Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 11 (2013) No 1, p 7-13
DOI 10.2478/v10136-012-0015-x
Therapeutic efficacy of a novel bispyridinium oxime K203 and commonly used oximes (HI-6, obidoxime, trimedoxime, methoxime) in soman-poisoned male rats and mice
Jiri Kassa, Jana Zdarova Karasova, Marketa Krejciova
Address: Jiri Kassa, Faculty of Military Health Sciences, Trebesska 1575, 500 01 Hradec Kralove, Czech Republic
kassa@pmfhk.cz
Received 12th March 2012.
Published online 2nd April 2012.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
Declaration of interests
Acknowledgements
References
SUMMARY
The potency of a novel oxime K203 in reactivating soman-inhibited acetylcholinesterase and reducing acute toxicity of soman was compared with commonly used oximes (HI-6, obidoxime, trimedoxime, methoxime) using in vivo methods. The study determining percentage of reactivation of soman-inhibited blood and tissue acetylcholinesterase in rats showed that the potency of the oxime K203 to reactivate soman-inhibited acetycholinesterase in the peripheral compartment is slightly higher than obidoxime and trimedoxime, especially in the diaphragm, slightly lower than methoxime and markedly lower compared to the oxime HI-6. The reactivating efficacy of the oximes studied in the peripheral compartment roughly corresponds to their potency to reduce acute toxicity of soman in mice. Based on the obtained data, we can conclude that the oxime K203 is not suitable for the replacement of the oxime HI-6 for the antidotal treatment of acute soman poisoning due to its relatively low potency to counteract acute toxicity of soman.
KEY WORDS
soman; acetylcholinesterase; K203; HI-6; obidoxime; trimedoxime; methoxime; rats; mice
INTRODUCTION
Organophosphorus nerve agents exert their toxic
effects by the phosphylation and subsequent
inactivation of acetylcholinesterase (AChE, EC
3.1.1.7). The inactivation of AChE allows the
accumulation of acetylcholine in the cholinergic
synapses of the central and peripheral nervous
systems. Subsequent widespread overstimulation of
cholinergic receptors is manifested as salivation,
lacrimation, sweating, diarrhea, urination, muscular
twitching and fibrilation and ultimately tonic/clonic
convulsions (Lotti 2000, Bajgar 2004). In addition,
nerve agent-induced centrally mediated seizures can
rapidly progress to status epilepticus and contribute to
profound brain damage and cardiac pathology
(Tryphonas and Clement 1995, McDonough and Shih
1997).
The medical countermeasures of poisoning with
nerve agents is usually based on a combined
administration of a muscarinic cholinergic receptor
antagonist to block the overstimulation of cholinergic
receptors by acetylcholine and an oxime to reactivate
nerve agent-inhibited AChE. Generally, anti-cholinergics (mainly atropine) are used for relieving
muscarinic signs and symptoms whereas AChE
reactivators (called oximes) are used for reactivation
of nerve agent-inhibited AChE (Lotti 2000, Bajgar
2004). While a lot of these reactivators are
sufficiently effective to reactivate sarin- or
VX-inhibited AChE, their potency to reactivate
soman-, cyclosarin- or tabun-inhibited AChE is
generally low (Kassa 2002, Marrs et al. 2006).
Therefore, the development of a sufficiently effective
AChE reactivator against some nerve agents such as
tabun or soman is still very important.
Soman (pinacolyl methyl fluorophosphonate)
belongs to a highly toxic group of organophosphorus
compounds misused as chemical warfare agents for
military as well as for terroristic purposes.
Deleterious effects of soman are extraordinarily
difficult to antagonize due to the very rapid aging of
soman-inhibited AChE. The dealkylation of soman
bound on the active site of AChE makes the
nucleophilic attack of oximes almost impossible (Puu
et al. 1986, Jokanovic and Prostran 2009).
A novel bispyridinium asymmetric oxime K203
[1-(4-carbamoylpyridinium)-4-(4-hydroxyimino-methylpyridinium)-but-2-ene dibromide] (Fig. 1) was
primarily synthesized at our department (Musilek et
al. 2008) to increase the efficacy of antidotal
treatment of acute poisoning with tabun that was
found to be resistant to conventional oxime therapy
due to the conformational changes of AChE-tabun
complex prior aging process in AChE active site
(Ekstrom et al. 2006). As the oxime K203 was found
to be promising reactivator of tabun-inhibited AChE
(Kassa et al. 2008, Kovarik et al. 2009), we decided
to evaluate the reactivating and therapeutic efficacy
of K203 against other nerve agents including soman,
because we are still searching for a broad-spectrum
oxime able to sufficiently counteract acute toxicity of
all nerve agents regardless of their chemical structure.
The aim of this study was to determine the
reactivating and therapeutic efficacy of a novel
bispyridinium oxime K203 in comparison with
commonly used oximes (HI-6, obidoxime,
trimedoxime, methoxime) against soman in rats and
mice.
MATERIAL AND METHODS
Animals
Male albino Wistar rats weighing 220-260 g and
NMRI male mice weighing between 20 and 25 g were
purchased from VELAZ (Prague, Czech Republic).
They were kept in an air-conditioned room with the
light from 07:00 to 19:00 h and were allowed access
to standard food and tap water ad libitum. The rats
and mice were divided into groups of 8 animals.
Handling of the experimental animals was done under
the supervision of the Ethics Committee of the
Faculty of Military Health Sciences, Czech Republic.
Chemicals
Soman was obtained from the Technical Institute in
Brno (Czech Republic) in compliance with
permission for the handling of chemical warfare
agents and it was 92% pure. Its purity was assayed by
acidimetric titration. All oximes (obidoxime,
trimedoxime, methoxime, HI-6, K203) were
synthesized at our Department of Toxicology of the
Faculty of Military Health Sciences (Czech Republic)
and they were more than 98% pure. The purity of
oximes was analyzed using a HPLC technique. All
other drugs and chemicals of analytical grade were
obtained commercially and used without further
purification. All compounds were administered
intramuscularly (i.m.) at a volume of 1 ml/kg body
weight (b.w.) to rats and 10 ml/kg b.w. to mice.
In vivo experiments
Before starting the evaluation of reactivating and
therapeutic efficacy of oximes, the acute toxicity of
tested oximes was evaluated in rats and mice by the
assessment of their LD50 values and their 95%
confidence limits using probit-logarithmical analysis
of death occuring within 24 h after i. m.
administration of each oxime at five different doses
with eight animals per dose (Tallarida and Murray
1987).
To evaluate the reactivating efficacy of the
oximes, the rats were injected i.m. with either
atropine (21 mg/kg) alone or atropine (21 mg/kg) in
combination with one of the oximes studied in
equitoxic doses corresponding to 5% of their LD50
5 min before receiving soman i.m. at a dose of
74 microg/kg (LD50). The prophylactic administration of
antidotes was used because this procedure is suitable
for a mechanistic study that compares the reactivating
efficacy of various oximes. The technique should give
better results than the treatment of animals after
poisoning and reduce the influence of aging of
soman-AChE complex (Clement et al. 1992). The rats
were decapitated and exsanguinated to obtain the
blood and tissues 30 min subsequent to soman
poisoning. AChE activity was measured in hemolyzed
blood and homogenized tissue (diaphragm and brain)
by the standard spectrophotometric method (Ellman
et al. 1961). The AChE activity was expressed as
microkat/kg or l (mol substrate hydrolyzed/kg wet tissue
or l blood within 1 s). The untreated control values for
blood, diaphragm and brain AChE activity were
obtained from rats administered with saline instead of
soman and antidotes (saline control). The reactivation
% extent was calculated using the AChE activity
values: {1- [((saline control) - (oxime +
atropine))/((saline control) - (atropine control))]} x
100 (Clement et al. 1992).
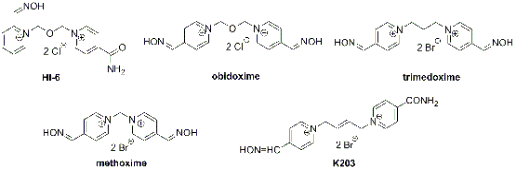
Fig. 1. Chemical structure of oximes.
The potency of oximes in combination with
atropine to eliminate soman-induced lethal effects in
mice was determined as follows. The LD50 value of
soman and its 95% confidence limit in
soman-poisoned mice treated with atropine alone
(21 mg/kg) at 1 min after i.m. administration of
soman was assessed using probit-logarithmical
analysis of death occuring within 24 h after i.m.
administration of soman at five different doses with
eight mice per dose (Tallarida and Murray 1987).
Then, soman-poisoned mice were treated i.m. with
one of tested oximes at equitoxic doses (5% LD50) in
combination with atropine (21 mg/kg) at 1 min after
i. m. challenge of soman. The LD50 values of soman
and their 95% confidence limits in soman-poisoned
mice treated with an oxime in combination with
atropine were assessed by the same method. The
efficacy of tested oximes was expressed as protective
ratio (LD50 value of soman in mice protected by the
combination of oxime and atropine/LD50 value of
soman in mice protected by atropine alone).
Statistical evaluation
The differences between groups were calculated using
means ± SD and differences were tested by one-way
ANOVA test with Scheffe's post hoc test at the
significance level 2alpha=0.05.
RESULTS
The acute i.m. toxicity of tested oximes is
summarized in Table 1. The results show that the
acute toxicity of the newly developed oxime K203 is
slightly lower than the acute toxicity of obidoxime
and trimedoxime but it is markedly higher than the
acute toxicity of methoxime and the oxime HI-6.
According to our results, the oxime HI-6 can be
considered to be the least toxic for both animal
species.
The ability of oximes to reactivate soman-
inhibited AChE in rat blood, diaphragm and brain in
vivo is shown in Table 2. The newly developed oxime
K203 seems to be a week reactivator of
soman-inhibited AChE in blood, diaphragm as well as
in brain. It is able to somewhat reactivate
soman-inhibited AChE in blood, diaphragm as well as
in brain but the increase of AChE activity is relatively
small and not significant compared to the activity of
soman-inhibited AChE in poisoned rats treated with
atropine alone. Its reactivating efficacy is slightly
lower compared to methoxime in blood, diaphragm
and brain, however, the difference between the
reactivating efficacy of K203 and methoxime is very
small. Obidoxime and trimedoxime are also weak
reactivators of soman-inhibited AChE, especially in
diaphragm and brain where both currently available
oximes are completely ineffective. On the other hand,
the oxime HI-6 is a relatively good reactivator of
soman-inhibited AChE in the peripheral compartment
(blood, diaphragm) where its ability to reactivate
soman-inhibited AChE is significantly higher
compared to all other studied oximes. However, it is
not able to reactivate soman-inhibited AChE in the
brain as obidoxime and trimedoxime.
Table 1. LD50 values of oximes following i.m. administration in male rats and mice.
| Oximes |
LD50 (mg/kg) ± 95% confidence limit | | Rats |
Mice | | K203 |
326.4 (285.4-373.2) |
137.8 (116.2-163.3) | | HI-6 |
781.3 (738.4-826.6) |
501.1 (437.0-573.7) | | Obidoxime |
240.7 (180.5-267.2) |
115.2 (89.6-133.8) | | Trimedoxime |
258.2 (220.4-267.2) |
105.8 (93.3-112.2) | | Methoxime |
442.2 (421.2-477.8) |
453.3 (406.5-504.4) |
Table 2. Percentage of reactivation of soman-inhibited AChE by oximes in male rat blood, diaphragm and brain in vivo.
| Treatment |
AChE activity (kat/l or kat/kg) | | Blood |
Diaphragm |
Brain | | Atropine |
5.09±1.65a |
7.15±2.56a |
46.32±24.19a | | Atropine + K203 (% reactivationb) |
5.98±1.20 (4.8) |
8.60±1.32 (14.1) |
49.66±23.63 (3.5) | | Atropine + HI-6 (% reactivation) |
9.71±0.78 (31,9*x) |
12.15±2.02 (51.2*x) |
43,70±27.30 (0) | | Atropine + obidoxime (% reactivation) |
7.59±1.25 (17.3) |
6.75±2.52 (0) |
43.44±13.40 (0) | | Atropine + trimedoxime (% reactivation) |
5.10±1.03 (0.1) |
6.70±2.06 (0) |
42.93±21.78 (0) | | Atropine + methoxime (% reactivation) |
5.79±1.19 (6.2) |
9.15±1.06 (20.5) |
53.20±26.82 (7.1) |
a means ± SD, N = 8. The untreated control value (saline control) for rat blood AChE activity was 16.58 kat/L, for diaphragm
AChE activity 16.91 kat/kg and for brain AChE activity 142.70 kat/kg
b percent reactivation was determined using the AChE activity values: {1- [((saline control) - (oxime + atropine))/((saline control)
- (atropine control))]} x 100
* significantly different from the atropine group
x significantly different from the atropine + obidoxime (trimedoxime, methoxime, K203) group
These results roughly correlate with the
therapeutic potency of the oximes tested against lethal
soman poisoning in mice (Table 3). Soman-poisoned
mice showed wide spectrum of clinical signs of
poisoning including muscarinic (salivation) and
niconitic (tonic-clonic convulsions) signs within a
few minutes regardless of the type of antidotes. They
died within 40-60 minutes after poisoning with
soman. The oxime K203 was able to decrease the
acute toxicity of soman approximately 1.4-fold and,
thus, its therapeutic efficacy corresponds to the
effectiveness of obidoxime and trimedoxime. On the
other hand, its potency to reduce acute lethal toxic
effects of soman in mice is slightly lower than the
therapeutic efficacy of methoxime and significantly
lower than the therapeutic efficacy of the oxime HI-6
that is able to decrease the acute toxicity of soman
2.8-fold. Thus, the oxime HI-6 showed significantly
higher potency to reduce acute lethal toxic effects of
soman in mice in comparison with other studied
oximes.
Table 3. The influence of the type of oxime on the potency of antidotal treatment to reduce acute toxicity of soman in male
mice.
| Treatment |
LD50 (g/kg) ± 95% confidence limit |
Protective ratio | | Atropine |
74.3 (62.1-88.9) |
- | | K203 + atropine |
109.4 (98.9-125.5)* |
1.47 | | HI-6 + atropine |
211.4 (158.1-282.8)*x |
2.85 | | Obidoxime + atropine |
111.0 (99.1-127.4)* |
1.49 | | Trimedoxime + atropine |
110.5 (102.5-128.6)* |
1.49 | | Methoxime + atropine |
133.4 (109.9-181.2)* |
1.80 |
* significantly different from the group treated by atropine alone
x significantly different from the group treated by atropine in combination with obidoxime (trimedoxime, K203)
DISCUSSION
Based on the previously published results, the novel
oxime K203 seems to be effective reactivator of
tabun-inhibited AChE and it is considered to be
suitable oxime for the antidotal treatment of acute
tabun poisonings (Kassa et al. 2008, Kovarik et al.
2009). However, to reach the satisfactorily effective
antidotal treatment of nerve agent poisoning, the
broad-spectrum oxime, sufficiently effective against
all nerve agents regardless of their chemical structure,
should be found. As no broad spectrum oxime has
been developed till now (Marrs et al. 2006, Kassa et
al. 2007, Szinicz et al. 2007), it is important to know
if some of newly developed oximes is able to
sufficiently protect organisms against all nerve
agents. Therefore, the evaluation of the ability of
novel oximes to reactivate nerve agent-inhibited
AChE and protect against acute signs and symptoms
of nerve agents regardless of their chemical structure
is necessary.
In this paper, the potency of the novel oxime
K203 to reactivate soman-inhibited AChE and protect
against acute toxicity of soman was evaluated in
comparison with chosen currently available oximes
including the oxime HI-6 that is considered to be the
best oxime against soman (Kassa and Cabal 1999,
Lundy et al. 2006). Our results demonstrate that the
reactivating and therapeutic efficacy of the oxime
K203 roughly corresponds to the efficacy of
obidoxime and trimedoxime but it is slightly less
effective than methoxime and significantly less
effective than the oxime HI-6. These results might not
be explained solely by significant differences in the
reactivating efficacy of the oximes studied in the
peripheral compartment (Shih 1993, Worek et al.
1998) but also by other antidotal mechanisms
observed after administration of the oxime HI-6, such
as direct antimuscarinic action and restoration of
neuromuscular transmission (van Helden et al. 1996).
There are several studies demonstrating that the
ability of HI-6 to antagonize soman-induced toxic
effects is significantly higher compared to other
commonly used oximes (Kassa and Cabal 1999,
Kassa 2002, Lundy et al. 2006, Marrs et al. 2006,
Jokanovic and Prostran 2009, Kuca et al. 2009). The
relatively high therapeutic potency of the oxime HI-6
may be due to various antidotal mechanisms based on
reactivation of phosphonylated AChE, direct
antimuscarinic and ganglion blocking actions,
restoration of neuromuscular transmission, retardation
of the formation of the aged inhibitor-enzyme
complex and inhibition of acetylcholine release (van
Helden et al. 1996). Thus, only the oxime HI-6
appears to be able to sufficiently protect experimental
animals from soman-induced adverse effects and
improve survival of soman-poisoned animals (Kassa
and Cabal 1999, Lundy et al. 2006).
On the other hand, the oxime HI-6 as well as the
other oximes studied including the oxime K203 are
not able to sufficiently reactivate soman-inhibited
AChE in the central nervous system due to difficult
penetration through the blood-brain barrier. The lack
of penetration of the oximes through the blood-brain
barrier is caused by the quaternary structure of
oximes (Lundy et al. 1990, Lorke et al. 2008, Zdarova
Karasova et al. 2011).
The above described data confirm that HI-6 is a
significantly more efficacious oxime than other
currently available oximes in the case of the antidotal
treatment of severe soman poisoning although its
therapeutic efficacy is also rather limited probably
because of the lack of its central reactivation efficacy.
On the contrary, the newly developed oxime K203
seems to be significantly less efficacious to reactivate
soman-inhibited AChE in rats and reduce lethal toxic
effects of soman in mice than the oxime HI-6 and,
therefore, it is not suitable for the replacement of the
oxime HI-6 for the treatment of acute soman
poisonings.
DECLARATION OF INTERESTS
The authors report no conflict of interest. The authors
alone are responsible for the content and writing of
the paper.
ACKNOWLEDGEMENTS
The authors wish to thank Mrs Jana Uhlirova for her
skilful technical assistance. The study was supported
by a long-term organization development plan 1011.
REFERENCES
Bajgar J. Organophosphate/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem. 38: 151-216, 2004.
[CrossRef]
Clement JG, Hansen AS, Boulet CA. Efficacy of HLo-7 and pyrimidoxime as antidotes of nerve agent poisoning in mice. Arch Toxicol. 66: 216-219, 1992.
[CrossRef]
[PubMed]
Ekstrom F, Akfur C, Tunemalm AK, Lundberg S. Structural changes of phenylalanine 338 and histidine 447 revealed by the crystal structures of tabun-inhibited murine acetylcholinesterase. Biochemistry. 45: 74-81, 2006.
[CrossRef]
[PubMed]
Ellman GL, Courtney DK, Andres V, Jr., Feartherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7: 88-93, 1961.
[CrossRef]
Jokanovic M, Prostran M. Pyridinium oximes as cholinesterase reactivators. Structure-activity relationship and efficacy in the treatment of poisoning with organophosphorus compounds. Curr Med Chem. 16: 2177-2188, 2009.
[CrossRef]
[PubMed]
Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J Toxicol Clin Toxicol. 40: 803-816, 2002.
[CrossRef]
[PubMed]
Kassa J, Cabal J. A comparison of the efficacy of a new asymmetric bispyridinium oxime BI-6 with currently available oximes and H oximes against soman by in vitro and in vivo methods. Toxicology. 132: 111-118, 1999.
[CrossRef]
Kassa J, Kuca K, Bartosova L, Kunesova G. The development of new structural analogues of oximes for the antidotal treatment of poisoning by nerve agents and the comparison of their reactivating and therapeutic efficacy with currently available oximes. Curr Org Chem. 11: 267-283, 2007.
[CrossRef]
Kassa J, Karasova J, Musilek K, Kuca K. An evaluation of therapeutic and reactivating effects of newly developed oximes (K156, K203) and commonly used oximes (obidoxime, trimedoxime, HI-6) in tabun-poisoned rats and mice. Toxicology. 243: 311-316, 2008.
[CrossRef]
[PubMed]
Kovarik Z, Vrdoljak AL, Berend S, Katalinic M, Kuca K, Musilek K, Radic B. Evaluation of oxime K203 as antidote in tabun poisoning (in Croatian). Arh Hig Rada Toksikol. 60: 19-26, 2009.
[CrossRef]
[PubMed]
Kuca K, Musilek K, Jun D, Pohanka M, Zdarova Karasova J, Novotny L, Musilova L. Could oxime HI-6 really be considered as "broad-spectrum" antidote? J Appl Biomed. 7: 143-149, 2009.
[JAB]
Lorke DE, Kalasz H, Petroianu GA, Tekes K. Entry of oximes into the brain: A review. Curr Med Chem. 15: 743-753, 2008.
[CrossRef]
[PubMed]
Lotti M. Organophosphorus compounds. In Spencer PS, Schaumburg HH (eds.): Experimental and Clinical Neurotoxicology. Oxford University Press, New York 2000, pp. 898-925.
Lundy PM, Hand BT, Broxup BR, Yipchuck G, Hamilton MG. Distribution of the bispyridinium oxime [14C] HI-6 in male and female rats. Arch Toxicol. 64: 377-382, 1990.
[CrossRef]
[PubMed]
Lundy PM, Raveh L, Amitai G. Development of the bisquaternary oxime HI-6 toward clinical use in the treatment of organophosphate nerve agent poisoning. Toxicol Rev. 25: 231-243, 2006.
[CrossRef]
[PubMed]
Marrs TC, Rice P, Vale JA. The role of oximes in the treatment of nerve agent poisoning in civilian casualties. Toxicol Rev. 25: 297-323, 2006.
[CrossRef]
[PubMed]
McDonough JR, Shih T-M. Neuropharmacological mechanisms of nerve agent - induced seizure and neuropathology. Neurosci Biobehav Rev. 21: 559-579, 1997.
[CrossRef]
Musilek K, Holas O, Kuca K, Jun D, Dohnal V, Opletalova V, Dolezal M. Synthesis of monooxime-monocarbamoyl bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against tabun- and paraoxon-inhibited acetylcholinesterase. J Enzyme Inhib Med Chem. 23: 70-76, 2008.
[CrossRef]
[PubMed]
Puu G, Artursson E, Bucht G. Reactivation of nerve agent inhibited acetylcholinesterases by HI-6 and obidoxime. Biochem Pharmacol. 35: 1505-1510, 1986.
[CrossRef]
Shih T-M. Comparison of several oximes on reactivation of soman-inhibited blood, brain and tissue cholinesterase activity in rats. Arch Toxicol. 67: 637-646, 1993.
[CrossRef]
[PubMed]
Szinicz L, Worek F, Thiermann H, Kehe K, Eckert S, Eyer P. Development of antidotes: problems and strategies. Toxicology. 233: 23-30, 2007.
[CrossRef]
[PubMed]
Tallarida R, Murray R. Manual of Pharmacological Calculation with Computer Programs. Springer-Verlag, New York 1987.
Tryphonas L, Clement JG. Histomorphogenesis of soman-induced encephalocardio- myopathy in Sprangue-Dawley rats. Toxicol Pathol. 23: 393-397, 1995.
[CrossRef]
[PubMed]
van Helden HPM, Busker RW, Melchers BPC, Bruijnzeel PLB. Pharmacological effects of oximes: how relevant are they? Arch Toxicol. 70: 779–786, 1996.
[CrossRef]
[PubMed]
Worek F, Widmann R, Knopff O, Szinicz L. Reactivating potency of obidoxime, pralidoxime, HI-6 and HLo-7 in human erythrocyte acetylcholinesterase inhibited by highly toxic organophosphorus compounds. Arch Toxicol. 72: 237-243, 1998.
[CrossRef]
[PubMed]
Zdarova Karasova J, Zemek F, Bajgar J, Vasatova M, Prochazka P, Novotny L, Kuca K. Partition of bispyridinium oximes (trimedoxime, K074) administered in therapeutic doses into different parts of the rat brain. J Pharm Biomed Anal. 54: 1082-1087, 2011.
[CrossRef]
[PubMed]
|
BACK
|


