Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 10 (2012), No 4, p 169-176
DOI 10.2478/v10136-012-0013-z
Expression of Mycobacterium tuberculosis proteins MPT63 and MPT83 as a fusion: purification, refolding and immunological characterization
Taras Redchuk, Natalia Korotkevich, Oksana Gorbatiuk, Pavlo Gilchuk, Andrii Kaberniuk, Olena Oliynyk, Denis Kolibo, Serhiy Komisarenko
Address: Taras Redchuk, Palladin Institute of Biochemistry (NASU), 9 Leontovicha Street, Kyiv, 01601, Ukraine
rtakyiv@gmail.com
Received 3rd January 2012.
Revised 20th February 2012.
Published online 22nd February 2012.
Full text article (pdf)
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
Acknowledgements
References
SUMMARY
Proteins MPT63 and MPT83 which are common for both Mycobacterium tuberculosis and Mycobacterium bovis, due to their high immunogenicity, are thought to play a promising role in the development of immunodiagnostic reagents and vaccines. To enhance the antigenic and immunogenic properties of these proteins, fragments of the mpt83 and mpt63 genes were fused in tandem. In this article we present an effective method for the MPT63-MPT83 fusion product purification by metal-affinity chromatography and in vitro refolding. Our results demonstrate that the antigenic properties of the recombinant proteins obtained are comparable to their native analogues. The anti-rMPT63 and anti-rMPT83 sera were found to be highly reactive against the rMPT63-MPT83 fusion protein, which suggests that the fusion protein retains the antigenic properties of the parent proteins. Our results may potentially contribute to the development of improved diagnostic tools or vaccines against human and/or cattle tuberculosis.
KEY WORDS
tuberculosis; Mycobacterium tuberculosis; MPT63; MPT83; fusion; antigen
INTRODUCTION
Tuberculosis in humans (TB) still remains one of the
most dangerous infectious diseases, killing near two
million people every year. This stems partially from
the imperfection of the available immunological
diagnostic methods, prevention, and treatment of TB,
despite the availability of the complete Mycobacterium tuberculosis genome sequence, which has
opened new ways for the identification and
characterisation of new antigenic targets (Fleischmann et al. 2002). The vast majority of contemporary
TB serological tests have been designed using
antigens specific for M. tuberculosis. However, single
antigen-based test systems have been shown to have
insufficient sensitivity and/or specificity, while
combinations of several antigens might essentially
improve the properties of such test-systems (Raja et
al. 2008). A particularly notable improvement may be
expected from combinations of secreted and
cell-associated antigens, as both these types of
antigen often become targets for immune response
and may play an important role in the pathogenesis of
infectious deseases.
In TB, there are two mycobacterial proteins that
seem to be possible candidates for the development of
complex-based test systems and vaccines. The
conserved MPT63 (Rv1926) and MPT83 (Rv2873)
proteins are found in species of the M. tuberculosis
complex (MTC), including virulent M. tuberculosis,
Mycobacterium africanum, Mycobacterium bovis, and
attenuated M. bovis BCG (sequences are identical for
all these species). MPT63 is one of the most abundant
secreted proteins of M. bovis BCG strains, as well as
pathogenic M. bovis/tuberculosis (Nagai et al. 1991).
Being uniquely specific to the M. tuberculosis
complex, MPT63 is a plausible candidate for
M. tuberculosis complex-specific diagnostics. Like
other secreted proteins of Mycobacterium (Horwitz et
al. 1995), MPT63 is a target for the immune response
in the infected host and can be important for TB
pathogenesis. Indeed some mycobacterial secreted
proteins appear to play an essential role in the
inhibition of phagosomal maturation during
phagocytosis and, thus, contribute to M. tuberculosis
survival and proliferation inside host macrophages
(Clemens and Horwitz 1995).
The structural similarity of MPT63 with
immunoglobulin folds and cell surface-binding
proteins (Goulding et al. 2002) hints at its
involvement in cell-host interactions and the ability to
affect phagocytosis during bacterial internalisation.
This could potentially explain the protective
properties of anti-MPT63 immune responses. For
instance, a DNA vaccine encoding both the MPT63
and ESAT-6 antigens has been demonstrated to
induce a strong protective response (McShane et al.
2001). The immunogenic properties of MPT63 could
be inferred from the high density of T-epitopes in its
N-terminal immunodominant region (Lee and
Horwitz 1999). Therefore, MPT63 has been proposed
as a target for vaccine design and diagnostic tools
development (Horwitz et al. 1995).
Another potential target antigen, MPT83, is a
highly immunogenic mycobacterial lipoprotein,
which has been identified as a cell surface-associated
antigen by electron microscopy and flow-cytometry
(Vosloo et al. 1997, Harboe et al. 1998). It has been
demonstrated that monoclonal antibodies against the
MPB83 surface antigen of M. bovis (an MPT83
homolog) increase the survival time of infected mice
and change the morphology of granulomas and the
distribution of acid-fast bacilli in the lungs (Chambers
et al. 2004). Vaccination with DNA or RNA
constructs expressing the M. tuberculosis MPT83
antigen are also capable of inducing both humoral
(Green et al. 2009) and T-cell immune responses
(Xue et al. 2004). Besides, the MPT83-encoding
DNA vaccine reduces M. bovis dissemination in a
mouse model and better protects kidneys against
infection than does BCG (Chambers et al. 2001).
The abovementioned suggests that a combination
of MPT63 and MPT83 proteins may be potentially
interesting for the development of TB
immunodiagnostic reagents and vaccines. To explore
this opportunity, we: (a) fused the antigen fragments
of the genes mpt83 and mpt63 in tandem and
expressed the construct in Escherichia coli; (b)
isolated all target proteins from bacterial cells in a
purified and soluble form, and (c) tested these
proteins for their capacity to induce specific
polyclonal antibodies that normally recognize their
native analogues.
MATERIALS AND METHODS
PCR amplification and cloning
Chromosomal DNA of M. bovis (BCG) was isolated
by the freeze-boil method and was used as a template
for PCR amplification. mpt83 DNA was amplified
with following primers: #1 5'-GGA TCC GAC ACC
CTC AAC GGC GGC GAG-3' and #2 5'-CTC GAG
CTG TGC CGG TGG CAT CAG TAC C-3'; mpt63
was amplified by nested PCR. The first set of primers
was: #3 5'-AGG GAC CAA TGA AGC TCA-3' and
#4 5'-TCT ACG GCT CCC AAA TCA-3'. The
nested primers were: #5 5'-CTG AGG ATC CAT
GAA GCT CAC CAC AAT GAT-3'and #6 5'-TCA
GCT CGA GCG GCT CCC AAA TCA GCA
GAT-3'. BamHI and XhoI restriction sites are
underlined. PCR was performed with the following
settings: 94 °C/1.5 min followed by 30 cycles
(94 °C/30 s, 52 °C/45 s, and 72 °C/45 s), and final
elongation 72 °C/7 min.
Splicing by the overlap extension PCR procedure
(SOE-PCR) was used to obtain a fused gene
containing the mpt83 and mpt63 gene fragments.
Extensions needed for splicing were added by an
additional PCR step with the 5' ATC ATT GTG GTG
AGC TTC ATC TGT GCC GGT GGC ATC AGT
ACC 3' primer instead of #2 for the mpt83 gene
fragment. The primer 5' GGT ACT GAT GCC ACC
GGC ACA GAT GAA GCT CAC CAC AAT GAT 3'
was used instead of #5 to extend the mpt63 gene
fragment. The obtained products were used for
splicing via 5 elongation cycles 95 °C/15 s,
55 °C/3 min, and 72 °C/40 s.
After the splicing procedure, the 5' GGA TCC
GAC ACC CTC AAC GGC GGC GAG 3' and 5'
TCA GCT CGA GCG GCT CCC AAA TCA GCA
GAT 3' primers were added and a standard PCR
scheme was performed (10 cycles 94 °C/30 s,
52 °C/45 s, and 72 °C/45 s).
The mpt63, mpt83, and mpt63-mpt83 amplified
DNA fragments were ligated with the E. coli
expression vector pET24a(+) (Novagen) using BamHI
and XhoI sites. The resulting plasmid was used to
transform E. coli Rosetta (DE3) host cells (Novagen)
for expression.
Fermentation and preparation of inclusion bodies
For protein expression, a modified auto-induction
protocol originally described by Studier (2005) was
applied as follows: E. coli BL21(DE3) cells
harbouring the required plasmid were incubated at
37 °C overnight in 2 ml of 2xYT medium containing
50 ug/ml kanamycin and 1% glucose. 1:1000
dilutions of the overnight culture were used to
inoculate 2 ml (small-scale expression) or 400 ml
(large-scale expression) fresh 2xYT medium
containing 50 ug/ml kanamycin, 25 mM (NH4)2SO4,
50 mM KH2PO4, 50 mM Na2HPO4, 1 mM MgSO4,
0.05% glucose, 0.2% alpha-lactose, 0.5% glycerol. The
cultures were allowed to express for 18-24 h at 37 °C
before the cells were harvested by centrifugation at
3000 g for 20 min. Final optical densities (OD600)
were typically within the range 9.0 to 18.0, depending
on induction time and expressed protein. Bacteria
were lysed on ice with 10 mM Tris-HCl buffer pH 8.0
(10 ml per 1 g w/w cells) containing 1 mg/ml chicken
egg lysozyme (Fluka), 5 mM MgSO4, DNase I
(50 ug/g cell paste) for 20 min. Cell lysates were
sonicated in the presence of 0.3% sodium
deoxycholate (SDC) and precipitated by spinning at
10,000 g for 20 min. Insoluble material was washed
once via sonication with Tris buffer without SDC,
pelleted by centrifugation and stored at -70 °C.
Proteins were separated by 12% SDS-PAGE. The
aliquots of supernatants and pellets were sampled and
mixed with electrophoresis loading buffer consisting
of 7% (w/v) SDS, 40% (v/v) glycerol, 0.25 M
Tris-HCl (pH 6.8), 0.0005% (w/v) bromophenol blue
and 100 mM DTT for SDS-PAGE analysis. Gels were
stained with Coomassie Brilliant Blue R250. Protein
concentrations were measured by densitometry using BSA iof known concentration as protein standard.
On-column purification and refolding of recombinant proteins
Pelleted inclusion bodies were solubilized in 20 mM
Tris-HCl (pH 8.0) containing 6 M guanidine-HCl,
100 mM Na2HPO4, 10 mM imidazole and 10 mM
2-mercaptoethanol for 1 h at room temperature and
filtered through a 0.45 um PVDF membrane filter
(Millipore). All solutions were filtered through
0.22 um nitrocellulose membrane filters (Millipore)
and degassed prior to chromatography. For
small-scale optimization experiments, a 1 ml HiTrap
chelating column loaded with Ni2+ ions was
connected to an FPLC system (Pharmacia) and
equilibrated with PBS buffer (pH 8.0) containing 8 M
urea and 10 mM imidazole (pump A) with the flow
rate of 0.5 ml/min. A 1 ml sample of solubilized
inclusion bodies (2 mg/ml) was applied from the
connected P1 pump (GE Helthcare) with the flow rate
of 0.2 ml/min, and the column was washed with the
equilibration buffer from the FPLC pump A until the
UV signal reached the base line. On-column refolding
was performed as follows. A 15-ml linear gradient
from the urea-containing buffer (pump A) to the
refolding buffer (PBS, pH 8.0 with 10 mM imidazole)
without urea (pump B) was applied with the flow rate
of 0.2 ml/min. The refolded protein was eluted in the
refolding buffer containing 0.3 M imidazole. For
large-scale refolding experiments, a 26/20 XK
column (GE Healthcare) packed with 20 ml of
Ni-NTA Superflow medium (Qiagen) was connected
to FPLC and refolding was performed using the
reagent system described above in a 200-ml linear
gradient of urea with the flow rate of 2.7 ml/min. The
effect of 50, 300, 500 mM imidazole in the elution
buffer on the recovery of refolded protein from the
column was investigated. All fractions were collected
and separated by SDS-PAGE. The purity of refolded
proteins and refolding yields were determined as
described above. The refolding yield was calculated
as the percentage of soluble target protein recovered
from inclusion bodies after refolding.
Production of rabbit antiserum against Mycobacterium antigens
Rabbit polyclonal antisera were prepared by
immunizing rabbits with rMPT63 and rMPT83. With
the first injection each animal received approximately
300 microg of protein mixed with complete Freund's
adjuvant (CFA, Sigma). Incomplete Freund's
adjuvant (IFA, Sigma) with the same amount of
protein was used for subsequent immunizations. The
mix was injected thrice at three intradermal sites and
once intramuscularly after a 15 days interval. Blood
samples were collected on the 7th day after the last
immunization.
Immunoblotting
Approximately 1 ug of each purified protein was used
for SDS-PAGE. Protein transfer from acrylamide gels
to nitrocellulose membranes Hybond-C Extra (GE
Helthcare, USA) was performed using the semi-dry
method. The membrane was blocked in PBS
containing 5% non-fat dry milk for 1 h at 37 °C
before the reaction with antiserum. The reaction with
rabbit polyclonal antibodies (dilution 1:100 in PBS
with 0.1% Tween) lasted for 1 h at 37 °C. Horse radish peroxidase conjugated with goat anti-rabbit
immunoglobulin G (Sigma Aldrich) was used as a
secondary antibody. Identification of target proteins
was verified by detecting the same bands with
Monoclonal Anti-polyHistidine-Peroxidase antibodies
(Sigma Aldrich). The antibody reactivity was
visualized with the diaminobenzidine.
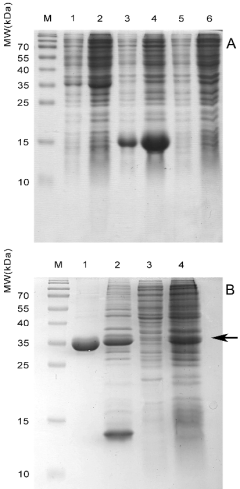
Fig. 1. (A) Small-scale expression of the recombinant
proteins rMPT63, rMPT83, and rMPT63-MPT83 fusion
on auto-induction medium. Lanes 1, 2 - soluble and
insoluble protein fractions from induced BL21(DE3) cells
carrying the plasmid pET-mpt63-mpt83. Lanes 3, 4 -
soluble and insoluble protein fractions from induced
BL21(DE3) cells carrying the plasmid pET-mpt83. Lanes
5, 6 - soluble and insoluble protein fractions from induced
BL21(DE3) cells carrying the plasmid pET-mpt63. The
amount of protein corresponding to 45 ul of induced E. coli
culture was loaded in each lane. (B) Expression and
purification of the rMPT63-MPT83 fusion protein. Lane 1
- purified and soluble rMPT63-MPT83 recovered from
inclusion bodies by on-column refolding. Lane 2 - isolated
and solubilized inclusion bodies that were used in
refolding. Lane 3 - cytoplasmic soluble fraction. Lane 4 -
total proteins of E. coli overproducing rMPT63-MPT83 in
large-scale experiments. The amount of protein
corresponding to 20-40 ul of induced E. coli culture was
loaded in each lane. M - molecular weight marker. The
arrow marks the position of the rMPT63-MPT83 fusion
protein.
RESULTS
Design of recombinant proteins
Fragments of 497 (mpt63 fragment) and 357 (mpt83
fragment) base pairs were prepared by PCR
amplification from M. bovis BCG (Russia)
chromosomal DNA. The amplified fragment of mpt63
corresponded to full-length CDS of the mpt63 gene.
The fragment amplified from mpt83 corresponded to
the sequence encoding 115-220 amino acids of the
Fasciclin-like domain (94-217aa) of the MPT83
protein. The fragment of 811 bp was obtained using
SOE-PCR. Purified amplicons were used to create
recombinant plasmids for expression of the target
proteins. Thus, the MPT83 sequence was positioned
in the gene of the predicted fusion protein sequence
after T7 tag residues introduced from the pET24a
vector DNA. A PolyHis tag was added from the
pET24a vector at the C terminus for the following
metal affinity purification. Sequencing of cloned
DNA showed that the fragments had been correctly
inserted and the sequences were identical to those
published (Accession No. NC_008769). Expression
was achieved using standard procedures and followed
by one-step metal-affinity chromatography
purification. The observed molecular masses of the
products obtained (16.0 kDa, 17.6 kDa, and
33.55 kDa for rMPT63, rMPT83, and
rMPT63-MPT83 fusion protein) corresponded to
those predicted (19 kDa, 14.5 kDa and 31 kDa,
respectively).
Expression, on-column purification and refolding of recombinant proteins
Initially, expression level optimization was performed
following the kit manufacturer's recommendations
(Novagen). It was found, however, that the expression
strain, induction temperature, time of incubation, and
IPTG concentration were not significant for the
expression in standard experimental conditions. To
enhance the expression, we decided to use the method
of protein production by auto-induction in high
density shaking cultures (as described in Materials
and methods).
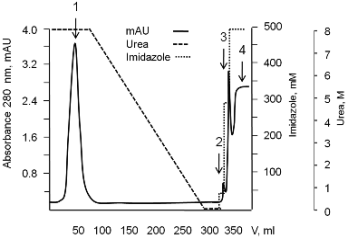
Fig. 2. Chromatogram of the purification and refolding process on 26/20 XK column packed with 20 ml of Ni-NTA
Superflow resin (the rMPT63-MPT83 fusion protein as an example). Solubilized inclusion bodies were applied to the column
loaded with a buffer containing urea, non-specifically bound proteins were washed out with the same buffer, and on-column
refolding was carried out as described in Materials and methods. 1 - proteins flowing through the column and washed with the
buffer containing urea; 2-4 - refolded protein eluted from the column with 50, 300, and 500 mM imidazole, respectively.
It was shown that most of the expressed
rMPT63-MPT83 fusion protein and rMPT83
accumulated as insoluble inclusion bodies (Fig. 1A).
We utilized the matrix-assistant refolding method to
obtain soluble and purified rMPT63-MPT83 fusion
protein. Solubilized under denaturing conditions the
protein was loaded into a metal affinity column and
refolded in a decreasing urea gradient using an
automated FPLC chromatography system as
described in "Materials and methods" (Fig. 2). The
purity of rMPT63-MPT83 after the refolding was
more than 90% based on gel densitometry data
(Fig. 1B). All the relevant data on the purification and
refolding experiments are summarized in Table 1.
Serological characterization of the obtained rMPT63 and rMPT83
The next stage of our investigation was serological
characterization of the obtained proteins: rMPT63,
rMPT83, and rMPT63-MPT83 fusion. Purified
rMPT63 and rMPT83 were used to obtain polyclonal
antiserum in rabbits. The specificity of the obtained
sera was tested by a series of Western blots. Their
reactivity with the antigen used for immunization was
strong in all cases. The same bands were detected
with monoclonal anti-polyhistidine-peroxidase
antibodies (Fig. 3A).
To compare the antigenic properties of the
obtained recombinant analogs with proteins isolated
directly from bacterial cells we screened M. bovis
BCG cell lysates and PPD for the presence of MPT63
and MPT83 (Fig. 3B).
A band of approximately 15 kDa (which
corresponds to the molecular weight of native
MPT63) was determined in a PPD sample incubated
with an anti-rMPT63 serum. This band was absent
from the lysate of lyophilized BCG perhaps because
of its active secretion outside the bacterial cell. This
data corroborates the thesis that MPT63 can be
detected in the culture liquid but not in whole-cell
extracts (Nagai et al. 1991).
In our experiments anti-MPT83 sera revealed a
band with elecrophoretic mobility which
corresponded to approx. 25 kDa in BCG cell lysate.
According to existing data the observed weight is
clearly indicative of native MPT83 (Charlet et al.
2005). The diffused band in PPD filtrate may have
been caused by unspecific binding of polyclonal
antibodies with degraded proteins from PPD. No
antigen recognition was observed with normal animal
and control animal (adjuvant administered without
protein) sera.
Serological characterization of the obtained rMPT63-rMPT83 fusion
To determine whether the fusion protein possesses the
antigenic properties of MPT63 and MPT83, it was
tested by both anti-rMPT63 and anti-rMPT83 sera.
The anti-polyhistidine monoclonal antibodies were
used to identify the recombinant product bands in Western blots. In all three assays (with anti-polyhistidine antibodies, anti-rMPT63, and
anti-rMPT83 sera) strong bands in the 30 kDa marker
area were detected (Fig. 3A).
Table 1. Comparison of expression, purification, and refolding of the recombinant proteins rMPT-63, rMPT-83, and rMPT63-MPT83 (data represent one from two/three independent experiments; large-scale expression protocol was used).
|
rMPT-63 |
rMPT-83 |
rMPT63-MPT83 | | Production yield (mg/l of E. coli) |
<20 |
670 |
271 | | Expression level from total E. coli proteins, % |
not determined |
49 |
13 | | Final OD600 of induced bacterial culture |
10.2 |
19.2 |
13.4 | | Recovery after isolation of inclusion bodies, % |
not determined |
82 |
95 | | Purity in inclusion bodies, % |
not determined |
83 |
27 | | Recovery from inclusion bodies after purification and refolding, % |
not determined |
96 |
92 | | Purity after refolding, % |
not determined |
95 |
93 | | Recovery of refolded protein from induced bacterial culture, % |
not determined |
79 |
87 |
DISCUSSION
One of the key challenges facing manufacturers of the
vaccines and diagnostic tools is the production of
pathogen proteins. The manufacturing process can be
very complex and even dangerous. Some of these
problems were resolved with the invention of
pathogen antigen production in non-pathogenic
heterologous expression systems. However this
powerful approach could be sometimes problematic
because some proteins are very difficult to express in
E. coli, including several proteins of M. tuberculosis.
Nevertheless, expression as a fusion protein may
increase the expression level of the "hard-to-obtain"
recombinant protein in the E. coli expression system
and the overall production yield (Mukherjee et al.
2003).
Comparison of the expression levels of rMPT63,
rMPT83, and the rMPT63-MPT83 fusion protein has
revealed a dramatically increased expression of
MPT63 as part of the rMPT63-MPT83 fusion protein.
In particular, the band corresponding rMPT63 protein
was undetectable by SDS-PAGE analysis of the
rMPT63 overexpressed cell lysates. Based on the
sensitivity of SDS-PAGE, we have concluded that
protein concentration is less than 20 mg per 1L of
E. coli culture. At the same time, accumulation of the
rMPT63-MPT83 fusion protein was approximately
13% of total E. coli proteins under optimal
conditions, which corresponded to 271 mg per 1L of
E. coli culture.
Expression of recombinant proteins as inclusion
bodies is a common method because of the high
quantity of the target protein in the total extracts, low
toxic influence on the host strain, and potential proper
protein folding by in vitro refolding procedures (Li et
al. 2004). A number of approaches for the recovery of
soluble proteins from inclusion bodies have been
proposed (Clark 2001, Vallejo and Rinas 2004), but
to date refolding still remains an empirical process,
which needs to be optimized in each individual caseto achieve reasonable yields of the protein in its
functional form (Tsumoto et al. 2003). In the present
study we utilize the matrix-assisted refolding method
to obtain a soluble and purified rMPT63-MPT83
fusion protein.
The on-column approach combined the
purification and refolding stages and allowed us to
obtain the target protein in multi-milligram quantities
using a scalable and cost effective procedure. Thus,
we developed an efficient method for obtaining a
soluble and purified form of the fusion protein
rMPT63-MPT83.
Another common problem of the recombinant
vaccine production is a misfolding of the proteins,
preventing their potential therapeutic use. A growing
body of evidence suggests that each novel vaccine
and diagnostic tool needs to be thoroughly analysed
for its conformational correspondence with the native analogs (see for an example Song et al. 2008). While
the biological function of MPT63 and MPT83
remains unknown, the only method for predicting
native protein folding is immunological characterization.
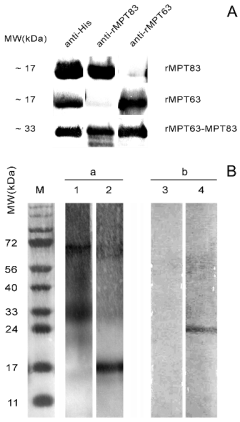
Fig. 3. (A) Western blot analysis of rMPT83, rMPT63,
and rMPT63-MPT83. anti-His - anti-polyhistidine-peroxidase antibodies; anti-rMPT83 - rabbit polyclonal anti-
rMPT83 sera. anti-rMPT63 - rabbit polyclonal anti-
rMPT63 sera. (B) Immunoblotting analysis of PPD (a) and
BCG Russia cell lysate (b). Lanes 1 and 4 - rabbit polyclonal anti-rMPT83 sera. Lanes 2 and 3 - rabbit polyclonal
anti-rMPT63 sera. M - molecular weight marker.
We have detected a strong reactivity of
anti-rMPT63 and anti-rMPT83 antibodies with their
parent proteins from BCG lysate (MPT83) and
M. tuberculosis culture filtrate (MPT63). These
results show the ability of recombinant proteins to
effectively induce production of polyclonal antibodies
that can recognize M. tuberculosis antigens.
Therefore, our results demonstrate a similarity
between the antigenic properties of the obtained
recombinant proteins and their native analogues that
is the biggest challenge facing the development of
vaccines and diagnostic tools.
Moreover, the results obtained with anti-rMPT63
and anti-rMPT83 sera also suggested that antibodies
against native MPT63 and MPT83 could be detected
using the recombinant fusion protein rMPT63-
MPT83.
In summary, our results may potentially create a
basis for further development of novel diagnostic tool
or subunit vaccines against human and cattle
tuberculosis.
ACKNOWLEDGEMENTS
We are grateful to Yevdokimova NYu, Rozhok AI
and Dubrovska AN for critical review of the manuscript.
REFERENCES
Chambers MA, Stagg D, Gavier-Widen D, Lowrie D, Newell D, Hewinson RG. A DNA vaccine encoding MPB83 from Mycobacterium bovis reduces M. bovis dissemination to the kidneys of mice and is expressed in primary cell cultures of the European badger (Meles meles). Res Vet Sci. 71: 119-126, 2001.
[CrossRef]
[PubMed]
Chambers MA, Gavier-Widen D, Hewinson RG. Antibody bound to the surface antigen MPB83 of Mycobacterium bovis enhances survival against high dose and low dose challenge. FEMS Immunol Med Microbiol. 41: 93-100, 2004.
[CrossRef]
Charlet D, Mostowy S, Alexander D, Sit L, Wiker HG, Behr MA. Reduced expression of antigenic proteins MPB70 and MPB83 in Mycobacterium bovis BCG strains due to a start codon mutation in sigK. Mol Microbiol. 56: 1302-1313, 2005.
[CrossRef]
[PubMed]
Clark ED. Protein refolding for industrial processes. Curr Opin Biotechnol. 12: 202-207, 2001.
[CrossRef]
Clemens DL, Horwitz MA. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 181: 257-270, 1995.
[CrossRef]
[PubMed]
Fleischmann RD, Alland D, Eisen JA, Carpenter L, White O, Peterson J, DeBoy R, Dodson R, Gwinn M, Haft D, Hickey E, Kolonay JF et al. Whole-genome comparison of Mycobacterium tuberculosis clinical and laboratory strains. J Bacteriol. 184: 5479-5490, 2002.
[CrossRef]
[PubMed]
Goulding CW, Parseghian A, Sawaya MR, Cascio D, Apostol MI, Gennaro ML, Eisenberg D. Crystal structure of a major secreted protein of Mycobacterium tuberculosis-MPT63 at 1.5-A resolution. Protein Sci. 11: 2887-2893, 2002.
[CrossRef]
[PubMed]
Green LR, Jones CC, Sherwood AL, Garkavi IV, Cangelosi GA, Thacker TC, Palmer MV, Waters WR, Rathe CV. Single-antigen serological testing for bovine tuberculosis. Clin Vaccine Immunol. 16: 1309-1313, 2009.
[CrossRef]
[PubMed]
Harboe M, Wiker HG, Ulvund G, Lund-Pedersen B, Andersen AB, Hewinson RG, Nagai S. MPB70 and MPB83 as indicators of protein localization in mycobacterial cells. Infect Immun. 66: 289-296, 1998.
[PubMed]
Horwitz MA, Lee BW, Dillon BJ, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 92: 1530-1534, 1995.
[CrossRef]
Lee BY, Horwitz MA. T-cell epitope mapping of the three most abundant extracellular proteins of Mycobacterium tuberculosis in outbred guinea pigs. Infect Immun. 67: 2665-2670, 1999.
[PubMed]
Li M, Su ZG, Janson JC. In vitro protein refolding by chromatographic procedures. Protein Expr Purif. 33: 1-10, 2004.
[CrossRef]
[PubMed]
McShane H, Brookes R, Gilbert SC, Hill AV. Enhanced immunogenicity of CD4(+) T-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect Immun. 69: 681-686, 2001.
[CrossRef]
[PubMed]
Mukherjee S, Daifalla N, Liu C, Campos-Neto A. Alternative approach to express Mycobacterium tuberculosis proteins in Escherichia coli. Biotechniques. 35: 34-36, 2003.
[PubMed]
Nagai S, Wiker HG, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 59: 372-382, 1991.
[PubMed]
Raja A, Ranganathan UD, Bethunaickan R. Improved diagnosis of pulmonary tuberculosis by detection of antibodies against multiple Mycobacterium tuberculosis antigens. Diagn Microbiol Infect Dis. 60: 361-368, 2008.
[CrossRef]
[PubMed]
Song L, Nakaar V, Kavita U, Price A, Huleatt J, Tang J, Jacobs A, Liu G, Huang Y, Desai P, Maksymiuk G, Takahashi V et al. Efficacious recombinant influenza vaccines produced by high yield bacterial expression: a solution to global pandemic and seasonal needs. PLoS One. 3: e2257, 2008.
[CrossRef]
[PubMed]
Studier FW. Protein production by auto-induction in high-density shaking cultures. Protein Expr Purif. 41: 207-234, 2005.
[CrossRef]
[PubMed]
Tsumoto K, Ejima D, Kumagai I, Arakawa T. Practical considerations in refolding proteins from inclusion bodies. Protein Expr Purif. 28: 1-8, 2003.
[CrossRef]
Vallejo LF, Rinas U. Strategies for the recovery of active proteins through refolding of bacterial inclusion body proteins. Microb Cell Fact. 3: 11, 2004.
[CrossRef]
[PubMed]
Vosloo W, Tippoo P, Hughes JE, Harriman N, Emms M, Beatty DW, Zappe H, Steyn LM. Characterisation of a lipoprotein in Mycobacterium bovis (BCG) with sequence similarity to the secreted protein MPB70. Gene. 188: 123-128, 1997.
[CrossRef]
Xue T, Stavropoulos E, Yang M, Ragno S, Vordermeier M, Chambers M, Hewinson G, Lowrie DB, Colston MJ, Tascon RE. RNA encoding the MPT83 antigen induces protective immune responses against Mycobacterium tuberculosis infection. Infect Immun. 72: 6324-6329, 2004.
[CrossRef]
[PubMed]
|
BACK
|




