SUMMARY
Enterovirus 71 (EV71) infection and its associated hand-foot-mouth disease is a significant public health problem. The purpose of this study is to develop a novel vaccine to prevent EV71 infection. Bacillus subtilis spores were engineered to express VP1 protein of EV71 with CotB as carrier protein. The recombination was tested in adult mice for the ability to induce immune responses. Mice were inoculated orally and intranasally simultaneously with the spores. The vaccine’s efficacy on stimulating immune responses was evaluated by measuring the titer of anti-VP1 IgG and IgA with enzyme-linked-immunosorbent serologic assay (ELISA), and the number of VP1-specific T cells by ELIS-POT. Serum titers of IgG (0.41±0.05 vs 0.20±0.07) and IgA (0.24±0.02 vs 0.11±0.01) in mice immunized with recombinant CotB-VP1 spores were higher than that of mice immunized with nonrecombinant spores 1A771. Splenocytes from the group of mice receiving VP1 spores vaccination contained 1.69±0.52/104 VP1-specific T cells, which was greater than the 0.06±0.06/104 cells from the group of mice receiving nonrecombinant spores vaccination. In conclusion, Bacillus subtilis spores displaying VP1 of EV71 are effective in stimulating cellular immunity and humoral immunity in mice.
KEY WORDS
enterovirus 71; immunity; vaccine; Bacillus subtilis spores
INTRODUCTION
Enterovirus 71 (EV71) is a member of the Human Enterovirus A species and belongs to the genus of Enterovirus from the family Picornaviridae. EV71 infection often results in hand-foot-mouth disease or herpangina. In the last four decades, several epidemic outbreaks have occurred across the globe, with most recent major outbreaks being in Asia where high mortality rates have been reported (Chan et al. 2000, Chen et al. 2007, Ooi et al. 2010, Tian et al. 2012). EV71 possesses four structural proteins VP1-VP4 that are necessary in the formation of the pentameric icosahedral capsid. The VP1-VP3 are located on the surface of virion (Oberste et al. 2003). VP1 is the prime structural protein with the most neutralizing epitopes, and the sequence of VP1 has high correlation with serotype (Carrillo et al. 1998, Shih et al. 2000, Yu et al. 2000, Foo et al. 2007). Studies have demonstrated that VP1 subunit vaccine expressed in either bacteria or transgenic plants could protect mice against enterovirus 71 infection (Wu et al. 2001, Chen et al. 2006, Chen et al. 2008, Lee and Chang 2010). Moreover, VP1 capsid protein has the ability to withstand the hydrochloric acid concentration in human gastric juice, so it can be given as an oral vaccine. Previous data has shown the feasibility of VP1 as an effective subunit vaccine for mucosal delivery (Luiz et al. 2008).
Recombinant Bacillus subtilis spores may be employed as safe and low cost live vaccine vehicles through mucosal immunity approach (Fais et al 1987, Kosaka et al. 1998, Isticato et al. 2001, Duc et al. 2004, Fakhry et al. 2008, Ceragioli et al. 2009, Cutting et al. 2009; Li et al. 2009, Knecht et al. 2011). Isticato et al. (2001) had successfully constructed a system to display biologically active C-terminal fragment of the tetanus toxin on the surface of B. subtilis spores. Others coat proteins of B. subtilis spores were also used as carrier proteins to display heterologous antigens on the surface of B. subtilis spores (Duc et al. 2004, Mauriello et al. 2004, Kim et al. 2007, Uyen et al. 2007, Hinc et al. 2010, Lee et al. 2010, Potot et al. 2010) and the recombinations successfully induced both local immunity and systemic immune responses. The mice orally immunized with the recombination spores can survive from the tetanus toxin challenge (Duc et al. 2004, Mauriello et al. 2004, Kim et al. 2007, Uyen et al. 2007, Hinc et al. 2010, Lee et al. 2010, Potot et al. 2010). Studies also show that the antigen displayed on the spore surface produces a more pronounced response than that on germinating spore (Barnes et al. 2007).
It is therefore an attractive proposition that different heterologous antigens can be displayed simultaneously on the spores by different carrier proteins, and that spores can be developed into a polyvalent vaccine. The purpose of this study was to investigate the feasibility of displaying VP1 protein of EV71 on the surface of B. subtilis spores, and to evaluate the humoral immunity and cellular immunity of this novel vaccine in a mice model that is immunized with the recombination spores.
MATERIALS AND METHODS
VP1 protein preparation and antiserum production
The bacteria which express VP1 protein (Yue et al. 2011) was induced by isopropyl-1-thio-beta-D-galactopyranoside for 4 h (Caruso et al. 1993). The bacteria cells were collected by centrifugation and broken by sonication. The VP1 protein was purified by Ni SepharoseTM High PerformanceHigh-performance Purification (GE) according to the instructions of the manufacturer. Purified VP1 protein was administered by subcutaneous route to BALB/c mouse (20 g, Shandong University, Jinan, China), followed by three boosts at 7-day intervals. Blood was collected at 3 days after the last injection. The antisera were pooled together and stored at -80 °C until analyzed. The titers were determined by enzyme-linked-immunosorbent serologic assay (ELISA).
Bacterial strains and transformation
B. subtilis strains and integration vector utilized are 1A771 and pDG1662 bought from Bacillus Genetic Stock Center. Plasmid amplification for nucleotide sequencing, subcloning experiments, and transformation of B. subtilis competent cells were performed with Escherichia coli strain DH5alpha;. E. coli competent cells (TransGen Biotech, Beijing China), and transformed according to the instructions.
Construction of gene fusions
The strategy for the construction of the gene fusions is shown in Fig. 1. To obtain cotB-based gene fusions, cotB DNA was first amplified by PCR using the B. subtilis chromosome as template. The amplification was primed with the synthetic oligonucleotides cotB1 and cotB2 oligonucleotides (5’gaattcACG GATTAGGCCG T T T GTCC3’ and 5’aagcttGGATGATTGATCATCTGAAG3’, respectively, annealing at -263-/-244 and +806/+825 of cotB; capital and small letters indicate bases of complementarity with cotB and an unpaired tail carrying a restriction site). The PCR products were visualized on ethidium bromide-stained agarose gels, which were purified by the EasyPure Quick Gel Extraction Kit (TransGen Biotech) as specified by the manufacturer. An amplification product of the expected size (1100 bp) was cloned into the pMD18-T vector (Takara) yielding plasmid pMD18-cotB. The VP1 DNA was amplified by RT-PCR using the enterovirus 71 genome as template, which was abtained by the QIAAmp@ Viral RNA Mini Kit (Qiagen). The amplification was primed with the synthetic oligonucleotides VP1s and VP1a oligonucleotides (5’aagcttGATAGGGTGGCAGATGTAAT3’ and 5’ggatcctcaGTTGGCTTTGAATAGGTAGTT3’, respectively, annealing at +4/+23 and +808/+828 of VP1; capital and small letters indicate bases of complementarity with VP1 and an unpaired tail carrying a restriction site and a stop codon) by a Quant One Step RT-PCR kit (TianGen Biotech). The amplification product of the expected size (840 bp) was cloned into the pMD18-T vector (Takara) yielding plasmid pMD18-VP1. pMD18-cotB and pMD18-VP1 were double restriction enzyme digested by EcoRI/HindIII and HindIII/BamHI respectively, and the 1100 bp and 840bp fragments were ligated by T4 DNA Ligase (Takara) to construct the cotB-VP1 gene fusion. The cotB-VP1 gene fusion was inserted into the plasmid pDG1662 previously digested with enzymes EcoRI/ BamHI to obtain plasmid pDG1662cotB-VP1.
Chromosomal integration
Plasmid pDG1662-cotB-VP1 linearized by digestion with NdeI and XhoI was used to transform competent cells of the B. subtilis strain 1A771. Seven Cmr clones named CV01-07 were tested by PCR using chromosomal DNA as template and oligonucleotides CotB1/CotB2, VP1s/VP1a and CotB1/VP1a to prime DNA amplification. All seven clones showed amplification products of 1100 bp, 840bp, 1940bp (Fig. 2), which indicates the occurrence of recombination events. One of them, CV02, was used for further studies.
Western-blot analyses
Sporulation of wild-type and recombinant strains was induced by exhaustion method. After 24-h incubation at 37 °C, spores were collected, washed five times, and purified by lysozyme treatment. The number of purified spores obtained was measured by direct counting with a Burker chamber under an optical microscope (Olympus CH with 40x lenses). 1010 purified spores were suspended in 100 microl of distilled water, centrifugating at 120 000 g for 10 min in room temperature, followed by suspension of 100 microl spores in decoating buffer (0.1M NaOH, 0.1M NaCl, 1% SDS, 0.1M DTT), and incubating at 70 °C for 1 h. The product was centrifugated at 120000g for 10min in room temperature, and the supernatant was extracted proteins. Extracted proteins were fractionated on 15% denaturing polyacrylamide gels, electrotransferred to nitrocellulose filters (Millipore), and used for western-blot analysis by standard procedures. Western-blot filters were visualized by ECL Western-blot Detection Kit (Beijing Solarbio Science & Technology, China) as specified by the manufacturer.
Immunofluorescence microscopy
B. subtilis strains (CV02, 1A771) were induced to spores by the same method as described above. Samples were collected by centrifugation at 120 000 g for 10 min at room temperature and purified by lysozyme (Mauriello et al. 2004). The samples were washed three times in PBST, and incubated with mouse anti-VP1 sera for 45 min at 37 °C, washed three times, and then incubated further with goat anti-mouse immunoglobulin G (IgG)-fluorescein isothiocyanate (Boster Biological Technology, Wuhan China) for 45 min at 37 °C. After three washings, the precipitation was resuspended in PBS and viewed under a Nikon Eclipse Ti-S fluorescence microscope.
Immunization of mice, sample collection and analysis
Male BALB/c mice (20 g) were housed in our animal facilities for the duration of the experiment. All animal procedures were in accordance with institutional guidelines. Spores (CV02, 1A771) were prepared and counted as described above. Two groups (sixteen mice in each group) were treated with 1.8 x 1010 spores orally (1.7 x 1010 spores/dose) and intranasally (1.0 x 109 spores/dose) twice a week for 4 weeks. The splenocytes were used to determinate the Interferon gamma (IFN-gamma) by Mouse IFN-gamma precoated ELIS-POT Kit (Dakewe Biotech Co., Shenzhen, China). Serum samples from the mice were collected from the retro-orbital plexus at week 4 and stored at -80 °C until analyzed. The presence of VP1-specific serum IgG antibodies was assayed by ELISA. Flat-bottom microtiter plates (high-binding capacity; JET, Canada) were coated with 100 microl of recombinant VP1 per well (1 microg/ml), and serum samples diluted (1:100 in duplicate were added. After 0.5 h incubation at 37 °C, the plates were washed and horseradish peroxidase-conjugate goat anti-mouse IgG (1:1,500, Boster Biological Technology) and HRP Conjugated Goat anti-Mouse IgA (alpha chain) (Immunology Consultants Laboratory, Oregon, USA) were added respectively. The horseradish peroxidase substrates were added, and the plates were read after 5 min at 450 nm by using an ELISA reader (Molecular Device, SPECTRA max 384 plus).
Statistical analysis
Samples were tested individually, and data were expressed as the mean ± standard error of the mean (SEM). Statistical significance was determined by independent sample t test, and test at the significance level 2alpha=0.05.
RESULT
The VP1 of Enterovirus 71 and the antiserum
The VP1 protein was expressed and purified by Ni SepharoseTM High Performance-High-performance Purification (GE) and used to raise polyclonal antibody in mice. The titer of the antiserum was 1:800 to 1:3200 (data not shown).
Construction and chromosomal integration of gene fusions
Protein CotB was used as carrier protein. The coding part of the VP1 gene of Enterovirus 71 was fused in frame with the coding part of cotB as specified below. Gene fusion retained the promoter of the cotB gene to ensure proper timing of expression during the sporulation process. When CotB was used as a carrier, only DNA encoding the N-terminal 275 amino acid residues of CotB was used (Fig. 1). Genetic stability was obtained by integrating the gene fusion on the B. subtilis chromosome into the coding sequence of the non-essential gene amyE which was included in the pDG1662 vector.
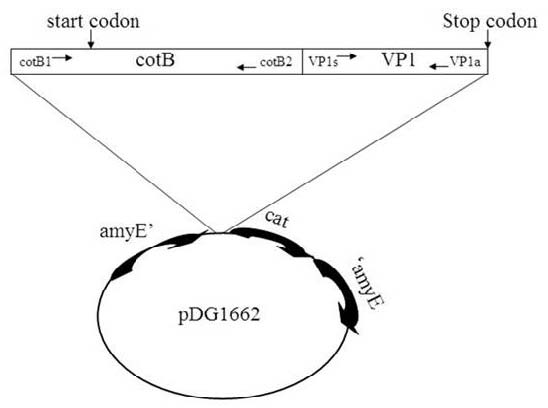
Fig. 1. Schematic representation of the gene fusion obtained and the place it integrated on. The coding region of the VP1 gene (4~828) was cloned in frame to nucleotide 1100 of cotB (-263~825) and integrated the gene fusion on pDG1662 vector.
The gene fusion was integrated on the B. subtilis chromosome after it was integrated on pDG1662 vector. This was identified by double enzymes digestion (Fig. 2). Seven clones identified by PCR (Fig. 3) were named CV01-07 and were used for further analysis. The recombinant strains and their isogenic parental strain 1A771 showed comparable sporulation but the spores of CV02 exfoliated more easily than those of 1A771 (not shown).
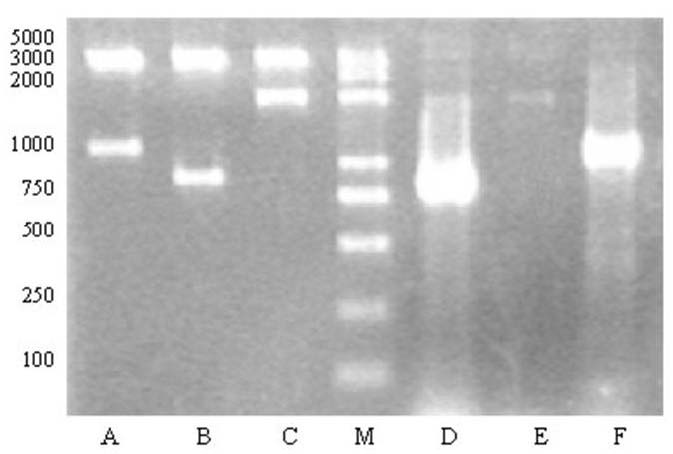
Fig. 2. Agarose gels electrophoresis analysis of double restriction enzyme digested plasmid pDG1662-cotB-VP1 and electrophoresis analysis of homologous recombination. (M) DNA marker; (A) pDG1662-cotB-VP1 digested by EcoRI/ HindIII; (B) pDG1662-cotB-VP1 digested by HindIII/BamHI; (C) pDG1662-cotB-VP1 digested by EcoRI/BamHI; (D) PCR product of VP1 fragment; (E) PCR product of the fusion of VP1 and cotB; (F) PCR product of cotB fragment.
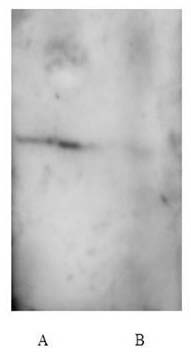
Fig. 3. Western blot analysis of CV2 (CotB-VP1) performed with anti-VP1 of spore coat proteins extracted from strains CV2 (A) and 1A771 (B).
Expression of VP1 on spores
Western blot analysis of spore coat proteins purified from recombinant strains carrying VP1 revealed the presence of an positive band which reacted with VP1 antibodies but that from wild-type was negative (Fig. 4), indicating the presence of VP1 on the spore.
Surface display of VP1
To analyze the surface exposure of CotB-fused VP1 molecules, sporulating cells of wild-type and the recombinant strains were analyzed by immunofluorescence microscopy with VP1-specific primary antibodies and anti-mouse IgG-FITC (Boster Biological Technology) as secondary antibody. While for recombination (CotB-VP1) a strong fluorescence signal was observed around spores and wild-type was negative (Fig. 5). These results indicate that CotB-VP1 is accessible to the antibody and could react with the special antibody. This is not surprising because CotB has the ability to display the protein fused with it on the surface of spores (Ceragioli et al. 2009).
Humoral immunity responses anti-VP1
To test induction of humoral immunity and cellular immunity responses, groups of sixteen mice were immunized orally and intranasally. As shown in Table 1, IgG and IgA in serum titers of mice immunized with CV02 (CotB-VP1) spores were higher than that of mice immunized with 1A771 (nonrecombinant spores, P<0.05), and the difference was also observed with the titer of IgA in irrigating solution of lung. There was no statistically significant difference in the IgA titer between the study and control group mice in the irrigating solution of intestine (P>0.05).
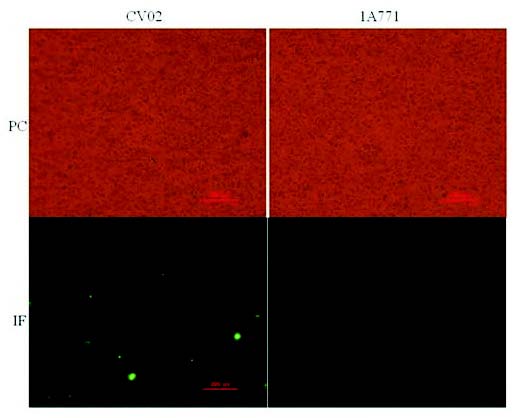
Fig. 4. Immunofluorescence microscopy analysis. Recombination (CV02) and wild-type (1A771) spores are visualized by phase contrast (PC) and by immunofluorescence (IF) microscopy. Samples were labeled with mouse anti-VP1 antisera, followed by anti-mouse IgG-FITC.
Fig. 5. ELIS-POT analysis of cellular immunity. (A) Splenocytes from mice immunized with CV02; (B) Splenocytes from mice immunized with 1A771; (C) positive control; (D) negative control.
Table 1. Comparison of serum and irrigation solution IgG and IgA in mice immunized with recombinant spores (study) and with non-recombinant spores (control).
| N | Serum (IgG) | Serum (IgA) | Irrigating solution of intestine (IgA) | Irrigating solution of lung (IgA) | | Study | 16 | 0.41±0.05* | 0.24±0.02* | 0.15±0.02 | 0.22±0.03* |
|---|
| Control | 16 | 0.20±0.07 | 0.11±0.01 | 0.14±0.05 | 0.16±0.02 |
|---|
* Statistically significant as compared with controls.
Cellular immunity response to VP1
Splenocytes from the group of mice receiving VP1 spores vaccination contained 1.69±0.52/104 VP1specificTcells, which was greater than he 0.06±0.06/104 cells from the group of mice receiving nonrecombinant spores vaccination (statistically significant, Fig. 5), which demonstrated that immunization with spores expressing CotB-VP1 elicited clear VP1-specific cell-mediated immune response.
DISCUSSION
In recent years, bacterial surface displays have received considerable attention in the fields of vaccine delivery. B. subtilis spore has the potential to be developed into a vaccine-delivery vehicle with the advantage of high stability. A number of studies have been conducted to develop the display systems of B. subtilis spores and to evaluate the different coat proteins as carrier (Challacombe 1983, Robinson et al. 1997, Ciabattini et al. 2004). These studies have found that recombinant B. subtilis spores have the characteristics for a vaccine, such as safety, stability, easy preparation, and low cost.
As VP1 of EV71 contains important antigenic sites that contribute to the neutralization of the virus (Carrillo et al. 1998), VP1 has been considered as a major antigenic coat protein and has the potential to act as an antiviral subunit vaccine (Lee et al. 2010). In this study, for the first time, we established the display of VP1 protein of EV71 on the cell surface of B. subtilis spore and studied its feasibility and effectiveness as vaccine in mice. We constructed a fusion gene including part of cotB (-263/+825bp) and part of VP1 (+4/+828bp), and the fusion was integrated on pDG1662 vector which could integrate the object gene on the B. subtilis chromosome by homologous recombination. The cotB transcriptional and translational signals integrated onto the B. subtilis chromosome ensured not only correct timing of expression during sporulation, but also the stable expression of the fusion proteins on the surface of spores, and the retention of a biologically active conformation of the VP1 protein which were strongly indicated by western-blot and immunofluorescence. All these were consistent with the surface display features from a previous report on the same spores (Ceragioli et al. 2009). The relatively low fluorescence levels observed when the recombinant spores were reacted with specific anti-VP1 antibodies (Fig. 5) were probably due to the presence on the spore surface of CotB together with CotB-VP (Ceragioli et al. 2009). The phenomenon that CV02 and 1A771 showed comparable sporulation but different efficiency of exfoliation is probably because that VP1 affected the connection between spore and sporangium.
Administration of the vaccine to the mucosa was performed via the oral and intranasal routes simultaneously. Oral strategies have been used by other investigators in the past (Duc et al. 2007, Mauriello et al. 2007). With the stimulation of VP1 protein, the number of lymphocytes secreting INF-gamma from the CV02 group was much greater than that from the 1A771 group. There was also significant difference in the serum IgG titers against VP1 between CV02 and 1A771 groups, but the titer was lower than the titers of antibody against TTFC (Duc et al. 2003) and anthrax protective antigen (Ogra et al. 1980). The level of IgA in serum and in irrigating lung solution also showed significant variations. These characters of immune responses are probably due to either the immune response having a clear Th1 bias (Barnes et al. 2007) or the different properties of the objective proteins, such as acid-alkali stability and the resistance to different proteases because using different protein displayed on spores to immunize mice getting different outcome (Lai et al 2003, Mauriello et al. 2004). The intestinal tract exposing to too many antigens may lead to the relative immune tolerance to VP1.
There are many questions remaining unanswered in this study. Although the immune-stimulating effect of this novel vaccine have been tested in mice, the effect of the vaccine on EV71 infected serum from animal models or humans is yet to be determined. In addition, immune responses or characteristics have some unique circadian rhythms, and many immune characters are altered in older human subject (Berger 2011). Whether the vaccine in the present study exerts the same stimulating effect on humeral or cellular immune responses in different age groups remains to be seen.
In conclusion, this study demonstrated that VP1 displayed on spores elicits both cellular and humoral immune responses, although the levels of immune responses were not very high. The most encouraging aspect of this work is that the recombinant spores are a potential vaccine against EV71 infection. Whether a longer period of vaccine with the spores is able to confer a better protection against EV71 infection requires further investigation. Future studies are also required to assess the immunological effects of combined displays with other toxins.
REFERENCES
Barnes AG, Cerovic V, Hobson PS, Klavinskis LS. Bacillus subtilis spores: a novel microparticle adjuvant which can instruct a balanced Th1 and Th2 immune response to specific antigen. Eur J Immunol. 37: 1538-1547, 2007.
[CrossRef]
[PubMed]
Berger J. The age of biomedicine: current trends in traditional subjects. J Appl Biomed. 9: 57-61, 2011.
[CrossRef]
[JAB]
Carrillo C, Wigdorovitz A, Oliveros JC, Zamorano PI, Sadir AM, Gómez N, Salinas J, Escribano JM, Borca MV. Protective Immune response to foot-and-mouth disease virus with VP1 expressed in transgenic plants. J Virol. 72: 1688-1690, 1998.
[PubMed]
Caruso A, Flamminio G, Folghera S, Peroni L, Foresti I, Balsari A, Turano A. Expression of activation markers on peripheral-blood lymphocytes following oral administration of Bacillus subtilis spores. Int J Immunopharmacol. 15: 87-92, 1993.
[CrossRef]
Ceragioli M, Cangiano G, Esin S, Ghelardi E, Ricca E, Senesi S. Phagocytosis, germination and killing of Bacillus subtilis spores presenting heterologous antigens in human macrophages. Microbiology. 155: 338-346, 2009.
[CrossRef]
[PubMed]
Challacombe SJ. Salivary antibodies and systemic tolerance in mice after oral immunization with bacterial antigens. Ann N Y Acad Sci. 409: 177-193, 1983.
[CrossRef]
[PubMed]
Chan L, Parashar U, Lye M, Ong FG, Zaki SR, Alexander JP, Ho KK, Han LL, Pallansch MA, Suleiman AB, Jegathesan M, Anderson LJ. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: clinical and pathological characteristics of the disease. Clin Infect Dis. 31: 678-683, 2000.
[CrossRef]
[PubMed]
Chen HF, Chang MH, Chiang BL, Jeng ST. Oral immunization of mice using transgenic tomato fruit expressing VP1 protein from enterovirus 71. Vaccine. 24: 2944-2951, 2006.
[CrossRef]
[PubMed]
Chen HL, Huang JY, Chu TW, Tsai TC, Hung CM, Lin CC, Liu FC, Wang LC, Chen YJ, Lin MF, Chen CM. Expression of VP1 protein in the milk of transgenic mice: A potential oral vaccine protects against enterovirus 71 infection. Vaccine. 26: 2882-2889, 2008.
[CrossRef]
[PubMed]
Chen SC, Chang HL, Yan TR, Cheng YT, Chen KT. An eight-year study of epidemiologic features of enterovirus 71 infection in Taiwan. Am J Trop Med Hyg. 77: 188-191, 2007.
[PubMed]
Ciabattini A, Parigi R, Isticato R, Oggioni MR, Pozzi G. Oral priming of mice by recombinant spores of Bacillus subtilis. Vaccine. 22: 4139-4143, 2004.
[CrossRef]
[PubMed]
Cutting SM, Hong HA, Baccigalupi L, Ricca E. Oral vaccine delivery by recombinant spore probiotics. Int Rev Immunol. 28: 487-505, 2009.
[CrossRef]
[PubMed]
Duc le H, Hong HA, Cutting SM. Germination of the spore in the gastrointestinal tract provides a novel route for heterologous antigen delivery. Vaccine. 21: 4215-4224, 2003.
[CrossRef]
Duc le H, Hong HA, Uyen NQ, Cutting SM. Intracellular fate and immunogenicity of B. subtilis spores. Vaccine. 22: 1873-1885, 2004.
[PubMed]
Duc le H, Hong HA, Atkins HS, Flick-Smith HC, Durrani Z, Rijpkema S, Titball RW, Cutting SM. Immunization against anthrax using Bacillus subtilis spores expressing the anthrax protective antigen. Vaccine. 25: 346-355, 2007.
[PubMed]
Fais S, Pallone F, Nava C, Magnani M. Lymphocyte activation by B. subtilis spores. Boll Ist Sieroter Milan. 66: 391-394, 1987.
[PubMed]
Fakhry S, Sorrentini I, Ricca E, De Felice M, Baccigalupi L. Characterization of spore forming Bacilli isolated from the human gastrointestinal tract. J Appl Microbiol. 105: 2178-2186, 2008.
[CrossRef]
[PubMed]
Foo DG, Alonso S, Phoon MC, Ramachandran NP, Chow VT, Poh CL. Identification of neutralizing linear epitopes from the VP1 capsid protein of Enterovirus 71 using synthetic peptides. Virus Res. 125: 61-68, 2007.
[CrossRef]
[PubMed]
Hinc K, Isticato R, Dembek M, Karczewska J, Iwanicki A, Peszyńska-Sularz G, De Felice M, Obuchowski M, Ricca E. Expression and display of UreA of Helicobacter acinonychis on the surface of Bacillus subtilis spores. Microb Cell Fact. 9: 2, 2010.
[CrossRef]
[PubMed]
Isticato R, Cangiano G, Tran HT, Ciabattini A, Medaglini D, Oggioni MR, De Felice M, Pozzi G, Ricca E. Surface Display of Recombinant Proteins on Bacillus subtilis Spores. J Bacteriol. 183: 6294-6301, 2001.
[CrossRef]
[PubMed]
Kim JH, Roh C, Lee CW, Kyung D, Choi SK, Jung HC, Pan JG, Kim BG. Bacterial surface display of GFP(uv) on Bacillus subtilis spores. J Microbiol Biotechnol. 17: 677-680, 2007.
[PubMed]
Knecht LD, Pasini P, Daunert S. Bacterial spores as platforms for bioanalytical and biomedical applications. Anal Bioanal Chem. 400: 977-989, 2011.
[CrossRef]
[PubMed]
Kosaka T, Maeda T, Nakada Y, Yukawa M, Tanaka S. Effect of Bacillus subtilis spore administration on activation of macrophages and natural killer cells in mice. Vet Microbiol. 60: 215-225, 1998.
[CrossRef]
Lai EM, Phadke ND, Kachman MT, Giorno R, Vazquez S, Vazquez JA, Maddock JR, Driks A. Proteomic analysis of the spore coats of Bacillus subtilis and Bacillus anthracis. J Bacteriol. 185: 1443-1454, 2003.
[CrossRef]
[PubMed]
Lee MS, Chang LY. Development of enterovirus 71 vaccines. Expert Rev Vaccines. 9: 149-156, 2010.
[CrossRef]
[PubMed]
Lee S, Belitsky BR, Brinker JP, Kerstein KO, Brown DW, Clements JD, Keusch GT, Tzipori S, Sonenshein AL, Herrmann JE. Development of a Bacillus subtilis-Based Rotavirus Vaccine. Clin Vaccine Immunol. 17: 1647-1655, 2010.
[CrossRef]
[PubMed]
Li L, Hu X, Wu Z, Xiong S, Zhou Z, Wang X, Xu J, Lu F, Yu X. Immunogenicity of self-adjuvanticity oral vaccine candidate based on use of Bacillus subtilis spore displaying Schistosoma japonicum 26 KDa GST protein. Parasitol Res. 105: 1643-1651, 2009.
[CrossRef]
[PubMed]
Luiz WB, Cavalcante RC, Paccez JD, Souza RD, Sbrogio-Almeida ME, Ferreira RC, Ferreira LC. Boosting systemic and secreted antibody responses in mice orally immunized with recombinant Bacillus subtilis strains following parenteral priming with a DNA vaccine encoding the enterotoxigenic Escherichia coli (ETEC) CFA/I fimbriae B subunit. Vaccine. 26: 3998-4005, 2008.
[CrossRef]
[PubMed]
Mauriello EM, Duc le H, Isticato R, Cangiano G, Hong HA, De Felice M, Ricca E, Cutting SM. Display of heterologous antigens on the Bacillus subtilis spore coat using CotC as a fusion partner. Vaccine. 22: 1177-1187, 2004.
[CrossRef]
[PubMed]
Mauriello EM, Cangiano G, Maurano F, Saggese V, De Felice M, Rossi M, Ricca E. Germination-independent induction of cellular immune response by Bacillus subtilis spores displaying the C fragment of the tetanus toxin. Vaccine. 25: 788-793, 2007.
[CrossRef]
[PubMed]
Oberste M S, Nix W A, Maher K, Pallansch M A. Improved molecular identification of enteroviruses by RT-PCR and amplicon sequencing. J Clin Virol. 26: 375-377, 2003.
[CrossRef]
Ogra PL, Fishaut M, Gallagher MR. Viral vaccination via the mucosal routes. Rev Infect Dis. 2: 352-369, 1980.
[CrossRef]
[PubMed]
Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 9: 1097-1105, 2010.
[CrossRef]
Potot S, Serra CR, Henriques AO, Schyns G. Display of Recombinant Proteins on Bacillus subtilis Spores, Using a Coat-Associated Enzyme as the Carrier. Appl Environ Microb. 76: 5926-5933, 2010.
[CrossRef]
[PubMed]
Robinson K, Chamberlain LM, Schofield KM, Wells JM, Le Page RW. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat Biotechnol. 15: 653-657, 1997.
[CrossRef]
[PubMed]
Shih SR, Li YS, Chiou CC, Suen PC, Lin TY, Chang LY, Huang YC, Tsao KC, Ning HC, Wu TZ, Chan EC. Expression of capsid protein VP1 for use as antigen for the diagnosis of enterovirus 71 infection. J Med Virol. 61: 228-234, 2003.
[CrossRef]
Tian H, Yang QZ, Liang J, Dong SY, Liu ZJ, Wang LX. Clinical features and management outcomes of severe hand, foot, and mouth disease from an inland Chinese community. Med Princ Pract. 21: 355-359, 2012.
[CrossRef]
[PubMed]
Uyen NQ, Hong HA, Cutting SM. Enhanced immunisation and expression strategies using bacterial spores as heat-stable vaccine delivery vehicles. Vaccine. 25: 356-365, 2007.
[CrossRef]
[PubMed]
Wu CN, Lin YC, Fann C, Liao NS, Shih SR, Ho MS. Protection against lethal enterovirus 71 infection in newborn mice by passive immunization with subunit VP1 vaccines and inactivated virus. Vaccine. 20: 895-904, 2001.
[CrossRef]
Yu CK, Chen CC, Chen CL, Wang JR, Liu CC, Yan JJ, Su IJ. Neutralizing antibody provided protection against enterovirus type 71 lethal challenge in neonatal mice. J Biomed Sci. 7: 523-528, 2000.
[CrossRef]
[PubMed]
Yue YY, Li ZH, Li P, Song NN, Zhao YH, Meng H. Prokaryotic expression and initial identification of enterovirus 71 structural protein VP1. Shandong Med J. 51: 22-24, 2011.
|






