Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 4, p 187-193
DOI 10.2478/v10136-012-0010-2
Acute poisoning with sarin causes alteration in oxidative homeostasis and biochemical markers in Wistar rats
Miroslav Pohanka, Jaroslav Romanek, Jiri Pikula
Address: Miroslav Pohanka, Faculty of Military Health Sciences, University of Defense, Trebesska 1575, 500 01 Hradec Kralove, Czech Republic
miroslav.pohanka@gmail.com
Received 8th December 2011.
Revised 30th January 2012.
Published online 31st January 2012.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
Acknowledgements
References
SUMMARY
Sarin is a potent inhibitor of acetylcholinesterase (AChE). It is known as an agent of chemical warfare and is one of a number of nerve agents misused for chemical terrorism, e.g. on the Tokyo subway attacks. Though effect of sarin on the cholinergic system is well-known, long-term adverse effects and the role of oxidative stress in sarin toxicity remain unknown. The experiment reported here was carried out on laboratory Wistar rats intramuscularly exposed to 0.5-50% of sarin LD50 for one hour. A complex biochemical examination of plasma samples and an assessment of oxidative stress in the liver, kidney, spleen, cerebellum and frontal lobe were performed after euthanasia of the animals. By means of these biochemical markers, we were able to observe the induction of hyperglycaemia in a dose-dependent manner. Other biochemical markers such as transaminases were influenced in a non-standard manner as sarin probably acted as an inhibitor of these markers. Oxidative stress markers and an assessment of AChE activity showed an unequal impact of sarin on different tissues. Significant inhibition of AChE was found in the cerebellum and frontal lobe. Besides this, alterations in reduced glutathione, ferric reducing antioxidant power (FRAP) and thiobarbituric acid reactive substances (TBARS) were proven. In particular, an accumulation occurred of reduced glutathione in the frontal lobe, whereas depletion of FRAP was found in the kidney and spleen, and a strong increase in TBARS occurred in the spleen in a dose-dependent manner. We infer that sarin extensively influences oxidative homeostasis. Surprisingly, the central nervous system seems to be more resistant than the other organs.
KEY WORDS
sarin; nerve agents; chemical warfare; acetylcholinesterase; oxidative stress; antioxidant; biochemistry
Abbreviations:
Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRE, creatinine; Glu, glucose; IP, inorganic phosphate; T-Bil, total bilirubin; TP, total protein.
INTRODUCTION
Sarin (IUPAC name: propan-2-yl methylphospho-nofluoridate) is a highly toxic nerve agent with the
reported median lethal dose (LD50) 103 microg/kg for
subcutaneously exposed rats (Brimblecomb et al.
1970). Like the other nerve agents, it is an irreversible
inhibitor of enzymes acetylcholinesterase (AChE) and
butyrylcholinesterase (BuChE). The irreversible
inhibition of AChE causes hyper-stimulation of
nicotinic as well as muscarinic acetylcholine
receptors, as the neurotransmitter acetylcholine is not
degraded (Marrs 1993). After exposure, symptoms of
acute toxicity such as tremors, seizures and
hypothermia follow (Abu-Qare and Abou-Donia
2002). Exposure to sarin can be resolved by oxime
reactivators such as obidoxime or HI-6. Application
of the reactivators results in a reverse of
cholinesterase activities (Kassa and Cabal 1999).
Sarin has a prominent role as one of the most
important nerve agents. In the 1991 Gulf War,
soldiers were accidentally exposed to sarin and
cyclosarin during the disposal of military stockpiles
at Khamisiyah (Proctor et al. 2006). Some years later,
sarin was used for terrorist purposes by Aum
Shinrikyo in Japan in 1994 and 1995 (Yanagisawa et
al. 2006).
The complex effects of nerve agents on the
nervous system remain unknown. Nerve agents have
been reported as inductors of neuronal cell apoptosis.
Surprisingly, N-methyl-D-aspartate receptor
pathways are also involved in the pathological
process after poisoning by sarin (Wang et al. 2008).
Sarin has been shown to be an initiator of oxidative
stress, and as a marker of DNA oxidative
degradation; 8-hydroxy-2´-deoxyguanosine, was
significantly elevated after intramuscular
administration of the toxin into rats (Abu-Qare and
Abou-Donia 2001). Our primary intention was aimed
at identification of the adverse effects of sarin in a
Wistar rat model. For experimental purposes, we
chose a complex set of tests suitable for oxidative
stress scoring in tissues. Additionally, we applied a
standard assessment of biochemical plasma markers
in order to estimate organ-specific pathologies. We
aimed the experiment to answer the question whether
the toxic effects of sarin can also be followed by
oxidative stress and imbalance in antioxidants.
Answering this question would be an initial step in
research into a non causative therapy in nerve agents
poisoning.
MATERIAL AND METHODS
Animal model and samples collection
40 male Wistar rats were purchased from the Velaz
Company (Prague, Czech Republic). The rats were
six weeks old at the beginning of the experiment and
weighed 192±5 g. For the entire experiment, the rats
were kept in an air conditioned room at temperature
22±2 °C, humidity 50±10% and light period 12 hours
per a day. Feed and drinking water were provided ad
libitum. The entire experiment was approved and
supervised by the Ethical Committee of the Faculty of
Military Health Sciences, University of Defence,
Hradec Kralove, Czech Republic.
Sarin was obtained from the Military Technical
Institute of the former Czechoslovakia. The Faculty
of Military Health Sciences has permission for the
manipulation of nerve agents from the government
institution SUJB, the official representative of the
Organisation for the Prohibition of Chemical
Weapons (OPCW) in the Czech Republic. The LD50
was assessed in a separate experiment to be 0.472
mg/kg (mortality followed up to 24 hours).
Animals were exposed intramuscularly in the
pelvic area of the hindlimb to sarin at doses 0
(controls; saline only), 0.5, 2, 10 and 50% of LD50.
After one hour, animals were sacrificed by carbon
dioxide narcosis. Blood, kidney, liver, brain and
spleen were collected from the sacrificed animals.
The frontal lobe and cerebellum were collected from
the brain. Blood was kept in heparinised tubes
(Dialab, Prague, Czech Republic) and plasma was
collected after blood centrifugation at 3,000G for
15 minutes. The collected tissues were homogenised
using an Ultra-Turrax mill (Ika Werke, Staufen,
Germany). A total of 100 mg of freshly collected
tissue was mixed with 1 ml of saline solution for one
minute.
Ex vivo assay
Ferric reducing antioxidant power (FRAP),
glutathione reductase (GR), reduced glutathione
(GSH) and thiobarbituric acid reactive substances
(TBARS) were assessed as oxidative stress markers.
Measurement procedures were in compliance with
reported papers (Pohanka et al. 2010, 2011a). AChE
activity in tissues was measured in compliance with
another paper (Pohanka et al. 2011b). The following
plasma biochemistry markers were assayed by
SPOTCHEM TM EZ SP-4430 (Arkray, Japan).
Statistics
A group size was eight specimens. The Bonferroni
test (Origin 8 SR2, OriginLab Corporation,
Northampton, MA, USA) was used at the significance
level 2alpha = 0.05.
RESULTS
The poisoned animals had no conspicuous
manifestation when poisoned by doses 0.5 and 2%
LD50. The animals exposed to sarin in doses 10 and
50% exerted tonic-clonic seizures starting five to ten
minutes after poisoning and lasting up to the
sacrificing.
Biochemistry markers in plasma
The assayed biochemistry markers are depicted as
Table 1. Albumin was significantly elevated at 10 and
50% of the LD50 of sarin. Alanine aminotransferase
and aspartate aminotransferase were significantly
decreased when sarin was administered at a dose
0.5% of LD50 and more. Sarin had no significant
effect on total protein levels and calcium in plasma.
Blood urea nitrogen was significantly decreased at
10 and 50% of the LD50 of sarin, while glucose was
significantly elevated at 50% of LD50. Creatinine was
significantly decreased at doses above 0.5% of LD50
and at 2% of LD50. Inorganic phosphate was
significantly decreased when the sarin dose exceeded
2% of LD50.
Table 1. Selected biochemical markers.
| Sarin dose
(% LD50) |
0 (controls) |
0.5 |
2 |
10 |
50 | | Alb (g/l) |
26.5±5.0 |
31.5±2.0 |
32.0±5.7 |
34.5±3.7 (*) |
33.4±3.9 (*) | | ALT (microkat/l) |
1.03±0.19 |
0.688±0.086 (*) |
0.475±0.12 (*) |
0.455±0.12 (*) |
0.329±119 (*) | | AST (microkat/l) |
5.51±1.48 |
3.54±0.70 (*) |
2.09±0.55 (*) |
2.18±0.46 (*) |
2.27±0.46 (*) | | BUN (mmol/l) |
9.40±1.14 |
8.83±1.59 |
7.71±0.68 |
6.98±1.61 (*) |
7.11±0.70 (*) | | Ca (mmol/l) |
2.53±0.21 |
2.59±0.13 |
2.58±0.22 |
2.53±0.17 |
2.61±0.23 | | CRE (micromol/l) |
77.5±19.6 |
58.3±10.4 (*) |
45.5±7.3 (*) |
45.6±13.6 (*) |
34.9±5.5 (*) | | Glu (mmol/l) |
7.15±1.17 |
7.50±0.84 |
7.23±0.78 |
7.86±0.67 |
11.3±2.0 (*) | | IP (mmol/l) |
2.56±0,29 |
2.27±0.17 |
2.04±0.27 (*) |
1.78±0.19 (*) |
1.57±0.32 (*) | | T-Bil (micromol/l) |
30.9±6.7 |
18.8±3.8 (*) |
17.8±1.5 (*) |
16.4±4.4 (*) |
11.9±1.9 (*) | | TP (g/l) |
57.9±2.9 |
56.5±3.0 |
57.1±7.4 |
56.6±5.0 |
63.1±8.4 |
* Mean ± standard deviation. (*) Significant as compared with control.
Acetylcholinesterase
The activity of AChE was tested in all collected
organs. The liver, kidney and spleen had low AChE
activity. The effect of sarin was not recognisable due
to the low activity and high relative standard
deviations. The assessed activity of AChE in the
frontal lobe and cerebellum is depicted in Fig. 1. No
significant effect was seen at the lowest dose of sarin
(0.5% of LD50), i.e. no inhibition of AChE was
observed in either the cerebellum or frontal lobe. An
increased dose of sarin caused significant inhibition
of AChE. In the frontal lobe, between 70-80%
inhibition was observed and the alteration was
significant. The sarin effect on the cerebellum was
greater than that observed in the frontal lobe.
Inhibition reached 87.9% at the highest dose of sarin.
In the cerebellum, the effect was significant at sarin
doses 2% of LD50 and higher. Higher doses caused a
significant decrease in AChE activity in the
cerebellum.
Markers of oxidative stress
According to the statistical test used, sarin
significantly influenced the balance between
antioxidants and oxidative stress in regard to TBARS,
GSH and FRAP. However, GR activity was not
significantly altered by sarin in any of the examined
organs. The GSH level was significantly increased in
a dose-dependent manner in the frontal lobe and liver
(Fig. 2). The increase was up 40% in the liver and 70% in the frontal lobe. The increase in the kidney
was insignificant. The livers and cerebellum did not
show evidence of sarin on the GSH level. In contrast
to GSH, the FRAP level was constant in the frontal
lobe, cerebellum and liver in exposed animals
(Fig. 3). The kidney and spleen were extensively
influenced at the highest dose of sarin. Low molecular
weight antioxidants were depleted in the kidney and
spleen with the highest sarin dose. Nearly 90% of low
molecular weight antioxidants disappeared in the
kidney and spleen after the administration of 50% of
the sarin LD50.
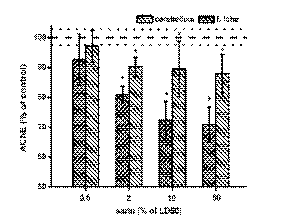
Fig. 1. Alteration in the acetylcholinesterase (AChE) activity in exposed animals. Dashed line lay in the control value (100%
of AChE activity), dotted lines determinate standard deviation for controls. Error bars represent the standard deviation for n =
8 specimens. * Significant compared with control.
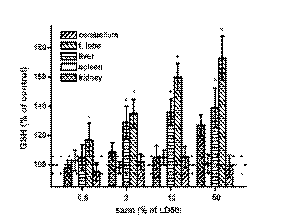
Fig. 2. Alteration in the reduced glutathione (GSH) level in exposed animals. Dashed line lay in the control value (100% of
GSH level), dotted lines determinate standard deviation for controls. Other symbols as in Fig. 1.
TBARS (depicted in Fig. 4) were increased in a
dose-dependent manner in all organs after the
application of sarin. However, the increase was the
greatest and significant only in the spleen. TBARS in
the spleen were significantly increased after the
administration 10% of the sarin LD50 and (an
approximate increase of 30% against controls) and
very significantly increased after the administration of
50% of the sarin LD50 (an approximate increase of
50% against the controls).
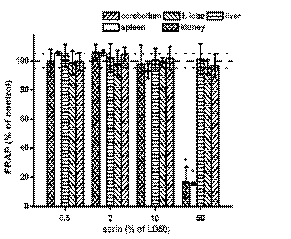
Fig. 3. Alteration in the ferric reducing antioxidant power (FRAP). Dashed line lay in the control value (100% of FRAP
level), dotted lines determinate standard deviation for controls. Error bars represent the standard deviation for n = 8 specimens.
Other symbols as in Fig. 1.
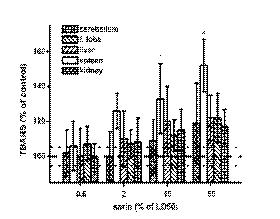
Fig. 4. Alteration in the thiobarbituric acid reactive substances (TBARS) level in exposed animals. Dashed line lay in the
control value (100% of TBARS level), dotted lines determinate standard deviation for controls. Error bars represent the standard
deviation for n = 8 specimens. Other symbols as in Fig. 1.
DISCUSSION
Sarin is a representative nerve agent. As reported by
many authors, the toxicity of sarin is based on the
inhibition of AChE followed by cholinergic crisis
(Niven and Roop, 2004). We decided to investigate
AChE activity in the organs rather than blood while
establishing the impact of sarin on the endangered
organs. The decrease of AChE activity in organs was
expected when considering the known mechanism of
sarin molecular mechanism of toxicity (Pohanka
2011a). However, the other assessed markers had not
been investigated previously and the findings were
unpredictable., We were especially surprised by the
alterations in plasma biochemical markers. The strong
decreases in alanine aminotransferase, aspartate
aminotransferase, blood urea nitrogen, creatinine and
total bilirubin have no common biochemical cause as
most of the pathologies diagnosed by the markers are
distinguished by an increase in their plasmatic values.
However, the molecular mechanism of sarin action on
the markers can be inferred. Sarin is a reactive
compound, and esterification of tyrosine residues in
peptides has been reported (Schopfer et al. 2010). The
biological effect of esterification was hypothesised
but unproven by Li et al. (2009), e.g. aspartate
aminotransferase has a tyrosine residue in its active
site (McPhalen et al. 1992). We infer that sarin
influenced the assessed markers by an inhibitory
mechanism. This should be taken into account when
treating human subjects accidentally exposed to sarin.
On the other hand, the unexpected decrease in some
biochemical markers can serve as a novel marker for
clinical toxicologists due to the fact that the decrease
is specific for the poisoning. However, experiment
focusing on diagnosis should be carried out before
considering it as a marker. Unlike the other
biochemical markers, glucose and albumin levels
increased in plasma. A description of the effect of
sarin on the albumin level has not been found in
several searched databases. An increase in glucose
can be attributed to stress conditions. This
phenomenon is in compliance with searched literature
(Tiruvoipati et al. 2011). Hyperglycaemia and
glycosuria were also reported to be pathological
consequences of accidental exposure to organo-phosphorus and carbamate pesticides (Shobha and
Prakash 2000).
Alterations in oxidative homeostasis were found
to extensively overweigh the inhibition of AChE. The
TBARS level indicates lipid peroxidation by the
detection of low molecular weight antioxidants as
they respond to a degradation product of lipids,
malondialdehyde and can be interpreted as a lack of
antioxidants for oxidative stress covering (Lykkesfeldt 2007, Pohanka 2011b). In the spleen, the
significant accumulation of TBARS was accompanied
by a depletion in low molecular weight antioxidants
represented by FRAP. This means that the organ was
more vulnerable to oxidative stress induced by sarin.
The central nervous system, a part of the body
extensively influenced by sarin, seems to be resistant
to oxidative insult as no extensive increase in TBARS
or depletion in low molecular weight antioxidants was
found. On the contrary, reduced glutathione was
found to be accumulated in the frontal lobe, even
though no significant increase in glutathione
reductase, the enzyme which reduces glutathione
from the oxidised state (Lowes and Galley 2011), was
seen.
Our findings show that sarin alone has only a
limited effect on oxidative stress and correspond with
the study by Abu-Qare and Abou-Donia (2001) where
a small effect of sarin on the oxidation of DNA
represented by 8-hydroxy-2´-deoxyguanosine was
proven. It seems to be more plausible that sarin
induces alterations in oxidative homeostasis and
regulation rather than a simple implication in reactive
oxygen species generation. The long-term adverse
effects of sarin, e.g. reported in a rat model by Allon
et al. (2011), are a pathological consequence of
exposure. The long-term effects remain even after the
reconstitution of new cholinesterase molecules, as the
approximate half-life of butyrylcholinesterase is
12 days (Ostergaard et al. 1988). In the central
nervous system of rats, new mRNA for AChE is
created shortly after exposure to 0.5% of the LD50 of
sarin (Damodaran et al. 2003). We have shown that
alterations in oxidative homeostasis are seen
simultaneously with AChE inhibition, and this starts
early after exposure. Owing to the results of this
study, we infer the changes in oxidative homeostasis
to be a relevant consequence following sarin
exposure. On the other hand, direct induction of
oxidative stress was proven only in the spleen, and
the central nervous system seems to be resistant to
sarin-induced oxidative insult. When considering the
present findings, sarin causes the body to be more
vulnerable to other adverse effects once oxidative
homeostasis is impaired. This conclusion is in
compliance with the results presented by Abu-Qare
and Abou-Donia (2001).
ACKNOWLEDGEMENTS
The Faculty of Military Health Sciences, University
of Defence is gratefully acknowledged for the specific
research project "Assay of biochemical and
pharmacokinetic parameters of selected inhibitors of
acetylcholinesterase" and institutional research: A
long-term organization development plan 1011
(Ministry of Education, Youth and Sport of Czech
Republic) is gratefully acknowledged.
REFERENCES
Abu-Qare AW, Abou-Donia MB. Combined exposure to sarin and pyridostigmine bromide increase levels of rat urinary 3-nitrotyrosine and 8-hydroxy-2´-deoxyguanosine, biomarkers of oxidative stress. Toxicol Lett. 123: 51-58, 2001.
[CrossRef]
Abu-Qare AW, Abou-Donia MB. Sarin: health effects, metabolism, and methods of analysis. Food Chem Toxicol. 40: 1327-1333, 2002.
[CrossRef]
Allon N, Chapman S, Egozi I, Rabinovitz I, Kapon J, Weissman BA, Yacov G, Bloch-Shilderman E, Grauer E. Deterioration in brain and heart functions following a single sub-lethal (0.8 LCt50) inhalation exposure of rats to sarin vapor: a putative mechanism of the long term toxicity. Toxicol Appl Pharmacol. 253: 31-37, 2011.
[CrossRef]
[PubMed]
Brimblecomb RW, Green DM, Stratton JA, Thompson PB. The protective actions of some anticholinergic drugs in sarin poisoning. Brit J Pharmacol. 39: 822-830, 1970.
[PubMed]
Damodaran TV, Jones KH, Patel AG, Abou-Donia MB. Sarin (nerve agent GB)-induced differential expression of mRNA coding for the acetylcholinesterase gene in the rat central nervous system. Biochem Pharmacol. 65: 2041-2047, 2003.
[CrossRef]
Kassa J, Cabal J. A comparison of the efficacy of a new asymmetric bispyridinium oxime BI-6 with presently used oximes and H oximes against sarin by in vitro and in vivo methods. Hum Exp Toxicol. 18: 560-565, 1999.
[CrossRef]
Li B, Schopfer LM, Grigoryan H, Thompson CM, Hinrichs SH, Hinrichs SH, Masson P, Lockridge O. Tyrosines of human and mouse transferrin covalently labeled by organophosphorus agents: a new motif for binding to proteins that have no acitve site serine. Toxicol Sci. 107: 144-155, 2009.
[CrossRef]
[PubMed]
Lowes DA, Galley HF. Mitochondrial protection by the thioredoxin-2 and glutathione systems in an in vitro endothelial model of sepsis. Biochem J. 436: 123-132, 2011.
[CrossRef]
[PubMed]
Lykkesfeldt J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin Chim Acta. 380: 50-58, 2007.
[CrossRef]
[PubMed]
Marrs TC. Organophosphate poisoning. Pharmacol Therapy. 58: 51-66, 1993.
[CrossRef]
McPhalen CA, Vincent MG, Jansonius JN. X-ray structure refinement and comparison of three forms of mitochondrial aspartate aminotransferase. J Mol Biol. 225: 495-517, 1992.
[CrossRef]
Niven AS, Roop SA. Inhalational exposure to nerve agents. Respir Care Clin N Am. 10: 59-74, 2004.
[CrossRef]
Ostergaard D, Viby-Moogensen J, Hanel HK, Skovgaard LT. Half-life of plasma cholinesterase. Acta Anaesthesiol Scand. 32: 266-269, 1988.
[CrossRef]
Pohanka M. Cholinesterases, a target of pharmacology and toxicology. Biomed Pap Olomouc. 155: 219-223, 2011a.
[CrossRef]
Pohanka M. Alzheimer's disease and related neurodegenerative disorders: implication and counteracting of melatonin. J Appl Biomed. 9: 185-196, 2011b.
[CrossRef]
[JAB]
Pohanka M, Sobotka J, Jilkova M, Stetina R. Oxidative stress after sulfur mustard intoxication and its reduction by melatonin: efficacy of antioxidant therapy during serious intoxication. Drug Chem Toxicol. 34: 85-91, 2010.
[CrossRef]
[PubMed]
Pohanka M, Sobotka J, Svobodova H, Stetina R. Investigation of oxidative stress in blood, brain, kidney, and liver after oxime antidote HI-6 application in a mouse experimental model. Drug Chem Toxicol. 34: 255-260, 2011a.
[CrossRef]
[PubMed]
Pohanka M, Novotny L, Pikula J. Metrifonate alters antioxidant levels and caspase activity in cerebral cortex of Wistar rats. Toxicol Mech Meth. 21: 585-590, 2011b.
[CrossRef]
[PubMed]
Proctor SP, Heaton KJ, Heeren T, White RF. Effects of sarin and cyclosarin exposure during the 1991 Gulf War on neurobehavioral functioning in US army veterans. Neurotoxicology. 27: 931-939, 2006.
[CrossRef]
[PubMed]
Schopfer LM, Grigoryan H, Li B, Nachon F, Masson P, Lockridge O. Mass spectral characterization of organophosphate-labeled, tyrosine-containing peptides: characteristic mass fragments and a new binding motif for organophosphates. J Chromatogr B Analyt Technol Biomed Life Sci. 878: 1297-1311, 2010.
[CrossRef]
[PubMed]
Shobha TR, Prakash O. Glycosuria in organophosphate and carbamate poisoning. J Assoc Physicians India. 48: 1197-1199, 2000.
[PubMed]
Tiruvoipati R, Chiezey B, Lewis D, Ong K, Villanueva E, Haji K, Botha J. Stress hyperglycemia may not be harmful in critically ill patients with sepsis. J Crit Care. 27: 153-158, 2011.
[CrossRef]
[PubMed]
Wang Y, Weiss MT, Yin J, Tenn CC, Nelson PD, Mikler JR. Protective effects of N-methyl-D-aspartate receptor antagonism on VX-induced neuronal cell death in cultured rat cortical neurons. Neurotox Res. 13: 163-172, 2008.
[CrossRef]
[PubMed]
Yanagisawa N, Morita H, Nakajima T. Sarin experiences in Japan: acute toxicity and long-term effects. J Neurol Sci. 249: 76-85, 2006.
[CrossRef]
[PubMed]
|
BACK
|





