, which was left to cool to room temperature at
a rate of 1°C min-1. After elaboration, the powder particles, sized between 40-63 microm, were compressed in a perfectly
isostatic manner. The prepared implants were sterilized by gamma-irradiation from a 60Co source gamma irradiation at a
dose of 25 Gy (Equinox, UK) using standard procedures for medical devices.
Cells culture
The human osteosarcoma cell line Saos-2, and the endothelial cell line Eahy926, were cultured under standard
conditions (37 °C, 5% CO2/95% air, 100% humidity) in DMEM (Sigma Chemical Co, St. Louis, MO) containing
an antibiotic (1% Penicillin 100 microg/ml) (Gibco Laboratories) and supplemented with 10% (v/v) foetal bovine serum
(FBS) and 2 mM L-glutamine, Gibco Laboratories).
SulfoRhodamin B (SRB) assay
The SulfoRhodamin B (SRB) assay (Voigt 2005) was used to measure cell proliferation based on the total cellular
protein. Measurements were made by a SRB assay (Sulfo-rhodamine B). The Saos-2 and EAhy926 cells were fixed
with 10% (w/v) cold trichloroacetic acid for 1 h, then washed, dried, and stained for 30 min with 300 microl of 0.4% SRB
(w/v in 1% acetic acid) under mechanical agitation. Unbound dye was removed by four washes. With 1% acetic acid,
protein-bound dye was extracted with 200 microl of Tris base (10 mM, pH 10.5), for 5 min under mechanical agitation.
Finally, 100 microl of each well was used for the determination of the optical density at 492 nm.
Animal model
Female Wistar rats (16-19 weeks of age), obtained from the central pharmacy, Tunisia, and bred in the central animal
house, were used in this study. The rats were acclimatized to their new environment for 7 days before the beginning
of the study and were fed on a pellet diet (Sicco, Sfax, Tunisia) and water ad libitum. All the animals were kept under
climate-controlled conditions (25 °C; 55% humidity; 12 h of light alternating with 12 h of darkness). The handling
of the animals was approved by the Tunisian ethical committee for the care and use of laboratory animals. All rats
were randomly divided into five groups (5 animals per group), the first group (I) used as negative control (T). Sixty
days after bilateral ovariectomy, Groups II, III, IV and V used respectively as positive control (OVX), were
implanted with BG (OVX-BG), BG-Sr (OVX-BG-Sr); the last one presented empty defects (OVX-NI ).
Surgical and postoperative protocol
All surgical interventions were performed under general anaesthesia in aseptic conditions. Anaesthesia was induced
with xylazine [7 to 10 mg/kg (i.P) ROMPUN® 2%] and ketamine [70 to 100 mg/kg (i.m) imalgene®] depending on
the body weight. The pre-operative preparation of the surgical sites was routinely carried out by cleaning with 96%
alcohol and antiseptic solutions (PROLABO; AnalaR Normapur®, France). The resulting bone defects were irrigated
profusely with physiological saline solution (0.9 wt. % NaCl; Ref.091214; Siphal, Tunisia) to eliminate bone debris.
A drilled hole, 3-mm diameter and 4-mm deep, was created on the lateral aspect of the femoral condyle using a
refrigerated drill to avoid necrosis. The drill-hole was filled with 10mg of BG-Sr in OVX-BG-Sr group and with 10
mg of BG in OVX-BG group. The filling was done carefully in a retrograde fashion to ensure both minimal inclusion
of air bubbles and direct implant - bone contact. The closure of the wounds was performed in layers (i.e. fascias and
the subcutaneous tissue), using resorbable material (Vicryl 3/0; Ethicon, Germany) in a continuous fashion. After
the surgical operation, all rats received subcutaneous analgesiea (Carprofen 10 mg/kg I CRimadyl®) for three
postoperative days and they were allowed unrestricted mobility: during this period, they were checked daily for
clinical lameness or other complications. On days 4, 7, 15, 30 and 60 after implant insertion, all rats were sacrificed
and specimens were harvested for biological and physico-chemical evaluation.
Haematological and biochemical assays
White blood cell (WBC) counts, red blood cell (RBC) counts, the mean corpuscular volume (MCV), haemoglobin
concentration (Hb), haematocrit (Ht) and platelet counts were measured by the electronic automate coulter MAXM
(Beckman Coulter, Inc., Fullerton, USA). Calcium (Ca), phosphorus (P), and total alkaline phosphatase (ALP) were
examined using commercial Biomaghreb kits (Tunisia).
Tissue preparation
The implanted femoral condyles of all groups were minced and homogenized (100 mg/ml) at 4 °C in 0.1 mol/l Tris-HCl buffer pH 7.4 and centrifuged at
3,000G for 10 min.
Oxidative stress measurements
Lipid peroxidation was measured by the quantification of thiobarbituric acid-reactive substances (TBARS)
determined by the method of Buege and Aust (1984). The activity of SOD was assayed by the spectrophotometric
method of Marklund (Marklund and Marklund 1975). The GPx activity was measured by the method described by
Pagila and Valentine (1967). CAT was assayed calorimetrically at 240 nm and expressed as moles of H2O2 consumed
per minute per milligram of protein, as described by Aebi (1984). The level of total protein was determined by the
method of Lowry and al. using bovine serum albumin as the standard at 660 nm (Lowry et al. 1951).
Physico-chemical analyses
Femoral condyles were dried for 24 h at 65 °C weighed accurately and placed in 25 ml tubes with 2 ml of added nitric acid. One milliliter of 30%
H2O2 was placed in the tube after 10 min. The volume of the mixture was made up to 500
ml with distilled water. Standard solutions of Ca, P, Sr, Na, K and Zn, were used to prepare the working standard
solution, and a blank solution. The element concentrations were detected using inductively coupled plasma optical
emission spectrometry ICP-OES (Ciros; Spectro Analytical Instrument, Germany). Scanning electron microscopy
(SEM) (Jeol JSM 6301F) was used to identify morphological changes between bone, BG-Sr and BG. The collected
samples were prefixed with 2.5% glutaraldehyde solution (phosphate buffer solution, pH 7.4) overnight, and then
washed with a phosphate buffer solution (pH 7.4), before being post fixed with a 2% osmic acid solution (phosphate
buffer solution, pH 7.4) for 90 min and dehydrated with an alcohol evaporating system. The samples were carried
out with a freeze-dryer (JFD-300 Electron Optic Laboratory) and a vapour deposition system (JFC-1200).
Histological studies
After 15 days the implanted femoral condyles were harvested from each rat and fixed in Burdack, (formalin). The
time delay was selected to assess the performance of the biomaterials on bone formation before degradation. Samples
were included in a mixture of methylmethacrylate (MMA) and glycolmthacrylate (GMA) without prior decalci-fication. Sections 6 to 7 microm thick were
debited along a transverse plane using a sliding microtome (Reichert-Jung).
Statistical analysis
The statistical analysis of the data was carried out using the Student's t-test. The determinations were performed from 5 animals per group. All values
were expressed as means ± SE at the significance level 2alpha=0.05.
RESULTS
In vitro study
Cell proliferation at SulfoRhodamin B (SRB) assay
As illustrated in Fig. 1A, cell proliferation studies in the presence of BG extracts showed that Saos-2 osteoblast and
Eahy 926 endothelial were enhanced by 112% compared to those of the controls. After contact of BG-Sr, cell
proliferation (Fig. 1B) showed an increase of 120% at SaOS2 osteoblast and 127% at EAhy926 when compared to
those of the controls in 6 days of incubation and in culture media diluted to 0.2%. However, after 3 days of
incubation, in almost all experiments, a decrease in the cell number was noted. This led to a renewed conditioned
medium after 72 h.
In vivo study
WBC, RBC, MCV, Hb, Ht, platelet counts and serum Ca and P showed no significant difference in all treated
animals as compared to the controls. Moreover, the OVX group exhibited a significant decrease in serum ALP
4.24±1.51 when compared to that of the controls 6.39±1.04 (Table 1). In the other treated groups, ALP demonstrated
no significant variations as compared to those of OVX and controls 60 days after surgery.
Table 1. Alkaline phosphatase (ALP) activity in ovariectomised female Wistar rats presented empty defects (OVX-NI), received
strontium-doped bioglass (OVX-BG-Sr) and bioglass (OVX-BG). Animals were given free access to food and tap water
and were allowed unrestricted mobility. After 60 days post operation, blood samples were taken by cardiac puncture under light
diethyl-ether anesthesia. Other experimental conditions are described in the text.
|
T |
OVX |
OVX-NI |
OVX-Bg-Sr |
OVX-BG | | ALP (kat/l) |
6.39±1.04 |
4.24±1.51* |
4.15±1.65 |
4.43±1.39 |
4.17±1.64 |
Data represent mean ± standard deviation to the mean value of controls. * Significant as compared with control rats.
Measured oxidative stress in bone
As illustrated in Fig. 2A, B, C, the data on the CAT,
SOD and GPX activities in the femoral condyle of
OVX rats showed a highly significant decrease when
compared to those of control rats. Also, ovariectomy
significantly elevated the MDA levels (Fig. 3). In the
same way, after 4,7,15 days, OVX-BG-Sr, OVX-BG
rats exhibited a highly significant decrease of SOD,
GPX and CAT activities as compared to OVX rats.
Oxidative stress increased MDA levels in OVX-NI,
OVX-BG and OVX-BG-Sr rats by 180% 165% and
164% respectively. After 60 days, OVX-BG-Sr and
OVX-BG showed a particular increase in SOD, GPX,
CAT activities when compared to other groups and a
significant decrease of MDA. This positive action
was more pronounced in OVX-BG-Sr than in
OVX-BG. In fact, after comparison with OVX-BG
groups, an increase of OVX-BG-Sr in the SOD, CAT
and GPX activities of 13.7%, 8.13% and 14%
respectively, was shown at the end of the experiment.
Moreover, a decrease in MDA by 5% was detected in
OVX-BG-Sr as compared to the OVX-BG groups.

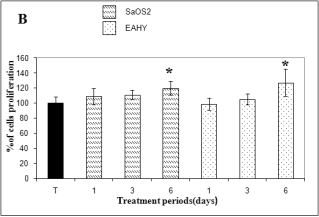
Fig. 1. SulfoRhodamin B (SRB) assay of Saos-2 human
osteosarcoma and Eahy 926 endothelial cells treated with
dissolution ions from bioglass (BG) (A) and
strontium-doped bioglass (BG-Sr) (B). Data represent mean
± standard deviation to the mean value of controls after 24
h, 3 and 6 days in culture media diluted to 0.2%. *
Significant as compared with controls rats.
A significant enhancement of enzymatic defenses was
observed especially in comparison with OVX groups.
A pronounced increase of the SOD, CAT and GPX
activities by 169%, 154% and 82% respectively was
shown. Moreover a very important decrease in MDA
by 28% was detected in OVX-BG-Sr when compared
to the OVX groups. Nevertheless, the antioxidative
activities did not attain the normal value of the control
rats.
ICP-OES of newly-formed bone
The P and Na concentrations changed somewhat but
the variations were not very important over 60 days.
As shown in Fig. 4A, the Ca concentrations in both
BG-Sr and BG matrix increased gradually in the
cancellous bone tissues. On the other hand, Fig. 4B
which gives the Ca/P value of the newly-formed bone
for each period, shows that the ratio value was close
to 1.30 when the glass matrix presented poor
degradation and near to that of biological apatite
1.66 after 30 and 60 days of implantation. The
increase in the Ca/P ratio over time is an indication of
progressive mineralization. In addition, as shown in
Fig. 4C Sr was slightly detected at about 60.04 ug/g
in the treated BG rats. Sr was barely taken up by
osteoid tissue or by bone cells or marrow cells in the
first period, but 60 days after surgery, it exhibited
144 ug/g when the control bone amount was
154.30 ug/g. As an overall consequence of BG-Sr
biodegradation, the release of Sr into the extracellular
fluid as a result of erosion surface showed that Sr
presented 152 ug/g by the end of treatment. The Sr/Ca
ratio could clarify the progression of Sr deposition in
the newly-formed bone. As shown in Fig. 4D, a
higher Sr/Ca ratio, equivalent to 6.20, was observed
in the new bone of the BG-Sr groups 60 days after surgery. However, the ratio didn't seem to be in the
same order of magnitude as those of controls which
were about 6.33. The absolute value of the Sr/Ca ratio
is low, because Sr is a trace element. On the other
hand, as shown in Fig. 4E, a small quantity of zinc
(Zn) which the bioactive glass did not contain was
detected in the newly-deposited bone tissue at about
0.12 mg/g after 30 days and this quantity represented
only about half that of the control (0.25 mg/g). Zn
appeared throughout the bioactive glass implant area
and increased progressively during the implantation
period to reach about 0.20 mg/g after 60 days, but
never reached the amount found in bone. Finally,
Potassium (K) disturbance was revealed in BG-Sr as
well as in BG 1.06 and 1.05 mg/g respectively
(Fig. 4F). These levels are normally found in the
control rat bone and represent about 1.14 mg/g.
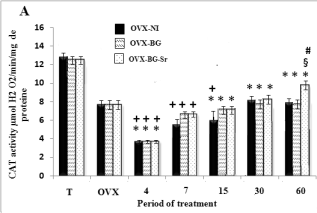
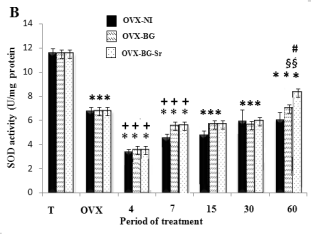
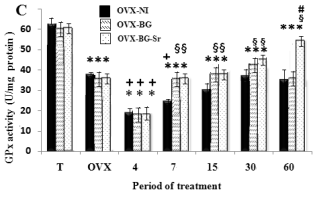
Fig. 2. Effects of bioglass (BG) and strontium-doped
bioglass (BG-Sr) on catalase (CAT) (A), superoxide
dismutase (SOD) (B) and glutathione peroxidase (GPx)
(C) activities in femoral condyle cells of ovariectomised
female Wistar rats for 4, 7, 15, 30 and 60 days. Values are
given as mean ± S.E. * Significantly less enzymatic activity
in the indicated group versus the control group, + versus the
ovariectomised group, § versus the ovariectomised group
with empty defects and # versus the ovariectomised group
and implanted with bioglass group.

Fig. 3. Effects of bioglass (BG) and strontium-doped
bioglass (BG-Sr) on malondialdehyde (MDA) level. *
Significantly higher level in the indicated group as
compared with the control group, + as compared with the
ovariectomised group, § lesser as compared with the
ovariectomised group with empty defects and # significant
as compared with the group implanted with bioglass.
SEM morphological analysis
SEM images showed cells anchored tightly on the
surface of BG-Sr (Fig. 5A, C, D), and exhibited
osteoblast morphology, indicating that cells had
finished attachment and were in the process of
spreading. Cells could spontaneously attach, spread
and proliferate well on the BG-Sr surface. However,
we noted an unstimulated layer of cells on the BG
surface (Fig. 5B). The apatite nucleation was
extensive on the BG-Sr surface (Fig. 6) and the
spread antennae of cells partially covered the apatite
nucleation. As a result, bone was formed by the
mineralization of an organic matrix including cells,
osteoid and proteins by the apatite nucleation and
growth. This structure indicates that Sr-incorporated
glass could not only stimulate apatite formation, but
also induce the adhesion and proliferation of
osteoblast cells, which might be the template for new
bone formation. In fact, the comparison with non
implanted and osteoporotic bone (Fig. 7A) showed
that the formation and mineralisation of bone matrix
implanted by BG-Sr was very accentuated (Fig. 7B,
C).
Histological evaluation of BG and BG-Sr implantation
New bone formation in all implanted defects was
observed in close contact with BG and BG-Sr (Fig.
8A, B). Both biomaterials were replaced by a large
amount of newly-formed bone. However, at the same
time clear differences were observed: the more
sporadic ingrowth within the periphery of the BG
implant showed a sparser osteoid deposition; the bone
surrounding BG-Sr presented a highly cellular layer,
more advanced ossification and a much larger
stimulation of osteoregeneration than those of the BG
group (Fig. 8C, D). This bone regeneration paralleled
the significant enhancements of the antioxidative
enzyme in the OVX-BG-Sr.
DISCUSSION
Recently, great interest has been focused on Sr as a
potential anti-osteoporotic factor (Collette et al.
2010). We hypothesise that the local presence of
0.1% Sr2+ at bone-to- bioactive glass implant interface
would be an excellent candidate for protective surface
treatment. Sr may contribute to the control of ROS imbalance during hormonal deficiency and healing
response. To our knowledge, there is no previous
report of a comprehensive study of antioxidant
defense systems in osteoporotic animals receiving
BG-Sr and BG.


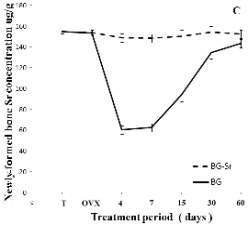
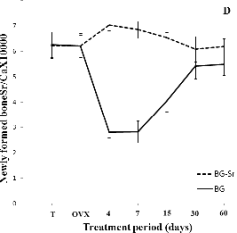
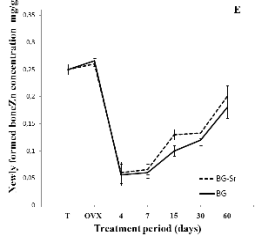
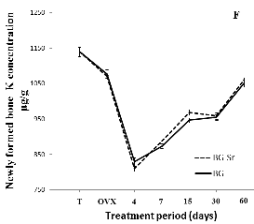
Fig. 4. Distribution of Ca (A), Ca/P (B), Sr (C), Sr/Ca
(D),Zn (E) and K (F) in newly-formed bone of
ovariectomised female Wistar rats implanted with bioglass
(BG) and strontium-doped bioglass (BG-Sr) for 4, 7, 15, 30
and 60 days.
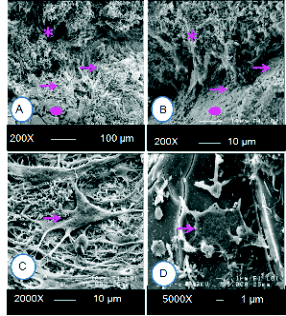
Fig. 5. SEM image in vivo showed increase of osteoblast
proliferation on 0.1% strontium-doped bioglass 15 days
post surgery (A), unstimulated osteoblast proliferation with
contact of bioglass (BG) (B). A large osteoblast attachment
on 0.1% strontium-doped bioglass (BG-Sr) (C, D). Arrows
indicate osteoblasts and bioglass osseointegration and *
bone. Circles indicate bioglass.

Fig. 6. Mineralized nodule formation on 0.1%
strontium-doped bioglass (BG-Sr) and development of
bioactive calcium phosphate layer over the surface of
strontium-doped bioglass (BG-Sr).
In previous work, the link between the toxic
effects of Sr-containing bioactive glasses and the
release of Sr2+ clarify was clarified (Oudadesse et al.
2011). After immersion in simulated body fluid
(SBF), the kinetic reaction between BG-Sr and the
surrounding synthetic fluids influences the formation
and the evolution of the newly-formed layers.
However, the intensity of these effects depends on the
content of Sr introduced in the glass matrix. Starting
from this observation, our in vitro study was aimed at
clarifying whether BG actually exerted toxic effects on cells, and whether Sr might play a role in such
cytotoxicity.

Fig. 7. SEM image of non implanted osteoporotic bone
(A), reparative events of bioglass (BG) showed the
regenerated bone (B). Regenerated bone surrounding
strontium-doped bioglass (BG-Sr) (60 days post surgery)
(C).
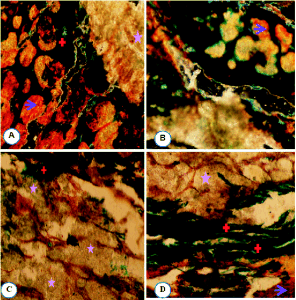
Fig. 8. Goldner's trichrome staining (10x objective).
Histological sections of the interface between bioglass
(BG) and bone (A, C), strontium-doped bioglass (BG-Sr)
and bone (B, D) showed osteostimulative properties and
acceleration of new bone formation surrounded
strontium-doped bioglass (BG-Sr). Arrows indicate
unmineralized bone (osteoid tissue). * Bioglass/strontium-doped bioglass; + mineralized tissue
at the same period (15 days post surgery).
In this study an SRB assay showed a better
enhancing effect on cell proliferation with Sr than
when cells were cultured in media with no Sr. The
response was optimal after 6 days of contact. The
need for several days of incubation was necessary for
the dissolution of bioactive glasses products and for
the expression of genes regulating the cell cycle (Jell
and Stevens 2006). It has a dual role, indirectly
promoting the adhesion of osteoblasts to the
hydroxyapatite layer (Anselme 2000) and directly
stimulating osteogenesis through Runx2 expression in
preosteoblastic cells (Zhu et al. 2007) or through the
expression of three key genes: Colla 1, Colla 2, and
Runx 2.33. The in vitro results pointed to the
biocompatibility and relative bioactivity of BG-Sr and
BG, confirming their ability to support the invasion of
cells (osteoblasts). However, after 3 days of
incubation, in almost all experiments, a decrease in
the cell numbers was recorded, which led to renewal
of the conditioned medium after 72 h. The mechanism
by which the bioglass modifies cell physiology is not
fully understood; the kinetics of release, the pH, the
synergy between the different chemical entities, and
the role of trace elements all remain hypotheses.
In the in vivo study, no adverse effects of BG-Sr,
or BG were observed in clinical examinations because
of the stability of the haematologic parameter, and the
serum levels of Ca and P. In contrast, the high level
of alkaline phosphatase activity in OVX-BG-Sr,
OVX-BG, amd OVX-NI when compared to the OVX
group reflected osteoblastic activity (Isomura et al.
2004). This might be due to a difference of bone
turnover, especially bone formation. A high ion
intensity of Ca, and P was detected in cancellous bone
shown by the high dissolution rate of BG-Sr. As the
glass matrix dissolved, various elements dispersed in
the glass and combined with elements in the bioactive
glass. The results clearly showed that during the
dissolution of the bioactive glass the different species
of ions were released selectively. Sodium was rapidly
exchanged with the surrounding tissues. Si, Ca and Sr
were released more slowly; P was released later
(Zreiqat et al. 2010). A series of physico-chemical
reactions at the material periphery led to the
precipitation of a Ca-P rich film on top of a pure
Si-layer through diffusive phenomena (Greenspan and
Hench 1976). In this study, 0.1% of Sr derived from
BG-Sr was taken up in the apatitic environments of
the crystals and was rapidly and easily exchanged
with Ca. After 30 days of exposure, the uptake of Sr
was observed in new cancellous bone tissues and
seemed more marked in newly-formed bone BG-Sr
than that of BG-bone. This can be explained by
higher Sr levels in the bone environment as a result of
bioglass matrix degradation. Most of the Sr amount
found inside the degraded layer of glass could prove
that this element was released slowly into the
biological environment, thus stimulating osteogenic
processes (Xue et al. 2006, Peng et al. 2009, Tsigkou
et al. 2009). Sr was an essential element associated
with bone growth and bone activity. With enough
time (8 weeks), new bone with higher Sr content was
transformed into mature bone.
The increase of Sr/Ca ratio in bone might be
explained by the fact that, in newly- formed bone, Sr
was not only incorporated into the crystals
(heteroionic substitutions), but also taken up onto
their surface (adsorption and exchange) (Grynpas et
al. 1996). The Ca/P ratio increased progressively but
never reached the amount found in the control bone.
This might be due to the replacement of Ca by Sr in
the newly- formed apatite crystals.
In addition, as reported by (Jallot et al. 2000) the
Si layer might be composed with Si (OH) groups
which induce heterogeneous nucleation of the apatite.
An appreciable amount of silicate ion was, however,
released from the bioglass. This was due to the
breaking of the Si-O-Si bonds such as dissolution
which led to an increase of Si at the glass periphery
and possibly to the formation of Si-OH and Si (OH)4
groups at the glass/bone interface. On the other hand,
the accumulation of Na or K might increase locally
the degree of bone environmental supersaturation.
This rapid bone clearance of Na revealed that the
glass became low in sodium as the bioactive glass
underwent degradation.
By the end of treatment, the low rate of Na+ could
be explained by the fact that the depositions of
hydroxyapatite on the bioactive glass surface
(Izquierdo-Barba et al. 2008) might well reduce the
rate of Na+ leaching but certainly did not eliminate it.
The presence of potassium was due to ion exchange
between the glass and the biological fluids (Jones et
al. 2007). As explanation of the increase level of Zn
contents during bone consolidation, authors (Ovesen
et al. 2009) demonstrated that collagen forming cells
invaded the blood clot and produced a specialised
form of collagen which wrapped itself around the
hole.
Slowly, the bone forming cells moved into the
collagen tissue called a callus. These cells laid down
the calcium, making the bone regain its strength. The
enzymes responsible for laying down the bone callus
were activated by Zn, and Osteon and Osteoid
structures are known to have a high Zn content
(Lusvardi et al. 2009). Our study suggested that
BG-Sr implantation into the rat cancellous bone could increase the amount of osteoblasts and osteoid
layer, and stimulate new bone formation as well.
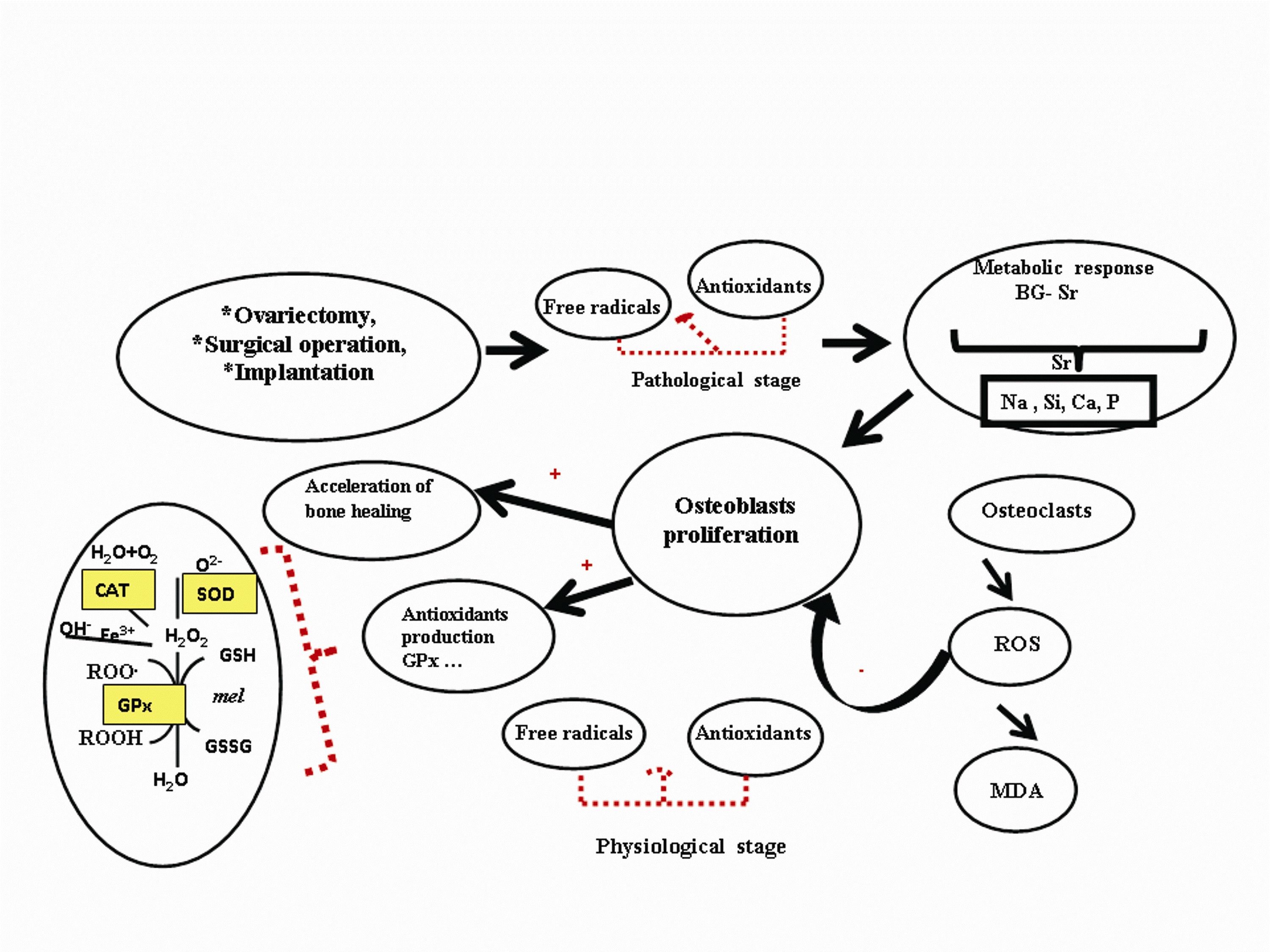
Fig. 9. Diagram of hypothetical mechanism of
strontium-doped bioglass (BG-Sr) biomedical material
on oxidative stress balance described in femoral condyle
of ovariectomised female Wistar rats.
Likewise, the present study emphasized the
impact of BG-Sr on the antioxidant system in
ovariectomised rats. As an effect of ovariectomy, we
reported a significant decrease in the antioxidant
levels in the femoral condyle of OVX rats, OVX
BG-Sr, and OVX-BG. These findings suggest that a
deficiency in ovarian hormones fails to combat
oxidative stress, and also that during tissue
regeneration, endothelial cells, by secreting cytokines
and expressing surface adhesion, play an important
role in the development of complications in
biomaterial implant through free radical-induction.
In fact, interactions at the material-macrophage
interface trigger macrophage activation including the
production of pro-inflammatory species (cytokines,
leukotrienes, etc.). Further GPx was found to suppress
osteoclastic differentiation (Lean et al. 2005).
Similarly, when GPx failed to eliminate H2O2 from
the cell. The accumulated H2O2 has been shown to
cause an inactivation of SOD and a decrease in GPx
in OVX animals (Sinet and Garber 1981) supports the
potential significance of our findings. The decrease in
SOD, CAT activities may result in more accumulation
of O2-, which was shown to inhibit other antioxidant
enzymes (Kono and Fridovich 1982).
The induction of oxidative stress in the period of
4, 7, 15 days could be explained in two ways. Cells
are exposed to two types of stress: i) imbalance
between production and neutralization of ROS due to
hormonal insufficiency ii) stress due to the post
surgery production of pro-inflammatory species. The
observation time of a 60-day treatment by bioactive
glass limited the increase in the level of MDA and
increased antioxidative acivities. These data suggest
that the healing response did not lead to failure of the
materials.
Regarding previous studies (Anderson et al.
2008), with biocompatible materials, the early
resolution of the acute and chronic inflammatory
responses occurred, with the chronic inflammatory
response usually lasting no longer than two weeks.
Besides, the persistence of the acute and/or
inflammatory responses beyond a three-week period
usually indicates an infection. The excellent
tolerability of BG-Sr showed that this chronic
inflammatory response was usually of short duration.
These results agree with our findings. The results
obtained after 60 days could be explained in three
ways, as described in Fig. 9: i) ions released from
BG-Sr works by helping resident cells in the bone to
restore the normal bone remodelling and enhance
metabolic activity in osteoblasts. These cells produce
antioxidants such as GPx to protect against ROS
(Sadeghi et al. 2008). ii) Sr was reported to be an
effective scavenger for oxidative radicals and this
antioxidant activity protects from damage and death
(Kaviarasan et al. 2008). iii) Sr released following the
BG-Sr dissolution might act synergistically with other
ions like silica, calcium, phosphate, and sodium ions
that contribute to a balanced oxidative status. That's
why BG-Sr was more efficient than BG.
In vitro studies agreed with in vivo findings and
showed that BG-Sr and BG stimulated
osteoprogenitors to differentiate into mature
osteoblasts producing bone-like nodules. Similarly, in
vivo studies showed the gradual degradation of
implanted bioglass with subsequent formation of new
bone at the implant surface and especially the
stabilization of oxidative status at a level close to that
of normal animals, suggesting antioxidative
properties of BG-Sr. Thus, this study might be the
first report indicating that BG-Sr could improve bone
regeneration and oxidative Status stabilization in
OVX rats.
In conclusion, it is important to note that BG-Sr
stimulated in vitro proliferation of SaOS-2, Eahy 926
endothelial cells. In vivo, BG-Sr had good bioactivity,
bone-bonding ability and oxidative balance stability
shown 60 days after surgery. The incorporation of
0.1% of Sr in BG may be an effective strategy for
creating materials for bone repair/antioxidation/
regeneration therapies. Although promising results
suggest possible clinical use of bioactive bone cement
for revision bone replacement procedure, further
investigations of long-term outcomes are necessary
before this bioactive bone cement can be applied in
human orthopedic surgery.
REFERENCES
Aebi H. Catalase in vitro. Methods Enzymol. 105: 121-126, 1984.
[CrossRef]
Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 20: 86-100, 2008.
[CrossRef]
[PubMed]
Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 21: 667-681, 2000.
[CrossRef]
Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 105: 302-310, 1984.
Caverzasio J, Thouverey C. Activation of FGF receptors is a new mechanism by which strontium ranelate induces osteoblastic cell growth. Cell Physiol
Biochem. 27: 243-250, 2011.
[CrossRef]
[PubMed]
Chesnut Ch 3rd, Silverman S, Andriano K, Genant H, Gimona A, Harris S, Kiel D, LeBoff M, Maricic M, Miller P, Moniz C, Peacock M et al. A
randomised trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic
fractures study. Am J Med. 109: 267-276, 2000.
[CrossRef]
Collette J, Bruyere O, Kaufman JM, Lorenc R, Felsenberg D, Spector TD, Diaz-Curiel M, Boonen S, Reginster J-Y. Vertebral anti-fracture efficacy of
strontium ranelate according to pre-treatment bone turnover. Osteoporos Int. 21: 233-241, 2010.
[CrossRef]
[PubMed]
Effah Kaufmann EA, Ducheyne P, Shapiro IM. Evaluation of osteoblast response to porous bioactive glass (45S5) substrates by RT-PCR analysis. Tissue
Eng. 6: 19-28, 2000.
[CrossRef]
[PubMed]
Gentleman E, Fredholm YC, Jell G, Lotfibakhshaiesh N, O'Donnell MD, Hill RG, Stevens MM. The effects of strontium-substituted bioactive glasses on
osteoblasts and osteoclasts in vitro. Biomaterials. 31: 3949-3956, 2010.
[CrossRef]
[PubMed]
Greenspan DC, Hench LL. Chemical and mechanical behaviour of bioglass-coated alumina. J Biomed Mater. Res. 10: 503-509, 1976.
[CrossRef]
[PubMed]
Grynpas MD, Hamilton E, Cheung R, Tsouderos Y, Deloffre P, Hott M, Marie PJ. Strontium Increases Vertebral Bone Volume in Rats at a Low Dose That Does
Not Induce Detectable Mineralization Defect. Bone. 18: 253-259, 1996.
[CrossRef]
Hamdy NA. Strontium ranelate improves bone microarchitecture in osteoporosis. Rheumatology. 48: 9-13, 2009.
[CrossRef]
[PubMed]
Hench LL. Bioceramics. J Am Ceram Soc. 81: 1705-1728, 1998.
[CrossRef]
Hench LL, Splinter RJ, Allen WC, Greenlee TK, Jr. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res Symp. 2:
117-141, 1971.
[CrossRef]
Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 28: 1456-1462,
2000.
[CrossRef]
Hojjatie B, Anusavice KJ. Three-dimensional finite element analysis of glass-ceramic dental crowns. J Biomech. 23: 1157-1166, 1990.
[CrossRef]
Isomura H, Fujie K, Shibata K, Inoue N, Iizuka T, Takebe G, Takahashi K, Nishihira J, Izumi H, Sakamoto W. Bone metabolism and oxidative stress in
postmenopausal rats with iron overload. Toxicology. 19: 93-100, 2004.
[CrossRef]
[PubMed]
Izquierdo-Barba I, Arcos D, Sakamoto Y, Terasaki O, Lopez-Noriega A, Vallet-Regi M. High-performance mesoporous boceramics mimicking bone
mineralization. Chem Mater. 20: 3191-3198, 2008.
[CrossRef]
Jallot E, Benhayoune H, Kilian L, Irigaray J L, Balossier G, Bonhomme P. Growth and dissolution of apatite precipitates formed in vivo on the
surface of a bioactive glass coating film and its relevance to bioactivity. J Phys D Appl Phys. 33: 2775-2780, 2000.
[CrossRef]
Jell G, Stevens MM. Gene activation by bioactive glasses. J Mater Sci Mater Med. 17: 997-1002. 2006.
[CrossRef]
[PubMed]
Jell G, Notingher I, Tsigkou O, Notingher P, Polak JM, Hench LL, Stevens MM. Bioactive glass-induced osteoblast differentiation: a noninvasive
spectroscopic study. J Biomed Mater Res A. 86: 31-40, 2008.
[CrossRef]
[PubMed]
Jones JR, Gentleman E, Polak J. Bioactive Glass Scaffolds for Bone Regeneration. Elements. 3: 393-399. 2007.
[CrossRef]
Kaviarasan K, Kalaiarasi P, Pugalendi V. Antioxidant efficacy of flavonoid-rich fraction from Spermacoce hispida in hyperlipidemic rats. J Appl Biomed.
6: 165-176, 2008.
[JAB]
Kono Y, Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 257: 5751-5754, 1982.
[PubMed]
Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation.
Endocrinology. 146: 728-735, 2005.
[CrossRef]
[PubMed]
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 193: 265-275, 1951.
[PubMed]
Lusvardi G, Zaffe D, Menabue L, Bertoldi C, Malavasi G, Consolo U. In vitro and in vivo behaviour of zinc-doped phosphosilicate glasses.
Acta Biomater. 5: 419-428, 2009.
[CrossRef]
[PubMed]
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and convenient assay for superoxide dismutase.
Eur J Biochem. 47: 469-474, 1975.
[CrossRef]
[PubMed]
Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, Cannata J, Balogh A, Lemmel EM, Pors-Nielsen S, Rizzoli R, Genant HK et al. The
effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Eng J Med. 350: 459-468, 2004.
[CrossRef]
[PubMed]
Oudadesse H, Dietrich E, Bui XV, Le Gal Y, Pellen P, Cathelineau G. Enhancement of cells proliferation and control of bioactivity of strontium doped
glass. Appl Surf Sci. 257: 8587-8593, 2011.
[CrossRef]
Ovesen J, Moller-Madsen B, Nielsen PT, Christensen PH, Simonsen O, Hoeck HC, Laursen MB, Thomsen JS. Differences in zinc status between patients with
osteoarthritis and osteoporosis. J Trace Elem Med Biol. 23: 1-8, 2009.
[CrossRef]
[PubMed]
Pagila D E, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 70:
158-169, 1967.
[PubMed]
Peng S, Zhou G, Luk KD, Cheung KM, Li Z, Lam WM, Zhou ZJ, Lu WW. Strontium promotes osteogenic differentiation of mesenchymal stem cells through the
Ras/MAPK signaling pathway. Cell Physiol Biochem. 23: 165-174, 2009.
[CrossRef]
[PubMed]
Sadeghi N, Oveisi MR, Jannat B, Hajimahmoodi. M, Jamshidi AR, Sajadian Z. Determination of plasma gluthatione reductase enzyme activity in osteoporotic
women. Daru. 16: 51-54, 2008.
Saito T, Kin Y, Koshino T. Osteogenic response of hydroxyapatite cement implanted into the femur of rats with experimentally induced osteoporosis.
Biomaterials. 23: 2711-2716, 2002.
[CrossRef]
Sanchez-Rodriguez MA, Ruiz-Ramos M, Correa-Munoz E, Mendoza-Nunez VM. Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as
characterized by antioxidant enzymes. BMC Musculoskelet Dis. 8: 124, 2007.
[CrossRef]
[PubMed]
Sinet PM, Garber P. Inactivation of human Cu, Zn superoxide dismutase during exposue to O-2 and H2O2. Arch Biochem Biophys. 212: 411-416, 1981.
[CrossRef]
Tsigkou O, Jones JR, Polak JM, Stevens MM. Differentiation of fetal osteoblasts and formation of mineralized bone nodules by 45S5 Bioglass conditioned
medium in the absence of osteogenic supplements. Biomaterials. 30: 3542-3550, 2009.
[CrossRef]
[PubMed]
Voigt W. Sulforhodamine B assay and chemosensitivity. Methods Mol Med. 110: 39-48, 2005.
[PubMed]
Xue W, Moore JL, Hosick HL, Bose S, Bandyopadhyay A, Lu WW. Osteoprecursor cell response to strontium-containing hydroxyapatite ceramics. J Biomed
Mater Res A. 79: 804-814, 2006.
[CrossRef]
[PubMed]
Zhu LL, Zaidi S, Peng Y, Zhou H, Moonga BS, Blesius A, Dupin-Roger I, Zaidi M, Sun L. Induction of a program gene expression during osteoblast
differentiation with strontium ranelate. Biochem Biophys Res Commun. 355: 307-311, 2007.
[CrossRef]
[PubMed]
Zreiqat H, Ramaswamy Y, Wu C, Paschalidis A, Lu Z, James B, Birkeb O, McDonald M, Little D, Dunstan CR. The incorporation of strontium and zinc into a
calcium-silicon ceramic for bone tissue engineering. Biomaterials. 31: 3175-3184, 2010.
[CrossRef]
[PubMed]
|
BACK
|


















