Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 11 (2013) No 1, p 27-32
DOI 10.2478/v10136-012-0016-9
Automated detection of organophosphate warfare gases (nerve agents) in air based on micro-SIA - lab-on-valve system
Ondrej Pavlicek, Miroslav Polasek, Martin Foltyn, Jiri Cabal
Address: Jiri Cabal, Faculty of Military Health Sciences, University of Defense, POB 35-T, 500 01 Hradec Kralove, Czech Republic
cabal@pmfhk.cz
Received 24th February 2012.
Revised 2nd April 2012.
Published online 6th April 2012.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results and Discussion
Conclusions
Acknowledgements
References
SUMMARY
Equipment for fast and accurate detection of organophosphate nerve agents is developed and tested. The method is based on the spectrophotometric
monitoring of the enzyme activity of butyrylcholinesterase after its contact with air in a special absorption unit (a “scrubber”) developed for the
purpose. The scrubber was made from a glass tube filled with glass beads (diam. 3 mm) and filled with approx. 5 ml of butyrylcholinesterase in a
phosphate buffer of pH 7.4. The air sample was bubbled through this solution for 20 s at a flow rate of 80 l hour-1. Thereafter 8 microl of
the enzyme solution were aspirated into the micro-SIA-LOV analyzer and the activity of the enzymes were evaluated by using Ellman’s reagent, i.e. 2.5
mmol l-1 butyrylthiocholine iodide and 0.25 mmol 5,5’-dithiobis (2-nitrobenzoic acid). The absorbance of the coloured reaction product was
measured at 412 nm after the reaction time of 60 s. The residue of the absorption liquid was washed away from the absorber and the system was washed
with the enzyme solution prior to next analysis. The contaminated air caused partial inhibition of the enzyme activity of the absorption liquid. The
activity of the contaminated sample was compared with the activity of the unaffected enzyme (blank measurement). The analysis was controlled by two
PCs. The effect of the concentration of analyte in the absorption liquid on the enzyme activity was tested for 10-5-10-9 mol
l-1 sarin. A single analysis (including the absorption step) took <130 s.
KEY WORDS
sequential injection analysis; lab-on-valve; organophosphate; cholinesterase; nerve agent; sarin
INTRODUCTION
Organophosphate-based warfare gases (nerve agents
such as sarin, soman, tabun and VX that interfere
with the central nervous system through inhibition of
cholinesterases) are potentially fatal chemicals that
are banned by the Chemical Weapons Convention
(CWC) (OPCW 1994). The relative simplicity of their
illegal production at minimal expense, release of sarin
vapours in the Tokyo subway system in 1995 and
several other events in the past decade have raised concerns that they could possibly be used for
large-scale terrorist attacks against civilians.
Therefore relatively inexpensive automated analytical
devices capable of rapid on-spot sensing of nerve
agents (NAGs) in air and giving early warning to
civilian responders or military personnel are of
interest. A number of sophisticated but costly
analytical methods (such as ion-mobility MS,
GC-MS, Surface Acoustic Wave Sensing and FT-IR)
have been officially approved by the CWC for routine detection and monitoring of CWC chemicals
(Mesillakso 2005).
In the present paper we attempt to utilize the
inhibitory effect of the NAGs on butyrylcholinesterase (BuChE) for their quantitative assay
through spectrophotometric measurement of the
decreased activity of BuChE (exposed to an NAG) by
Ellman's reaction (Ellman et al. 1961). This indicator
reaction involves interaction of the BuChE with
butyrylthiocholine (BTCh) that is hydrolysed to
thiocholine; thiocholine reacts with Ellman's reagent
[5,5´-dithio-bis(2-nitrobenzoic) acid] (DTNBA) to
produce yellow thionitrobenzoate exhibiting ma-ximum absorption at 412 nm (proportionally related
to the activity of the BuChE). The concept of
sequential injection analysis (SIA) in the lab-on-valve
(LOV) format developed by Ruzicka (2000) was
employed to automate the manipulation of reactant
solutions at microl levels.
MATERIAL AND METHODS
Chemicals
Lyophilized BuChE from equine serum was
purchased from SEVAC (Prague); BTCh, DTNBA,
insecticide tetraethyl pyrophosphate (TEPP),
insecticide 1-naphthyl-N-methylcarbamate (Sevin)
and anhydrous ethanol were obtained from Aldrich;
KH2PO4 and Na2HPO4.12H2O were obtained from
Lachema (Brno), sarin was obtained from the NAGs
collection of the Faculty of Military Health Sciences,
Hradec Kralove.
Apparatus
A PC-controlled micro-SIA apparatus equipped with
a lab-on-valve platform (FIAlab Instruments Inc.,
Bellevue, USA) comprising a 6-port selection valve,
a 1.0 ml syringe pump and USB2000 Ocean Optics
spectrophotometer was used as the basis of the NAG
detection setup. A glass scrubber packed with glass
beads (see Fig. 1) included three PC-controlled
valves; it was manufactured in the lab workshop. A
miniature air pump was used to bubble the sample air
through the scrubber. Air samples with defined sarin
concentrations were prepared by using a dynamic
evaporation chamber (Sevelova et al. 2003). For a
scheme of the measurement protocol see Table 1.
Optimization and procedures
Initially the SIA conditions for Ellman's reaction
were optimized away from the scrubber by using less
hazardous BuChE inhibitor solutions, namely Sevin
and TEPP, with respect to concentrations and volumes of reactants aspirated, time of BuChE -
inhibitor interaction (20-600 s) and inhibited BuChE
(Ellman's reagent + BTCh) incubation time
(15-120 s). Inhibition curves relating the percentage
of BuChE retained activity (EA%) to the concentration
of the inhibitors (10-9-10-4 mol l-1) were measured;
the EA% was calculated as (hx/h0).100 where the hx
and h0 are the peak heights of the thionitrobenzoate at
412 nm obtained with the inhibited and native
uninhibited enzyme injections respectively. After this,
the inhibition curve of BuChE with sarin solutions
was measured. Consequently the dependence of the
level of inhibition of enzyme on the time of BuChE -
sarin interaction (300-700 s) was also examined.
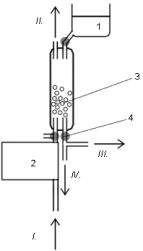
Fig. 1. Scrubber module. 1, fresh enzyme solution container; 2, air pump; 3, glass
beads and absorbing liquid; 4, PC-controlled pinch valves;
I, air inlet; II, air outlet; III, absorbing liquid to SIA;
IV, waste.
To study the effect of the air contaminated with
sarin on the EA%, the air sample containing 1 microg/l of
sarin was pumped through the scrubber (packed with
glass beads) containing 5 ml of absorbing liquid
(0.12 mg ml-1 of BuChE in phosphate buffer, pH
7.40) at the flow rate of 80 l.h-1 for 20 s. Thereafter
8 microl of the absorbing liquid and 2 microl of the reagent
(2.5 mmol BTCh + 0.25 mmol DTNBA) were
aspirated, allowed to react for 60 s and sent to the
detector at a flow rate of 4 microl s-1 (peak height hx); the
h0 was obtained by aspirating 8 microl of uninhibited
enzyme solution (0.12 mg ml-1 of BuChE in
phosphate buffer of pH 7.4) instead of the absorbing
liquid. Also in this measurement the dependence of
the level of inhibition of enzyme on the duration of
BuChE - sarin interaction (300-700 s) was evaluated.
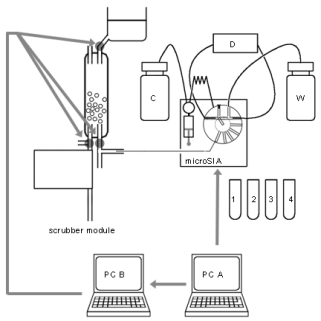
Fig. 2. Scheme of the system, connection of the absorber and SIA units. PC A, controlling micro-SIA-LOV; PC B, controlling the scrubber valves;
C, carrier solution; D, spectrophotometric detector; W, waste. In the given part of the program, PC A acts as an actuator giving a signal to PC B to
execute a new absorption cycle.
1, 2, 3, 4 - vials containing reagents and substrate solution.
RESULTS AND DISCUSSION
Inhibition curves
Optimum SIA conditions for the measurement of the
inhibition curves of BuChE with Sevin as a relatively
weak inhibitor (carbamate) and TEPP as much
stronger inhibitor (organophosphate) were the
following: carrier water, order of the aspirated zones:
4 microl of the inhibitor solution, 8 microl of 0.12 mg ml-1
BuChE in phosphate buffer pH 7.4, another 4 microl of
the same inhibitor solution (sandwich mode) and
inhibition time 60 s; thereafter 8 microl of reagent solution
(2.5 mmol l-1 BTCh + 0.25 mmol l-1 DTNBA)
aspirated and after the incubation time of 60 s the
zones with the yellow reaction product
thionitrobenzoate were pushed at 4 microl s-1 into the
detector channel of the LOV module. The inhibition
curves of Sevin (curve 1 and 2) and TEPP (curves 3
and 4) for inhibition times 60 s and 600 s are shown
in Fig. 3. It can be clearly seen that even at 60 s
inhibition time TEPP can be reliably detected
(causing 10% BuChE inhibition) at 0.1 micromol l-1
concentration levels with repeatability characterized
by RSD 3.6% (n=5).
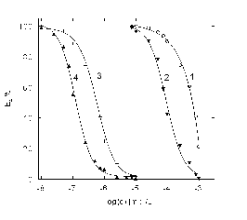
Fig. 3. Inhibition curves (dependence of EA% on the
concentration of inhibitor) of BuChE as measured by the
spectrophotometric SIA-LOV technique with inhibitor
solutions containing Sevin (1, 2) or TEPP (3, 4) at
inhibition times 60 s (1, 3) and 600 s (2, 4).
A sarin inhibition curve was obtained with
aqueous sarin solutions. Consequently, 0.2 ml of sarinsolution was added to 1.8 ml of the enzyme solution
(0.012 mg ml-1 BuChE in phosphate buffer, pH 7.4)
to obtain concentration scale 10-5 to 10-9 mol l-1 of
sarin. After 300 s inhibition, enzyme activity was
determined by SIA. 8 microl of this enzyme solution and 2 microl of reagent solution were aspirated, allowed to
react for 60 s and sent to detector at the flow rate
of 4 microl s-1. The enzyme activity was then determined
with the same samples in the same way at the times of
inhibition 400, 500, 600 and 700 s.
Table 1. Measurement protocol.
| Step |
SIA measurement protocol | | 1 |
SIA washes the detection cell, aspirates and flushes 500 microl of water | | 2 |
spectrometer performs a reference scan | | 3 |
SIA aspirates 300 microl water + 8 microl of blank enzyme solution + 2 microl of substrate - reagent
solution | | 4 |
enzyme - substrate interaction, delay 60 s | | 5 |
SIA delivers 75 microl to spectrophotometer | | 6 |
reaction product measurement at 412 nm | | 7 |
SIA flushes the holding coil and empty the detection cell | | 8 |
SIA aspirates 600 microl of enzyme solution from absorber and flushes it to prevent sample
contamination | | 9 |
SIA aspirates 300 microl water + 8 microl of enzyme from absorber | | 10 |
PC A, controlling SIA sends information to PC B, controlling absorber, to wash the scrubber from the
inhibited enzyme solution and to start a new cycle of absorption of sarin from the air | | 11 |
SIA aspirates 2 microl of substrate - reagent solution | | 12 |
enzyme - substrate interaction, delay 60 s | | 13 |
SIA delivers 75 microl to spectrophotometer | | 14 |
reaction product measurement at 412 nm | | 15 |
SIA flushes the holding coil and empty the detection cell | |
| | Step |
Absorber protocol | | 1 |
filling absorption space with approx. 5 ml of enzyme solution | | 2 |
air sample bubbles through for 20 s | | 3 |
enzyme solution is flushed to collection point, where is consequently aspirated by SIA | | 4 |
PC B is waiting for impulse from PC A | | 5 |
the residual enzyme solution is flushed into waste, the scrubber is washed by filling and emptying with the
enzyme solution into absorption space | | 6 |
new absorption process starts |
This observation of the dependence of inhibition
on the time for each concentration of sarin showed
that at a time of inhibition under 400 s it is not
possible to reach 0% enzyme activity. See Fig. 4.
The inhibition curve resulting from the SIA-LOV
detection of sarin in liquid samples under optimum
conditions indicated in the experimental section is
shown in Fig. 5.
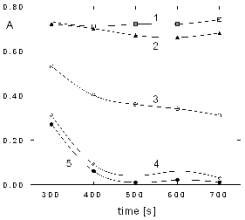
Fig. 4. Dependece of inhibition on the time of interaction
between the enzyme and sarin. A, absorbance of the reaction product at 412 nm;
concentrations of sarin [mol/l]: 1, 10-9; 2, 10-8; 3, 10-7;
4, 10-6 and 5, 10-5.
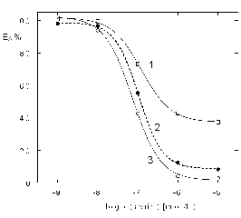
Fig. 5. The inhibition curves of BuChE with sarin in
liquid samples at different times of inhibition. Inhibition time: 1, 300 s; 2, 400 s and 3, 700 s.
Detection of sarin in air
In order to verify the ability to convert sarin from the
air sample (containing 1 microg l-1 of sarin) into a liquid
medium, solution of BuChE in phosphate buffer was
used as the liquid phase. The enzyme solution (placed
in the scrubber) was purged with the sarin-spiked air
sample for 20 s and consequently the enzyme activitywas determined in the way described above by the
reaction of 8 microl of enzyme solution and 2 microl of
reagent-substrate solution.
For 300-700 s time of inhibition 65-45% of the
original enzyme activity was retained. This is
equivalent to 0.1 micromol l of sarin in the enzyme
solution. This fact shows that only approx. 3% of
sarin has been recovered from the air sample.
Decreasing the air flow rate would be desirable for
improving the conversion efficiency but this was not
possible because of the technical limitations of the
absorber unit.
CONCLUSIONS
It can be concluded that under these conditions the
detectable threshold concentration of sarin in liquid
medium causing 10% inhibition of BuChE is
approximately 1.4 mg m-3 of sarin. Considering
approx. 3% efficiency of 20 s recovery of sarin from
air samples into liquid, the threshold concentration
corresponds 0.2 mg m-3 of sarin in the air. Effective
dose of sarin vapours causing miosis, the very first
recognizable effect of it, is ECt50 = 2 mg.min/m3,
while the lethal dose is LCt50 = 70 mg.min/m3 (Riegle
et al. 1994). Using proposed instrument, sarin vapours
can be safely detected. Our data compare well with
data appearing recently for a commercial acoustic
wave sensor (30 mg m-3) (Matsushita et al. 2005) or
portable ion mobility mass spectrometer (below 0.1
mg/m-3) (Maruko et al. 2006). Selectivity of the
proposed micro-SIA-LOV device to NAGs is limited
by the fact that any other strong BuChE inhibitors
(e.g., insecticides) will also give a response. On the
other hand, this fact can be considered an advantage
since any dangerous BuChE inhibitors can be
detected in this way. While the tested SIA-LOV
method using cholinesterase activity detection
according to Ellman's reaction is fast and sensitive
and the the scrubber - SIA-LOV enables fully
automated collection and testing of the air samples for
the presence of sarin, the consumption of enzyme
solution in the absorber unit and power consumption
are excessive. The low efficiency of the scrubber
decreases the sensitivity of the system.
ACKNOWLEDGEMENTS
This work was supported by the Czech Ministry of
Education, Research Project MSM 0021620822 and
grant SVV-2010-261-001. This work was supported
by the Czech Ministry of Defence, Project
OVUOFVZ200801.
REFERENCES
Ellman GL, Courtney DK, Andres V. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7: 88-90, 1961.
[CrossRef]
Maruko H, Sekiguchi H, Seto Y, Sato A. Detection performance of aspiration-type ion mobility spectrometer for chemical warfare agents. Bunseki Kagaku.
55: 191-197, 2006.
[CrossRef]
Matsushita K, Sekiguchi H, Seto Y. Performance of portable surface acoustic wave sensor array detector for chemical agents (in Japanese). Bunseki
Kagaku. 54: 83-88, 2005.
[CrossRef]
Mesillakso M. Chemical Weapons Convention Chemicals Analysis. Sample Collection, Preparation and Analytical Methods. J. Wiley & Sons, Chichester,
2005, pp. 67-77.
[CrossRef]
OPCW. Convention on the Prohibition, Stockpiling and Use of Chemical Weapons and on their Destruction. Organisation for the Prohibition of Chemical
Weapons, The Hague, 1994.
Riegle DW, D'Amato AM. U. S. Chemical and Biological Warfare-Related Dual Use Exports to Iraq and their Possible Impact on the Health Consequences of
the Gulf War. United States Senate, 103rd Congress, 2nd Session, May 25, 1994, http://www.gulfweb.org/bigdoc/report/appgb.html,
downloaded: 7th Feb 2012.
Ruzicka J. Lab-on-valve: Universal microflow analyzer based on sequential and bead injection. Analyst. 125: 1053-1060, 2000.
[CrossRef]
Sevelova L, Vachek J, Bajgar J. Inhalation apparatus for generating sarin and soman toxic vapors. Acta Medica (Hradec Kralove). 46: 157-161, 2003.
[PubMed]
|
BACK
|






