Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 11 (2013), No 1, p 15-26
DOI 10.2478/v10136-012-0014-y
Antiparasitic effects of Zingiber officinale (Ginger) extract against Toxoplasma gondii
WonHyung Choi, MeiHua Jiang, JongPhil Chu
Address: WonHyung Choi, Department of Medical Zoology, Kyung Hee University School of Medicine, 1 Hoegi-dong, Dongdaemun-gu, Seoul 130-701, Republic
of Korea
whchoi@khu.ac.kr
Received 8th December 2011.
Revised 28th February 2012.
Published online 1st March 2012.
Full text article (pdf)
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
Acknowledgements
References
SUMMARY
Zingiber officinale Roscoe, Ginger, has been used in folk medicine as a medicinal plant, as well as a spice and food in many countries. This
research was carried out to evaluate the antiparasitic effect of ginger root extract (GE) and GE/F1 (fraction 1 obtained from GE) against
Toxoplasma gondii (T. gondii) in vitro and in vivo. The effects of GE and GE/F1 against the proliferation of T.
gondii-infected C6 cells and T. gondii were evaluated by several indicators such as an MTT assay, nuclear staining, immunofluorescence
staining, apoptotic proteins and animal testing. GE/F1 strongly inhibited the proliferation of T. gondii-infected C6 cells and T. gondii
in a dose-dependent manner compared with sulfadiazine. After T. gondii invasion, C6 cells induced the activation of caspase-3, bax, p53 and p21
related to programmed cell death, and GE/F1 effectively suppressed the expression of caspase-3, bax, p53 and p21 causing cell death of the infected
host cells. In addition, INF-gamma, and IL-8 levels, and the viability of T. gondii-infected mice treated with GE/F1 (500 microg/ml) were not
changed or increased during the period of the experiment. These results demonstrate that GE/F1 not only induces anti-T. gondii effects causing
the inactivation of apoptotic proteins in infected host cells through the direct inhibition of T. gondii but also has antiparasitic properties
which inhibit inflammatory cytokine secretion in vivo.
KEY WORDS
apoptotic protein; C6 glioma cells; cytokine; p53; programmed cell death
INTRODUCTION
Toxoplasma gondii (T. gondii) is a protozoan parasite
which causes infection of the central nervous system
in HIV/AIDS patients and chronic toxoplasmosis in
young children or adults with an impaired immune
system (Luft and Remington 1992). T. gondii is a
typical infectious organism that contains
micro-organelles such as mitochondria, Golgi,
micropore, rhoptries, dense granules, and micronemes
(Joiner and Roos 2002). T. gondii causes infection
stages such as the tachyzoites, bradyzoites and
sporozoites in humans and animals, and also induces
the progressive proliferation pathway jn two stages
(the cyst stage and the oocyst stage) when it
proliferates in host cells. T. gondii forms a
parasitophorous vacuole membrane (PVM) after
invading host cells. PVM not only provides T. gondii
with nourishment but also protects T. gondii from
acidification (Dubey et al. 1998). A child can be
infected through placental infection which is
transmitted to the foetus from the mother infected by
T. gondii, and people may become infected by eating
raw or undercooked meat and contagion by sporula-ted oocysts in soil or cat faeces.
Typically, sulfadiazine, pyrimethamine, and
atovaquone are used in the clinic to treat T. gondii,
but their side effects are often evident. Until recently,
studies of medicinal plants for the treatment of
toxoplasmosis have been rare, and research on
effective and new substances of relatively low
toxicity is urgently needed. Zingiber officinale
Roscoe, commonly known as ginger, has been widely
used as an herbal medicine and in folk medicine, as
well as a spice and food in many countries around the
world. Furthermore, it has been used for the treatment
of human diseases such as common cold, cough,
dyspepsia, diarrhea, and headache particularly in
Asia. Ginger has been reported to have gastro-protective effects (Yamahara et al. 1988), and
inhibitory effects against nausea and vomiting (Ernst
and Pittler 2000), and is also known to have strong
anxiolytic and anti-emetic activities (Vishwakarma et
al. 2002). Ginger induces extensive bioactivities
including anti-inflammatory (Grzanna et al. 2005),
anticancer (Shukla and Singh 2007), antioxidant
(Jaqetia et al. 2003), and antimicrobial effects (Ficker
et al. 2003). Its main chemical components are natural
compounds such as gingerenones A, B, C (Endo et al.
1990), gingerdiol (Kikuzaki et al. 1992), 6-, 8-,
10-Gingerol (Hiserodt et al. 1998), paradol, shogaol
(Ma et al. 2004), and essential oil (Singh et al. 2008).
Recently, the 10-shogaol and 1-dehydro-
6-gingerdione isolated from ginger partially activate
the serotonin 5-HT1A receptor which is widely
expressed in the central nervous system (CNS)
(Nievergelt et al. 2010). Ginkgo biloba L. and
Glycyrrhiza glabra L. are known as G. biloba, and
G. glabra respectively. The extract of G. biloba
causes anti-inflammatory (Kotakadi et al. 2008),
molluscicidal (Yang et al. 2008), antimicrobial
(Mazzanti et al. 2000), and anticancer activities
(DeFeudis et al. 2003). The combination treatment of
ginger and G. biloba has been reported to cause
neuroprotective effects (anti-anxiety effects and
effects on the brain's learning power and memory) in
various behavioral tasks (Hasenöhrl et al. 1998). In
addition, this combination preparation also shows
memory-enhancing effects in aged animals (Topic et
al. 2002). G. glabra has a variety of biological effects
such as anticancer (Kanazawa et al. 2003),
antimicrobial (Gupta et al. 2008), and antiviral
activities (Fiore et al. 2008), and its extract has been
recently utilized as a food additive in the food
industry. These researches not only indicate the
various biological activities of medicinal plants but
also demonstrate their physiological effects in vitro
and in vivo.
An effective herbal medicine for the treatment of
toxoplasmosis has not been developed yet, even
though drugs such as sulfadiazine and pyrimethamine
have been used as anti-T. gondii drugs in hospitals.
For this reason, we investigated the anti-T. gondii
effects of ginger, G. glabra, and G. biloba that have
been used as medicinal plants and as food in many
countries.
MATERIALS AND METHODS
Materials
Fetal bovine serum (FBS), antibiotics and
trypsin-EDTA were purchased from Invitrogen
Corporation (Gibco®, U.S.A). RPMI medium 1640,
dimethyl sulfoxide (DMSO), MTT (3-(4,5-
dimethylthiazol-2-yl)-2,5-diohenyl-2H-tetrazolium
bromide; Thiazolyl blue), albumin bovine serum
(BSA), phosphate buffered saline (PBS), 0.4% trypan
blue solution, Hoechst 33342 and sodium dodecyl
sulfate (SDS) were purchased from Sigma Chemical
Co., Ltd. (St. Louis, MO, U.S.A). ECL western
blotting detection kit was purchased from Millipore
Korea Co., Ltd. Silica gel 60 (Kieselgel60, 230-400
mesh) was purchased from Merck Chemical Co., Ltd
(Germany). The Bio-Rad protein assay kit was
purchased from GenDEPOT Co., Ltd. (P.O. Box 454
Barker TX 77413 U.S.A). All other chemicals and
reagents were purchased from Merck Chemical Co.,
Ltd (Germany) and Sigma Chemical Co., Ltd. (St.
Louis, MO, U.S.A).
Preparation of extracts and fractions
The dried ginger, G. glabra, and G. biloba were
provided by the Oriental Medical Center, Kyung Hee
University (Seoul, Republic of Korea). The powdered
roots of ginger (2 kg) and G. glabra (2 kg), and the
powdered leaves of G. biloba (2 kg) were extracted
using 4 l of methanol at room temperature for 24 h
respectively. These extracts were filtered by filter
paper and vacuum pump, and then were evaporated
under reduced pressure using a concentrator in a
vacuum at 35 °C. Among these extracts, ginger
extract (GE) strongly inhibited the proliferation of C6
cells infected with T. gondii (T. gondii-infected C6
cells) and T. gondii compared with other extracts. GE
was dissolved with methanol, and it was coated with
silica gel. The coated GE was loaded onto a cotton
wool pad at the top of the silica gel column and
divided with various partitions by the silica gel
column chromatography method using CHCl3-MeOH
eluate. All fractions were analysed by thin layer
chromatography (TLC), and then we obtained nine
fractions (column fractions obtained from GE)
including GE/F1 (fraction 1 obtained from GE).
Among these fractions, GE/F1 strongly inhibited the
proliferation and viability of T. gondii-infected C6
cells and T. gondii. Sulfadiazine (SF) was used as a
treatment-positive group to evaluate whether or not
GE/F1 has an anti-proliferative effect against
T. gondii.
Animals
BALB-c/mice (4 weeks, n=150) were purchased from
DaeHan Bio-Link Co., Ltd. Korea, and all animals
were kept at 22±0.5 °C and 12 h-light/dark cycle in a
controlled environment of a central animal care
facility of the Kyung Hee University School of
Medicine. Food and water were provided ad libitum.
The facility was strictly maintained in accordance
with National guidelines of the National Institutes of
Health Guide for the Care and Use of Laboratory
Animals.
Cell lines and culture conditions of T. gondii
Rat C6 glioma cells (C6 cells) were purchased from
Korean Cell Line Bank at Seoul National University,
and Rat DI TNC1 normal brain cells (DI TNC1 cells)
were purchased from American Type Culture
Collection (U.S.A). Cells were cultured in RPMI
medium 1640 containing 2 mM L-glutamine,
supplemented with 10% fetal bovine serum (FBS),
100 units/ml penicillin and 100 microg /ml streptomycin
(Biofluids, Rockville, MD, U.S.A) in a humidified
atmosphere containing 5% CO2 in air at 37 °C. The
RH strain of Toxoplasma gondii (T. gondii) was
suspended with 1X PBS, which was injected in the
abdominal cavity of each BALB-c/mouse. Three days
after the injection, T. gondii was collected from the
peritoneal fluids of each mouse kept in the abdominal
cavities of the mice before use in the studies. In the in
vitro study, C6 cells were infected with T. gondii (C6
cells: T. gondii=1:10).
MTT assay on the proliferation and cell viability
To evaluate the effects of ginger, G. glabra, and
G. biloba extracts on the proliferation and viability of
T. gondii-infected C6 cells, we investigated the
viability of T. gondii-infected C6 cells exposed to
these extracts. C6 cells were seeded in a 24 well plate
(1 x 105 cells/well), which were infected with
T. gondii (1 x 106 tachyzoites/well) after 24 h. The
T. gondii-infected C6 cells were then incubated with
varying concentrations (30-240 microg/ml) of ginger,
G. glabra, and G. biloba extracts respectively, and
their viability was determined by MTT assay.
T. gondii was seeded in a 24 well plate (5 x 106/well),
which was incubated with a different concentration
(30-240 microg/ml) of GE, GE/F1, and SF for 24 h
respectively. The PR (the proliferation rate) of
T. gondii-infected C6 cells and T. gondii was
calculated as follows: OD control-OD test sample/OD control
x 100%. Optical absorbance was measured at a
wavelength of 570 nm using an ELISA leader.
Microscopic observation of T. gondii in C6 cells
C6 cells were seeded in a 24 well plate (5 x 104/well),
which were incubated at 37 °C for 24 h. C6 cells were
infected with T. gondii (5 x 105 tachyzoites/well), and
then T. gondii-infected C6 cells were treated with
240 microg/ml of GE/F1 for 24 h. The morphological
changes of C6 cells and T. gondii-infected C6 cells
were observed under a light microscope (Nikon
Eclipse TE 2000-U, Japan).
Nuclear staining of T. gondii-infected C6 cells
This assay was performed according to the method of
Latt and Stetten (1976). Hoechst 33342 is a
cell-permeable nuclear staining reagent which emits
blue fluorescence when it combines to dsDNA. C6
cells were seeded in a 24 well plate (5 x 104
cells/well), which were infected with T. gondii (5 x
105 tachyzoites/well). The T. gondii-infected C6 cells
were incubated with 120 microg/ml of GE/F1, and SF for
24 h respectively. After washing with 1X PBS, the
T. gondii-infected C6 cells were fixed in 1X PBS
containing 5% formaldehyde for 30 min, and then
washed with 1X PBS and stained with a final
concentration of 20 microM (Hoechst 33342) for 30 min
in the dark. After staining, the cells were washed with
1X PBS three times, and their nuclei were observed
under a UV fluorescent microscope (Nikon Eclipse
TE 2000-U, Japan).
Immunofluorescence of PVM in T. gondii-infected C6 cells
C6 cells were seeded onto cover slips in a 24 well
plate (5 x 104/well), which were infected with
T. gondii (5 x 105 tachyzoites/well). T. gondii-
infected C6 cells were incubated with 120 microg/ml of
GE/F1 and SF for 24 h respectively. After washing
with 1X PBS, they were fixed with 3% formaldehyde
for 10 min and 0.05% (v/v) Triton X-100 for 5 min.
The cells were blocked with 1X PBS containing 1%
BSA for 1 h at room temperature after washing. A
mouse monoclonal anti-PVM antibody was diluted
with 1:100 (v/v) using 1% BSA/PBS, and then the
cells were incubated with anti-PVM antibody solution
at room temperature for 1 h. After washing, goat
anti-mouse IgG-FITC-conjugated secondary antibody
was diluted with 1:100 (v/v) using 1X PBS, which
was added to each well. The cells were incubated at
room temperature for 1 h, and were washed with 1X
PBS three times. Their fluorescence was observed
under a UV fluorescent microscope (Nikon Eclipse
TE 2000-U, Japan).
Western blot analysis
C6 cells were incubated in a 6well plate (1 x
105/well) for 24 h, which were infected with T. gondii
(1 x 106 tachyzoites/well). T. gondii-infected C6 cells
were treated with different concentrations (60-120
microg/ml) of GE/F1 and SF for 24 h respectively, and
harvested for the purpose of a western blot analysis.
The pellets were lysed using RIPA lysis buffer (Elpis
Biotech). The protein concentrations were measured
at 595 nm using the Bio-Rad protein assay kit and 1%
BSA as standard. Equal amounts of protein were
loaded onto 12% SDS-PAGE and transferred to a
nitrocellulose membrane. The membranes were
blocked with 1X PBS (5% skim-milk) containing
0.05% Tween-20 at room temperature for 1 h, and
were also washed five times with 1X PBS containing
0.05% Tween-20. The membranes were incubated
with the corresponding primary antibodies to p53
(1:500), p21 (1:500), caspase-3 (1:500), bax (1:500)
and -actin (1:2,000) respectively, overnight at 4 °C.
After the membranes were washed with 1X PBS
containing 0.05% Tween-20, they were incubated
with goat anti-mouse, goat anti-rabbit or rabbit
anti-goat IgG HRP conjugated secondary antibodies
at room temperature for 2 h. After repeating the
washing step, protein bands were visualized using the
ECL western blotting analysis kit (Millipore
Corporation, Billerica, MA 01821 U.S.A). All
antibodies were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA), Cell Signaling (Cell
Signaling Technology, Inc., Danvers, MA), and R&D;
System (Minneapolis, MN, U.S.A).
The viability of T. gondii-infected mice
Sixty animals were divided into control (n=15) and
experimental groups (3 groups, n=45). T. gondii was
seeded in a 12 well plate (1 x 107/well), which was
incubated with 250, and 500 microg/ml of GE/F1 for 24 h.
T. gondii treated with 250 and 500 microg/ml of GE/F1
was harvested before the injection in the abdominal
cavity of each mouse in the experiment group, which
was washed with 1X PBS three times. The pellets
were also suspended with 1X PBS, which were
injected in the abdominal cavity of each
BALB-c/mouse in the experimental groups. Mice
infected with T. gondii (T. gondii-infected mice) were
used as an infection positive group, and all animals
were kept in a central animal care facility during the
experiment.
Measurements of IFN-, and IL-8
Ninety animals were divided into control (n=30) and
experimental groups (2 groups, n=60). T. gondii was
seeded in a 12 well plate (1 x 107/well), which was
incubated with 500 microg/ml of GE/F1 for 24 h.
T. gondii treated with 500 microg/ml of GE/F1 was
harvested before the injection into the abdominal
cavities of the mice, and it was washed with 1X PBS
three times. The pellets were also suspended with 1X
PBS, which were injected into the abdominal cavity
of each BALB-c/mouse in the experimental groups.
The T. gondii-infected mice were used as an infection
positive group. Blood samples were collected from
the left ventricle of heart of the anaesthetised mice
into heparinized syringes every day for 5 days after
the injection. Serum was separated by centrifugation
at 12000 rpm for 15 min at 4 °C, and stored in vials at
-70 °C until analysis for IFN-gamma, and IL-8. IFN-gamma
levels were measured with commercial sensitive
ELISA kits (SABiosciences, QIAGEN Company, MD
21703, USA), and IL-8 levels were measured with
commercial sensitive ELISA kit (COSMO BIO CO.,
LTD. Koto-ku, Tokyo 135-0016, Japan). All other
chemicals and reagents were purchased from Sigma
Chemical Co., Ltd. (St. Louis, MO, U.S.A). The
activity of IFN-gamma, and IL-8 in serum was measured
according to the manufacturer's protocol.
Statistical analysis
All results were expressed as mean ± S.E.M.
Statistical analysis of the data was performed using
Student's t-test and one-way analysis of variance
(ANOVA software version 14). The significance
level was 2alpha=0.05.
RESULTS
Effect of GE and GE/F1 on the proliferation of T. gondii-infected C6 cells and T. gondii
T. gondii has various micro-organelles such as
mitochondria and ER network. MTT is an established
assay performed in many laboratories. We evaluated
the effects of ginger, G. glabra and G. biloba extracts
on the cell proliferation and cell viability of T. gondii
using an MTT assay. After T. gondii-infected C6 cells
were incubated with various concentrations (30-240
g/ml) of the extracts for 24 h, their viability was
decreased in a dose- and time-dependent manner
(Fig. 1). In particular, GE strongly inhibited the
proliferation and viability of T. gondii-infected C6
cells compared with other extracts. T. gondi -infected
C6 cells treated with 240 microg/ml of GE/F1 for 24 h
exhibited antiproliferation including T. gondii fragmentation and a significant decrease in T. gondii, as
well as morphological changes including cell
shrinkage, membrane blebs, and cell fragmentation
compared with T. gondii-infected C6 cells (Fig. 2). As
shown in Figs 1-3, GE/F1 effectively inhibited the viability of T. gondii-infected C6 cells and T. gondii
compared with SF, which induced the
T. gondii-selective growth inhibitory effect against
T. gondii, and then PR was measured less than 45%
at concentrations of 240 microg/ml (Fig. 3). The 50%
inhibitory concentration (IC50) values of GE, GE/F1,
and SF against T. gondii were measured as 220.83,
205.56, and 276.81 microg/ml, respectively. These results
indicate that GE/F1 effectively caused antiproliferation of T. gondii-infected C6 cells through
the direct inhibition of T. gondii. On the other hand,
GE/F1 showed no obvious cytotoxicity against DI
TNC1 cells (Fig. 4).
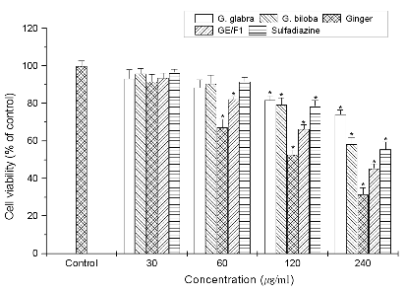
Fig. 1. The inhibitory effect of different extracts on the viability and proliferation of T. gondii-infected C6 cells. T.
gondii-infected C6 cells were incubated with concentrations (30-240 microg/ml) of ginger, G. glabra, G. biloba extracts, GE/F1,
and SF for 24 h, respectively. Results are expressed as a percentage of the control, and all data are presented as mean ± S.E.M.
* Statistically significant as compared with control.
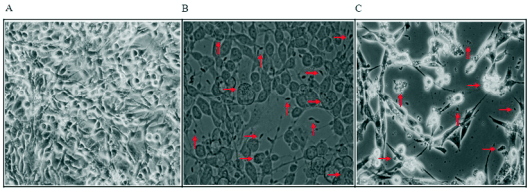
Fig. 2. Morphological changes of T. gondii-infected C6 cells. T. gondii-infected C6 cells were incubated with 240 microg/ml
of GE/F1 for 24 h. (A) Uninfected C6 cells. (B) T. gondii-infected C6 cells (the arrow shows tachyzoites into PVM formed by
proliferation of T. gondii in C6 cells after T. gondii invasion, and T. gondii). (C) T. gondii-infected C6 cells
treated with 240 microg/ml of GE/F1 (the arrow shows T. gondii fragmentation, and the morphological changes including cell shrinkage,
membrane blebs).
Inhibitory effect of GE/F1 on PVM formed by T. gondii proliferation in host cells
T. gondii has distinguishing features such as
anti-apoptosis through the inactivation of apoptotic
proteins during the early- and intermediate
proliferative phase in host cells after cell invasion.
The activation of PVM is accelerated in the
intermediate stage (the stage before release of T. gondii from PVM) of T. gondii in a time-dependent
manner. We evaluated the antiproliferative response
of T. gondii induced by interaction between PVM and
GE/F1 during the proliferation stage of T. gondii in
host cells. The morphological change of PVM and
nuclei of T. gondii was markedly decreased in
T. gondii-infected C6 cells treated with 120 microg/ml of
GE/F1 and SF respectively (Fig. 5). These results
show that GE/F1 selectively inhibited the
proliferation of T. gondii and PVM formed by
T. gondii invasion in infected host cells.
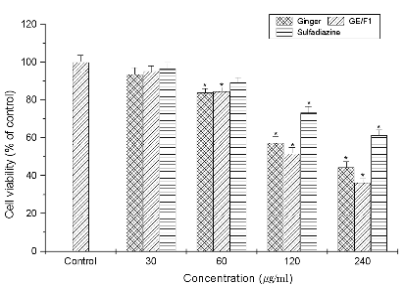
Fig. 3. The effects of GE, GE/F1, and SF on the proliferation of T. gondii. T. gondii was incubated with concentrations
(30-240 microg/ml) of GE, GE/F1, and SF for 24 h, respectively. Results are expressed as a percentage of the control, and all data
are presented as mean ± S.E.M. Symbols as in Fig. 1.
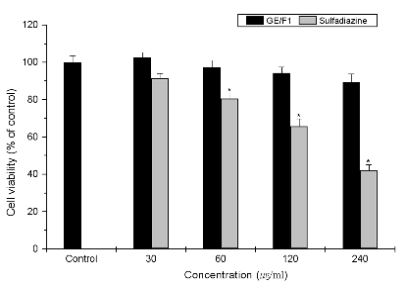
Fig. 4. Cytotoxicity of GE/F1 and SF on the viability of normal cells. DI TNC1 cells were treated with different concentrations (30-240
microg/ml) of GE/F1 and SF for 24 h respectively. Results are expressed as a percentage of the control, all data are presented as mean ± S.M.E.
Symbols as in Fig. 1.
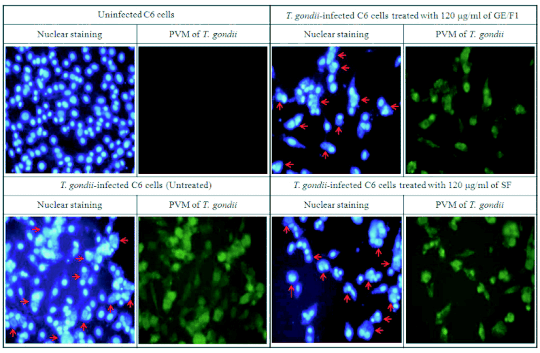
Fig. 5. Nuclear staining and immunofluorescence of T. gondii-infected C6 cells treated with GE/F1 and SF. C6 cells were
seeded onto cover slips in a 24 well plate (5 x 104/well), which were infected with T. gondii (5 x 105/well) after 24
h. T. gondii-infected C6 cells were incubated with 120 g/ml of GE/F1 and SF for 24 h, respectively. Green shows morphological
changes of PVM formed by T. gondii proliferation in C6 cells after T. gondii invasion. Fluorescence simultaneously shows
nucleus of T. gondii in PVM, and nucleus of C6 cells respectively (the arrow shows the nucleus of T. gondii into PVM formed
by proliferation of T. gondii in C6 cells).
Modulation of GE/F1 on apoptotic proteins in T. gondii-infected C6 Cells
The caspase-3, bax, p53 and p21 proteins play crucial
roles in the regulation of cell cycle progression during
cell division (Morgan 1995). In this study, we
evaluated the inhibitory effects of GE/F1 on the
apoptotic signalling proteins which cause cell cycle
arrest and apoptosis during the proliferation stage of
T. gondii in T. gondii-infected C6 cells. The p53 is a
tumor-suppressor protein of the cell death pathway,
and the p21 functions as a regulator of cell cycle
progression at G1/S or G2/M transition in response to
a variety of stress stimulation. The caspase-3 and bax
are indicator proteins of the mitochondria pathway of
the cell death mechanism. The expression of p53,
p21, caspase-3 and bax activated by intracellular
proliferation of T. gondii was markedly decreased in
T. gondii-infected C6 cells treated with GE/F1
compared with both T. gondii-infected C6 cells
treated with SF and T. gondii-infected C6 cells
(T. gondii infection positive group) (Fig. 6). These
results suggest that GE/F1 effectively blocked
apoptosis of C6 cells induced by T. gondii invasion
through the direct inhibition of T. gondii.
Effects of GE/F1 on the viability and proliferation of T. gondii in T. gondii-infected mice
T. gondii is a parasite which induces serious
infectious diseases such as brain injury and immune
deficiency in human and animals. For this reason, we
evaluated the antiproliferative effect of GE/F1 against
the proliferation and growth of T. gondii in
T. gondii-infected mice. The mice were observed
during the period of the experiment after the injection
of T. gondii treated with 250 and 500 microg/ml of GE/F1
for 24 h. As mentioned above, T. gondii-infected mice
treated with 500 microg/ml of GE/F1 showed higher
viability than T. gondii-infected mice and
T. gondii-infected mice treated with 250 microg/ml of
GE/F1, and the mice maintained the same activity and
dynamics as the control group. In addition, there was
a significant difference of the survival rate between
T. gondii-infected mice and T. gondii-infected mice
treated with GE/F1 (Fig. 7). These results
demonstrate that GE/F1 has anti-T. gondii effects and
unique properties which inhibit the proliferation and
growth of T. gondii.
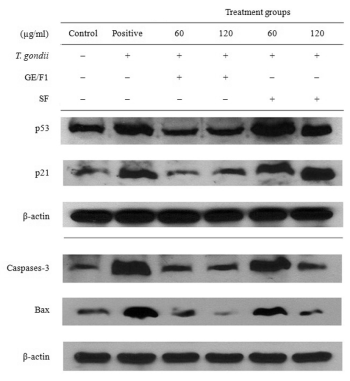
Fig. 6. Change of apoptotic signalling proteins of T. gondii-infected C6 cells. T. gondii-infected C6 cells were treated with
different concentrations (60-120 microg/ml) of GE/F1 and SF for 24 h respectively, and protein expression was measured using
western blot analysis.
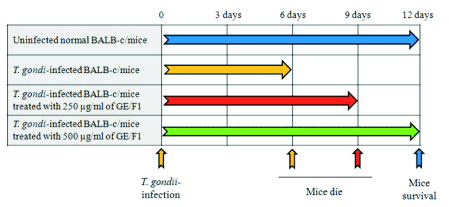
Fig. 7. Effect of GE/F1 on the viability of T. gondii-infected mice. T. gondii was incubated with 250 and 500 microg/ml of
GE/F1 for 24 h, and was injected into the abdominal cavity of each mouse in the experimental groups. Sixty animals were divided into control (n=15)
and experimental groups (3 groups, n=45).
Changes of cytokines in T. gondii-infected mice
Cytokines play in endocrine via specific cell surface
receptors on their target cells as key players in the
regulation of the immune response. They exert their
actions during tissue damage leading to inflammation
and infections such as parasites, bacteria, virus, and
endocrinological autoimmune diseases. Particularly,
the release of cytokines such as IFNs (INF-alpha, beta, gamma),
and IL-8 which are produced by infections and
inflammation is an important component of the immune response to secrete cytokines that help to
activate endocrine system. As shown in Fig. 8,
although secretions of INF-gamma, and IL-8 were slightly
increased on first day after the injection of T. gondii
treated with 500 microg/ml of GE/F1, their levels were not
increased during infection periods. On the other hand,
the concentrations of cytokines in the serum of
T. gondii-infected mice were rapidly increased
compared with the control group. These results
demonstrate that GE/F1 effectively inhibited the
secretions of INF-gamma and IL-8 which are rapidly
activated by infections, inflammation, and tissue
damage in endocrine system of mice.
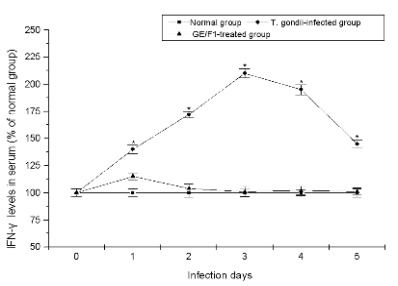
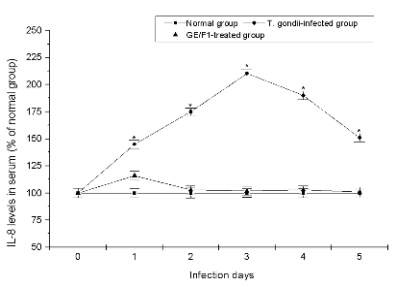
Fig. 8. The serum levels of IFN-gamma, and IL-8 of T. gondii-infected mice treated with GE/F1. . The serum levels of (A) IFN-gamma,
and (B) IL-8. T. gondii was treated with 500 microg/ml of GE/F1 for 24 h, and was injected into the abdominal cavity of each mouse in the
experimental group. The cytokines were measured using ELISA kits in a time-dependent manner. Results are expressed as a percentage of the control, and
all data are presented as mean ± S.M.E. * Statistically significant as compared with control.
DISCUSSION
T. gondii causes serious complications in AIDS/HIV
patients, as well as parasite symptoms into the brain
through extrinsic infection. T. gondii causes
spontaneous abortion in pregnant women and foetal
infection through the placenta during pregnancy. It is
also one of the important parasites which induce
zoonosis. When host cells are infected by a parasite,
the host cells activate protective systems including
the immune-response and defensive mechanism
against parasitic invasion and stimulation of secretion
released from the parasite. Particularly, IFN-gamma
produced by helper T-cells accelerates the
differentiation of T lymphocytes through the
signalling transport of the STAT1 and T-bet, and then
they activate anti-protozoa system which causes the
resistance to T. gondii invasion in vivo. However,
T. gondii inhibits the production of IL-4, the activity
of caspase-3 and the release of cytochrome-c from
mitochondria after invasion in host cells. T. gondii
suppresses apoptotic signals of host cells to induce
anti-apoptosis during its proliferation stage in host
cells (Laliberte and Carruthers 2008). In this study,
we evaluated the effect of GE/F1 which inhibits the
proliferation of T. gondii and the changes of
cytokines induced by T. gondii infection through
animal testing, and signalling pathways of apoptotic
proteins in host cells after T. gondii infection.
In general, apoptotic signalling pathways induce
apoptosis through the G1/S or G2/M transition of the
cell cycle by regulating the expression of genes.
Among apoptotic proteins, bax and caspase-3 are
important indicators related to the mitochondrial
signalling pathways that regulate the activation of the
apoptotic cascade mechanism, and their activity
results in the subsequent apoptotic progression (such
as caspase-9, -7, -6, Cyto-c, and CAD). The
overexpression of p53 induces an increase of bax and
p21 expression, and a suppression of bcl-2 expression
(Agarwal et al. 1998). Furthermore, p21 is a CKI that
inhibits the activity of Cyclin-Cdk complex at the G1
or G2 checkpoints of cell cycle, and the expression of
p21 is controlled by tumour suppressor p53 at G1
phase (El-Deiry et al. 1993, Harper et al. 1993, Gartel
and Radhakrishnan 2005, Choi et al. 2011). These
apoptotic proteins promote cell death and cell arrest
at the cell cycle. The spontaneous apoptosis of
T. gondii-infected cells is induced by the activation of
caspase-3, -7, -8 and -9 during the early apoptotic
stage of host cells after T. gondii invasion (Laliberte
and Carruthers 2008). However, T. gondii exerts
influence on the apoptotic progress and cell arrest
pathways of host cells after cell invasion. They also
proliferate using a three-phase cycle (S phase, late S
phase and G1 phase) which comprises 50-70% of the
parasite doubling time. T. gondii induces inactivation
of cell cycle initiator (cyclin-cdk complex and p21)
and apoptotic mediator (caspase families) through its
signalling pathways during the early- and
intermediate proliferative phase of T. gondii in host
cells. Furthermore, activation of the PI3K
(phosphoinositol 3 kinase) in infected cells leads to
inactivation of Bad and the inhibition of the forkhead
transcription factor (FKHR1) in the host cells
(Gubbels et al. 2008). In the present study, the
proliferation of T. gondii and PVM were remarkably
inhibited in T. gondii-infected C6 cells treated with
GE/F1 compared with T. gondii-infected C6 cells
(Fig. 5). Although the expression of bax, caspase-3,
p53 and p21 was increased in C6 cells after T. gondii
infection, their expression was decreased in a
concentration-dependent manner in infected cells
after treatment with GE/F1 (Fig. 6). On the other
hand, both p53 and p21 were significantly increased
in T. gondii-infected C6 cells and T. gondii-infected
C6 cells treated with SF compared with uninfected C6
cells.
The immune system in vivo recognizes the
presence of pathogens by several proteins and
cytokines. Cytokines such as IFN-gamma and IL-8 play
pivotal roles in a variety of immune responses of the
B-cells, T-cells, NK cells, mast cells, and
macrophages which are activated by tissue damage,
inflammation or infection of pathogens such as
bacteria, virus, and parasites. They are chemical
signals which are produced by macrophage, epithelial
and endothelial cells at the site of inflammation. We
evaluated the changes of cytokines in the serum of
T. gondii-infected mice. After the injection of
T. gondii treated with 500 microg/ml of GE/F1, the
concentrations of INF-gamma, and IL-8 in serum were not
increased during the period of parasitic infection
compared with infection positive group. Even though
cytokine levels of T. gondii-infected mice treated with
500 microg/ml of GE/F1were slightly increased on the
first day after injection, the increase of cytokines may
be a low response of the immune network induced
because immune cells recognize their presence as
foreign substances. In addition, T. gondii-infected
mice treated with GE/F1 (500 microg/ml) showed the
same viability as the control group during the
experiment. Taken together, these results indicate that
T. gondii-infected host cells induce activation of the
cell cycle initiator (p21) and apoptotic mediator (p53,
caspase-3 and bax) which cause host cell apoptosis
during the proliferative phase (the stage before the
release of T. gondii) of T. gondii. The results show
that T. gondii increases inflammatory cytokine
secretion in vivo after its infection.
In summary, the results of this study demonstrate
that GE/F1 not only has anti-T. gondii effects causing
the direct inhibition of T. gondii but also inhibits the
activation of apoptotic proteins (p53, p21, caspase-3,
and bax) induced by T. gondii proliferation in infected
host cells. The results also indicate that GE/F1 has
antiparasitic properties which are able to maintain the
survival of T. gondii-infected mice. Therefore, our
research findings provide substantial evidence that
GE/F1 can be used as a new candidate substance for
anti-T. gondii drug development through the blocking
of apoptotic proteins in vitro and inflammatory
cytokine inhibition in vivo, and that, in general,
extracts and substances derived from medicinal plants
may be used as useful medicinal resources in the
development of antiparasitic drugs.
ACKNOWLEDGEMENTS
The authors would like to express sincere gratitude to
the staffs of Kyung Hee University School of
Medicine for providing laboratory facilities and
research equipment during the course of the
experiment. All authors approved the final
manuscript, and the authors report no conflicts of
interest.
REFERENCES
Agarwal ML, Taylor WR, Chernov MV, Chernova OR, Stark, GR. The p53 network. J Biol Chem. 273: 1-4, 1998.
[CrossRef]
[PubMed]
Choi WH, Chu JP, Jiang MH, Baek SH, Park HD. Effects of Fraction obtained from Korean Corni Fructus extracts causing anti-proliferation and
p53-dependent apoptosis in A549 lung cancer cells. Nutr Cancer. 63: 121-129, 2011.
[PubMed]
DeFeudis FV, Papadopouls V, Drieu K. Ginkgo biloba extracts and cancer: a research area in its infancy. Fundam Clin Pharmacol. 17: 405-417,
2003.
[CrossRef]
[PubMed]
Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue
cysts. Clin Microbiol Rev. 11: 267-299, 1998.
[PubMed]
El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53
tumor suppression. Cell. 75: 817-825, 1993.
[CrossRef]
Endo K, Kanno E, Oshima Y. Structures of antifungal diarylheptenones, gingerenones A, B, C and isogingerenone B, isolated from the rhizomes of
Zingiber officinale. Phytochemistry. 29: 797-799, 1990.
[CrossRef]
Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br J Anaesth. 84: 367-371,
2000.
[CrossRef]
[PubMed]
Ficker CE, Arnason JT, Vindas PS, Alvarez LP, Akpaqana K, Gbeassor M, De SC, Smith ML. Inhibition of human pathogenic fungi by ethnobotanically
selected plant extracts. Mycoses. 46: 29-37, 2003.
[CrossRef]
[PubMed]
Fiore C, Eisenhut M, Krausse R, Raqazzi E, Pellati D, Armanini D. Antiviral effects of Glycyrrhiza species. Phytother Res. 22: 141-148, 2008.
[CrossRef]
[PubMed]
Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 65: 3980-3985, 2005.
[CrossRef]
[PubMed]
Grzanna R, Lindmark L, Frondoza CG. Ginger-an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 8: 125-132, 2005.
[CrossRef]
[PubMed]
Gubbels MJ, White M, Szatanek T. The cell cycle and Toxoplasma gondii cell division: Tightly knit or loosely stitched. Int J Parasitol. 38:
1343-1358, 2008.
[CrossRef]
[PubMed]
Gupta VK, Fatima A, Faridi U, Neqi AS, Shanker K, Kumar JK. Antimicrobial potential of Glycyrrhiza glabra roots. J Ethnopharmacol. 116:
377-380, 2008.
[CrossRef]
[PubMed]
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell.
75: 805-816, 1993.
[CrossRef]
Hasenohrl RU, Topic B, Frisch C, Hacker R, Mattern CM, Huston JP. Dissociation between anxiolytic and hypomnestic effects for combined extracts of
Zingiber officinale and Ginkgo biloba, as opposed to diazepam. Pharmacol Biochem Behav. 59: 527-535, 1998.
[CrossRef]
Hiserodt RD, Franzblau SG, Rosen RT. Isolation of 6-, 8-, and 10-Gingerol from Ginger Rhizome by HPLC and Preliminary Evaluation of Inhibition of
Mycobacterium avium and Mycobacterium tuberculosis. J Agric Food Chem. 46: 2504-2508, 1998.
[CrossRef]
Jaqetia GC, Baliqa MS, Venkatesh P, Ulloor JN. Influence of ginger rhizome (Zingiber officinale Ros) on survival, glutathione and lipid
peroxidation in mice after whole-body exposure to gamma radiation. Radiat Res. 160: 584-592, 2003.
[CrossRef]
[PubMed]
Joiner KA, Roos DS. Secretory traffic in the eukaryotic parasite Toxoplasma gondii: less is more. J Cell Biol. 157: 557-563, 2002.
[CrossRef]
[PubMed]
Kanazawa M, Satomi Y, Mizutani Y, Ukimura O, Kawauchi A, Sakai T. Isoliquiritigenin inhibits the growth of prostate cancer. Eur Urol. 43: 580-586,
2003.
[CrossRef]
Kikuzaki H, Tsai SM, Nakatani N. Gingerdiol related compounds from the rhizomes of Zingiber officinale. Phytochemistry. 31: 1783-1786,
1992.
[CrossRef]
Kotakadi VS, Jin Y, Hofseth AB, Ying L, Cui X, Volate S, Chumanevich A, Wood PA, Price RL, McNeal A, Singh UP, Singh NP et al. Ginkgo biloba
extract EGb 761 has anti-inflammatory properties and ameliorates colitis in mice by driving effector T cell apoptosis. Carcinogenesis. 29: 1799-1806,
2008.
[CrossRef]
[PubMed]
Laliberte J, Carruthers VB. Host cell manipulation by human pathogen Toxoplasma gondii. Cell Mol Life Sci. 65: 1900-1915, 2008.
[CrossRef]
[PubMed]
Latt SA, Stetten G. Spectral studies on 33258 Hoechst and related bisbenzimidazole dyes useful for fluorescent detection of deoxyribonucleic acid
synthesis. J Histochem Cytochem. 24: 24-33, 1976.
[CrossRef]
Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 15: 211-222, 1992.
[CrossRef]
[PubMed]
Ma J, Jin X, Yang L, Liu ZL. Diarylheptanoids from the rhizomes of Zingiber officinale. Phytochemistry. 65: 1137-1143, 2004.
[CrossRef]
[PubMed]
Mazzanti G, Mascellino MT, Battinelli L, Coluccia D, Manganaro M, Saso L. Antimicrobial investigation of semipurified fractions of Ginkgo
biloba leaves. J Ethnopharmacol. 71: 83-88, 2000.
[CrossRef]
Morgan DO. Principles of CDK regulation. Nature. 374: 131-134, 1995.
[CrossRef]
[PubMed]
Nievergelt A, Huonker P, Schoop R, Altmann KH, Gertsch J. Identification of serotonin 5-HT1A receptor partial agonists in ginger. Bioorg Med Chem. 18:
3345-3351, 2010.
[CrossRef]
Singh G, Kapoor IP, Singh P, de Heluani CS, de Lampasona MP, Catalan CA. Chemistry, antioxidant and antimicrobial investigations on essential oil and
oleoresins of Zingiber officinale. Food Chem Toxicol. 46: 3295-3302, 2008.
[CrossRef]
[PubMed]
Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 45: 683-690, 2007.
[CrossRef]
[PubMed]
Topic B, Hasenohrl RU, Hacker R, Huston JP. Enhanced conditioned inhibitory avoidance by a combined extract of Zingiber officinale and
Ginkgo biloba. Phytother Res. 16: 312-315, 2002.
[CrossRef]
[PubMed]
Vishwakarma SL, Pal SC, Kasture VS, Kasture SB. Anxiolytic and antiemetic activity of Zingiber officinale. Phytother Res. 16: 621-626,
2002.
[CrossRef]
[PubMed]
Yamahara J, Mochizuki M, Rong HQ, Matsuda H, Fujimura H. The anti-ulcer effect in rats of ginger constituents. J Ethnopharmacol. 23: 299-304,
1988.
[CrossRef]
Yang XM, Chen SX, Xia L, Chen J. Molluscicidal activity against Oncomelania hupensis of Ginkgo biloba. Fitoterapia. 79: 250-254, 2008.
[CrossRef]
[PubMed]
|
BACK
|










