Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 11 (2013), No 2, p 71-78
DOI 10.2478/v10136-012-0024-9
Circadian variation in hematological toxicity of the immunosuppressive agent "Mycophenolate Mofetil" in rats
Ichrak Dridi, Wafa Ben-Cherif, Karim Aouam, Mohsen Hassine, Mossadok Ben-Attia, Alain Reinberg, Naceur Abderrazak Boughattas
Address: Ichrak Dridi, Laboratory of Pharmacology, Faculty of Medecine, University of Monastir, 5019 Monastir, Tunisia
dridi.ichrak@yahoo.fr
Received 12th September 2012.
Revised 6th November 2012.
Published online 8th November 2012.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
Conclusions
Acknowledgements
References
SUMMARY
Because of biological rhythms, drug efficiency and toxicity vary according to the time of administration of the drug. This study investigates whether
the haematological toxicity of the immunosuppressive agent Mycophenolate Mofetil varies according to the circadian dosing-time in rats. 300 mg/kg of
Mycophenolate Mofetil was injected by i.p. route to different groups of animals at six different circadian stages (1, 7, 13, and 19 Hours After
Light Onset, HALO). Mycophenolate Mofetil treatment induced a significant decrease at 7 HALO in red blood cells (-18%), in haemoglobin rate (-15%) and
in white blood cells (-54%). These parameters followed a circadian rhythm with an acrophase located at the end of the light-rest phase. A significant
thrombocytopenia was observed according to MMF the circadian dosing-time. In the controls, the number of platelets followed a circadian rhythm.
Mycophenolate Mofetil modified this rhythm which became an 8-h ultradian rhythm. The data indicate that, the Mycophenolate Mofetil-induced
haematological toxicity was maximum when the drug was administered in the middle of the light-rest phase, which is physiologically analogous to the
end of the activity of the diurnal phase in human patients.
KEY WORDS
circadian rhythm; immunosuppressive agent; MMF; hematological toxicity; rat
INTRODUCTION
Chronobiological studies have been of great help in almost all medical fields but especially in chronopharmacology. The chronobiology studies examine
the relationship between the temporal factor and living beings (Reinberg 1992, Berger 2011). The temporal changes in drug effects include variations
in both the desired (chronoeffectiveness) and undesired (chronotoxicity) effects (Ben-Cherif et al. 2012). These biological responses to various drugs
follow circadian rhythms in experimental animals as well as in human beings. Many drugs vary in potency and/or toxicity associated with the
rhythmicity of biochemical, physiological, and behavioural processes. The choice of animal models is a revolutionary tool for chronobiological studies
that provide some obvious benefits (Ohkura et al. 2007a, b). Experiments, which would not be feasible on humans, can be conducted on animals and the
genetic background as well as most environmental factors can be controlled. Such models have served as powerful tools for understanding the circadian
rhythms of several biological variables.
The success of Mycophenolate Mofetil (MMF) in the field of solid organ transplantation (Neumann et al. 2003, Laskari et al. 2010) has made this drug
attractive for the treatment of several autoimmune diseases (Sollinger 1995, Laskari et al. 2010). It is an ester prodrug of mycophenolic acid (MPA).
Indeed, MMF is rapidly hydrolyzed in the gastrointestinal tract to MPA, which is quickly and almost completely absorbed (Bullingham et al. 1998). MPA,
the major metabolite and active component of MMF, is a potent, reversible, non competitive inhibitor of inosine monophosphate dehydrogenase, the key
rate-limiting enzyme involved in the de novo purine biosynthesis of guanosine nucleotides (Eugui and Allison 1993, Cantarovich el al. 2011). B
and T lymphocytes rely on this de novo pathway for the generation of guanosine nucleotides.
Among the side effects of immunosuppressive agents are haematological toxicity such as anaemia, due to bone marrow suppression or haemolysis (Danesi
and Del Tacca 2004), thrombocytopenia and leukopenia which is an important adverse event associated with MMF administration (Mackie et al. 1996, Virji
et al. 2001).
The present work is aimed at investigating whether murine haematolological MMF toxicity varies according to circadian dosing-time.
MATERIAL AND METHODS
A total of 80 male Wistar rats aged 6 to 8 weeks (SIPHAT, Tunisia) were synchronized for 3 weeks prior to the beginning of experiments in two
air-conditioned rooms. The rooms were specially designed for chronobiological investigations by having an inverted light regimen to explore several
circadian stages during the usual diurnal work span.
The animals were synchronized with an alternating 12 h light (L) and 12 h dark (D) cycle (light: 07:00 h to 19:00 h and dark: 19:00 h to 07:00 h). The
room temperature was maintained at 22±2 °C and the relative humidity was about 50-60%.
All experiments were performed according to the guidelines for the care and use of laboratory animals.
During all experiments, a standard rat diet (Purina Rat Chow; SICO, Sfax 3000, Tunisia) and water were provided ad libitum. The animals were
randomly divided into 4 groups of 20 rats each, which corresponded to the four explored circadian stages denoted as 1, 7, 13 and 19 Hours
After Light Onset (HALO).
The synchronization of animals was checked by assessing the circadian rhythm in rectal temperature which was measured with a digital thermometer. The
acrophase (peak time) was used as a marker rhythm index.
Drug
MMF is a white crystalline powder. It was kindly provided by Medis laboratories (Nabeul, Tunisia). MMF has an empirical formula of C23 H
31 NO7.
It was freshly prepared on each day before injections by adding an adequate volume of sterile distilled water to obtain the desired concentrations.
The drug was administered to the rats by i.p. route in a fixed fluid volume (10 ml/kg b.w.).
Study design
The study design is summarized in Table 1. A total of 80 rats were treated at four different circadian stages (1, 7, 13 and 19 HALO). The 20 control
rats (5 rats per time point) received a saline injection. The 60 MMF-treated rats (15 rats per time point) were injected a 300 mg/kg dose by
i.p. route.
Blood cell counts
The blood samples were taken three days after injection (maximum toxicity). All blood samples were withdrawn by cardiac puncture carried out under
anaesthesia, taking into account the circadian injection time. Blood was collected in EDTA-treated tubes. The count of red blood cell (RBC), white
blood cell (WBC) and platelets (PLT) per l was performed using an automatic counter (Medonic 650). Haemoglobin concentration (HGB) in g per l and
haematocrit (%) was calculated automatically.
Table 1. Main characteristics of the study investigating hematological chronotoxicity of Mycophenolate Mofetil in male Wistar rats.

Statistical analysis
Animal synchronization was verified by the Cosinor analysis (based on the least-squares method) of the 24 h body temperature marker rhythm to validate
the circadian rhythm (with a trial period t 1/4 24 h). A rhythm is validated by the rejection of the hypothesis whose amplitude is null. Rhythm
detection was considered statistically significant at the significance level 2alpha=0.05. The rhythm is then characterized by the following
parameters: i) the amplitude (A), ii) the mesor (M: the 24 h rhythm-adjusted mean) and iii) acrophase (Ø: the peak time of the cosine function)
(Halberg 1969, Nelson et al. 1979). A, M, and Ø are given with their 95% confidence limits when rhythm detection is statistically significant
(Sani et al. 2011).
All results were expressed as the mean values (m) with ± standard deviation (SD). Significance was calculated by one way-ANOVA at the
significance level 2alpha=0.05.
RESULTS
Rectal temperature
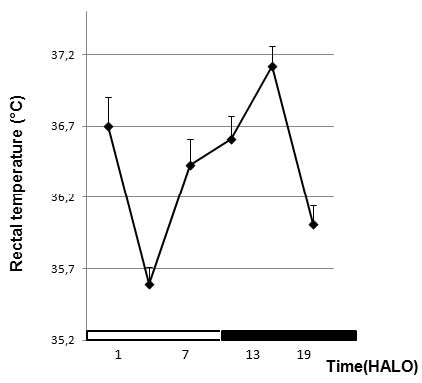
Fig. 1. Variation of temperature according to the circadian time before treatment.
MMF effect on the red blood picture
In controls, the maximum RBC counts (7.37±0.15 1012/l) and the HGB concentration (1.53±0.01 g/l) were located at 7 HALO. The
minimum ones were located at 19 HALO (5.93±0.35 1012) and (1.3±0.02 g/l) respectively.
A similar circadian rhythm in RBC counts and HGB concentration was observed in controls with an acrophase located at 8 HALO±0.26 h and 8.9
HALO±0.2 h respectively. The 24 hr-means of RBC count and HGB concentration decreased in MMF-treated rats (Fig. 2, Table 2).
MMF induced a statistically significant decrease in RBC counts (-15% to -18%) and HGB concentration (-13% to -15%) whatever the circadian time of MMF
injection (Fig. 2a, b). These two parameters followed a statistically significant circadian rhythm (tau = 24 h). A circadian variation of HCT rate was
validated significantly in controls (Fig. 2c) with the highest values observed at 13 HALO (39.7%±0.6) and the lowest ones at 19 HALO
(34.5%±0.5). In treated animals, the HCT rate decreased significantly only at 1 and 19 HALO (-16.3% vs. -28.7%) [F(79,76) =
6.36].
In controls and treated animals, the HCT rate followed a similar circadian rhythm with an acrophase located at the end of the light-rest phase (10
HALO±0.24 h vs. 9.9 HALO±0.15 h respectively).
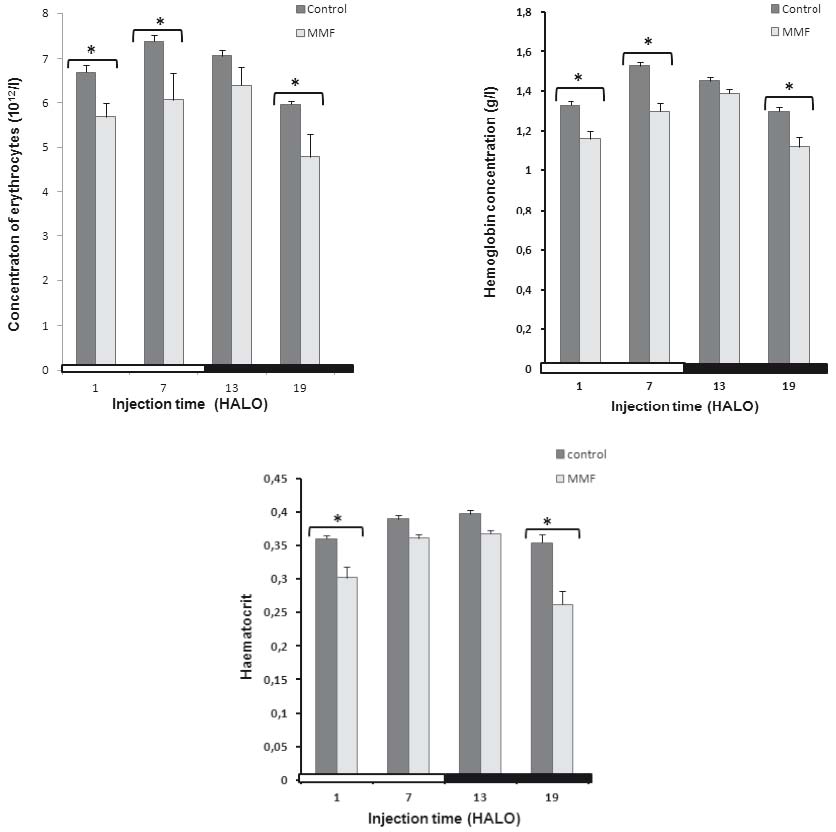
Fig. 2. Effect of Mycophenolate Mofetil (300 mg/kg i.p.) on the red blood picture; red blood cell counts (A) hemoglobin
concentration (B) and hematocrite rate (C) of male rats treated at 1, 7, 13 and 19 HALO; * statistically significant as compared with
controls.
MMF effect on leukocyte line
The circulating white blood cells (WBC) varied significantly according to a physiological circadian rhythm with an acrophase located in the middle of
the light-rest phase (Ø = 6.9±0.39) in control rats.
MMF treatment induced a decrease in the 24hr-mean of WBC counts in the treated rats (6.96±1.2 109/l) as compared to the controls
(10.2±1.6 109/l).
The WBC count decrease varied according to the MMF circadian dosing-time. It was statistically significant in treated animals at 7 (-54%) and 13 HALO
(-42%) [F(79,76) = 5.22].
No significant leucopenia was observed when MMF was injected at 1 and 19 HALO.
In MMF-treated animals, the cosinor analysis revealed a circadian rhythm in WBC counts with an acrophase shifted to the beginning of the light-rest
phase (2.13 HALO±1.25 h) as compared to controls (Table 2).
Table 2. Circadian rhythm parameters of five hematological variables in controls and Mycophenolate Mofetil-treated rats. Data represents the
estimated chronobiological parameters ± SD.
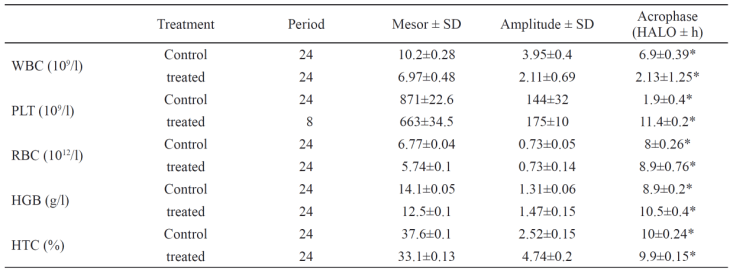
* Statistically significant
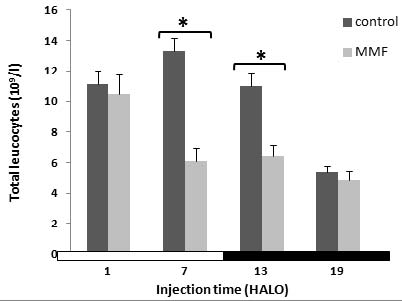
Fig. 3. Effect of Mycophenolate Mofetil (300 mg/kg i.p.) on white blood cell counts of rats treated at 1, 7, 13 and 19 HALO.
Impact of MMF treatment on thrombocyte line
The 24 hr-mean PLT count decreased significantly from 870.8±70.6 109/l in controls to 645.1±66 109/l in MMF
treated rats [F(79,76) = 6.64], irrespective of blood sampling-time.
In controls, the cosinor analysis of the physiological variation of PLT counts revealed a circadian rhythm with an acrophase located near the middle
of the light-rest phase (4.9 HALO±0.8 h) (Table 2).
MMF treatment induced a significant decrease in PLT counts at 7 HALO (-33%) [F7
(79,76) = 3.8). An absence of thrombocytopenia was observed when MMF was injected at the other circadian times. In MMF-treated rats no
significant 24 hr-rhythm was detected in PLT counts.
DISCUSSION
The administration of immunosuppressive drugs may be associated with a great deal of haematological toxicity (Danesi and Del Tacca 2004). The present
work aims to investigate whether murine haematological toxicity of the immunosuppressant agent MMF varies according to circadian dosing-time. The
circadian rhythm in the number of circulating blood cells in mammals is highly regular and reproducible (Haus et al. 1983). However, the complex
nature of this regulation has prevented the elucidation of the underlying mechanisms of circadian changes in circulating blood cells (Haus and
Smolensky 1999).
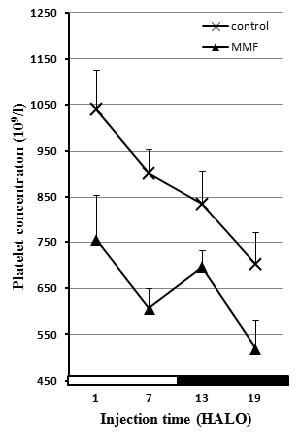
Fig. 4. Effect of Mycophenolate Mofetil (300 mg/kg i.p.) on the number of platelets of male rats treated at 1, 7, 13 and 19
HALO.
The results obtained in the present study suggest that the circadian time at which MMF is administered is important.
In each study, the chronobiological synchronization of animals is an essential step. It was verified using the rectal temperature as a marker rhythm.
The circadian peak of rectal temperature usually occurs in the middle of the dark-activity span of rats. The peak times of rectal temperature were
localized at 16.7 HALO±1.1 h. This peak location coincides with the occurrence of the highest physical activity in mice, i.e. during
the dark span (Khedhaier et al. 2003, Ben-Cherif et al. 2012).
This work has shown that MMF administration resulted in a change in the rates of various haematological parameters. It caused a decrease in the RBC
count, HGB concentration and HCT level. This reduction was significant when the drug was injected at 1, 7 and 19 HALO for the RBC count and HG
concentration. It was also significant at 1 and 19 HALO for HCT rate. This treatment caused anaemia at 1, 7 and 19 HALO, which is in accordance with
several previous studies that have shown MMF treatment induced haematological toxicity and could cause anaemia (EBPG 2002a).
The general mechanisms responsible for haematological toxicity following treatment with immunosuppressant agents, including MMF, range
fromcross-reactivitiesoftargetsoncirculatingelements (ATG/ALG, OKT3) to a direct lymphocytolytic effect (immunosuppressive corticosteroids) and
inhibition of critical signal transduction pathways, including phosphatidylinositol 3-kinase (PI3K), mitogenactivated protein kinase (MAPK), AKR mouse
T-cell lymphoma (AKT), mammalian target of rapamycin (mTOR)/p70S6 kinase (p70S6K), which are involved in the suppression of apoptosis, responses to
growth factors and cellular proliferation (sirolimus). Finally, direct inhibition of nucleic acid synthesis may result in bone marrow suppression, as
in the case of MMF and AZA. In particular, de novo purine and nucleic acid synthesis is suppressed by MPA, a selective, reversible,
noncompetitive antagonist of inosine monophosphate dehydrogenase (IMPDH) activity of bone marrow cells and lymphocytes (Weber et al. 2002). IMPDH
catalyses the rate-limiting step in the de novo synthesis of guanine nucleotides from inosine monophosphate (IMP). Therefore, the inhibition of
IMPDH activity by MPA, a major metabolite of MMF, may result in the induction of apoptosis or inhibition of cell proliferation (Danesi et al.
2000).
In the controls, RBC counts, HGB concentration and HCT level follow a circadian rhythm. MMF treatment did not alter the circadian rhythmicity of
these haematological parameters. This result suggests that the circadian rhythm in the number of blood cells is dependent on core components of the
circadian clock. Several hormones such as steroid hormones play an important role in generating the circadian rhythm of circulating blood cells
(Ottaway and Husband 1994) whose functioning are probably not affected by the immunosuppressive agent MMF, because it does not alter the rhythmicity
of these haematological parameters.
In comparing the amplitudes of these parameters, the amplitude of RBC counts is less marked in both controls and treated. It’s well known that
the amplitude of the RBC rhythm is not physiologically large (Haus 1996). This might be related to the circulating RBC, which is determined not only
by their clearance from the peripheral circulation in the spleen and the liver but also by their production in the bone marrow.
The acrophase of RBC counts, HGB concentration and HCT level are at the end of the resting phase in the controls and the animals treated with 300 mg/
kg of MMF. These parameters follow a circadian rhythm with nocturnal acrophases in horses (Piccione et al. 2005). There is evidence in the literature
that the acrophase of the diurnal RBC rhythm is near the light-dark transition in nocturnal mice (Ohkura et al. 2007b). The acrophase of circulating
RBC in diurnally active humans is also at the time of the light-dark transition (Haus et al. 1983).
MMF treatment caused a decrease in WBC number whatever the treatment time and the leucopenia index (%) depends on MMF injection in the 24-hr
scale.
A statistically significant leucopenia of -54% and -42% was observed at 7 and 13 HALO respectively. This finding is also in accordance with several
studies which showed that MMF caused leucopenia (EBPG 2002b). The subacute oral administration of a 10-20 mg/kg MMF dose for 2 weeks in male Lewis
rats induced a significant reduction of RBC and also causes a significant leucopenia with a lymphopenic effect (Pally et al. 2001). Danesi and Del
Tacca (2004) confirmed that MMF causes leucopenia.
The present work also showed that the number of white blood cells in controls and MMF treated animals followed a circadian rhythm with a peak at the
rest period and a trough at the active phase.
In control and treated animals the acrophase is located at 6.9 HALO±0.39 h and at 2.13 HALO±1.25 h respectively.
The study of Ohkura et al. (2007a) showed that the circadian rhythm of WBC in nocturnal mice peaks at the beginning of the light period. On the other
hand, the peak of circulating WBC in diurnally active humans is at the time of the light-dark transition (Haus et al. 1983). Thus, the difference of
about 180° between circadian rhythms in diurnally active humans and nocturnally active rodents is essentially applicable to the rhythm of WBC
numbers (Ohkura et al. 2007b).
Studies focusing on the relationship between the basal circadian rhythm of WBC numbers and immune rhythms using mouse models might help to establish
immunomodulatory therapies on a chronobiological basis (Pally et al. 2001). The numbers of circulating WBC involved in the immune defense of organisms
are subject to high-amplitude circadian rhythms (Haus and Smolensky 1999).
Tsutsumi et al. (1999) showed that the maximum of PLT counts is at 9:00 am (443±15.3 109/l) in the male Minipigs and this
haematological parameter follows a circadian rhythm.
The study of changes in platelet count has revealed a significant decrease whatever the treatment time with MMF. The index of the low platelets
(thrombocytopenia) depends on MMF injection time. However, MMF treatment at 7 HALO resulted in a higher thrombocytopenia (-32%). This is in agreement
with the work of several other authors’who have shown that MMF may induce thrombocytopenia (Mackie et al. 1996, Virji et al. 2001).
Our study also showed that in control animals, the number of platelets followed a circadian rhythm with an acrophase located at the beginning of the
resting phase (Ø = 1.9 HALO±0.4 h). MMF treatment altered this rhythm which became an ultradian rhythm (tau = 8 h).
CONCLUSIONS
In conclusion, the administration of immunosuppressive drugs such as MMF may be associated with a great deal of haematological toxicity (anemia,
leucopenia, thrombocytopenia) that could be mild and predictable coinciding with the time of MMF toxicity with an ultradian rhythm (8 h) shown in a
preliminary study (Dridi et al. 2012).
The safe administration of immunosuppressive drugs requires therapeutic drug monitoring and the knowledge of potential drug interactions that may
unpredictably be associated with the occurrence of severe haematological toxicity.
ACKNOWLEDGEMENTS
The authors would like to thank Mr Adel Rdissi for proof reading this article. This work was supported by "Le ministere de l'Enseignement Superieur et
de la Recherche Scientifique".
REFERENCES
Ben-Cherif W, Dridi I, Aouam K, Ben-Attia M, Reinberg A. Chronotolerance study of the antiepileptic drug valproic acid in mice. J Circadian Rhythms.
10: 3, 2012.
[CrossRef]
[PubMed]
Berger J. The age of biomedicine: current trends in traditional subjects. J Appl Biomed. 9: 57-61, 2011.
[CrossRef]
[JAB]
Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet. 34: 429-455, 1998.
[CrossRef]
[PubMed]
Cantarovich M, Brown NW, Ensom MH, Jain A, Kuypers DR, Van Gelder T, Tredger JM. Mycophenolate monitoring in liver, thoracic, pancreas, and small
bowel transplantation: a consensus report. Transplant Rev. 25: 65-77, 2011.
[CrossRef]
[PubMed]
Danesi R, Del Tacca M. Hematologic toxicity of immunosuppressive treatment. Transplant Proc. 36: 703-704, 2004.
[CrossRef]
[PubMed]
Danesi R, Mosca M, Boggi U, Mosca F, Del Tacca M. Genetics of drug response to immunosuppressive treatment and prospects for personalized therapy. Mol
Med Today. 6: 475-482, 2000.
[CrossRef]
Dridi I, Ben-Cherif W, Aouam K, Ben-Attia M, Reinberg A, Boughattas NA. Murine circadian time-dependent tolerance to the immunosuppressive agent
mycophenolate mofetil (MMF). Biol Rhythm Res. 1-9, 2012.
[CrossRef]
EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the
transplant recipient. IV.9.1. Anaemia. Nephrol Dial Transplant. 17: 48, 2002a.
EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the
transplant recipient. IV.9.2. Leukopenia. Nephrol Dial Transplant. 17: 49, 2002b.
[CrossRef]
Eugui EM, Allison AC. Immunosuppressive activity of mycophenolate mofetil. Ann NY Acad Sci. 685: 309-329, 1993.
[CrossRef]
[PubMed]
Halberg F. Chronobiology. Annu Rev Physiol. 31. 675-725, 1969.
[CrossRef]
[PubMed]
Haus E. Biologic rhythms in hematology. Pathol Biol. 44: 618-630, 1996.
[PubMed]
Haus E, Smolensky MH. Biologic rhythms in the immune system. Chronobiol Int. 16: 581-622, 1999.
[CrossRef]
[PubMed]
Haus E, Lakatua DJ, Swoyer J, Sackett-Lundeen L. Chronobiology in hematology and immunology. Am J Anat. 168: 467-517, 1983.
[CrossRef]
[PubMed]
Khedhaier A, Ben-Attia M, Gadacha W, Sani M, Bouzouita K, Chouchane L, Mechkouri M, Reinberg A, Boughattas NA. Circadian Rhythms in Toxic Effects of
the Serotonin Antagonist Ondansetron in Mice. Chronobiol Int. 20: 1103-1116, 2003.
[CrossRef]
[PubMed]
Laskari K, Mavragani CP, Tzioufas AG, Moutsopoulos HM. Mycophenolate mofetil as maintenance therapy for proliferative lupus nephritis: a long-term
observational prospective study. Arthritis Res Ther. 12: R208, 2010.
[CrossRef]
[PubMed]
Mackie F, Verran D, Horvath J, Tiller D. Severe thrombocytopenia with OKT3 use for steroid-resistant rejection in a cadaveric renal transplant
recipient. Nephrol Dial Transplant. 11: 2378, 1996.
[CrossRef]
[PubMed]
Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 6: 305-323, 1979.
[PubMed]
Neumann I, Haidinger M, Jager H, Grutzmacher H, Griesmacher A, Muller MM, Bayer PM, Meisl FT. Pharmacokinetics of Mycophenolate Mofetil in Patients
with Autoimmune Diseases Compared Renal Transplant Recipients. J Am Soc Nephrol. 14: 721-727, 2003.
[CrossRef]
[PubMed]
Ohkura K, Uto Y, Nagasawa H, Hori H. Effect of molecular chirality and side chain bulkiness on angiogenesis of haloacetylcarbamoyl-2-nitroimidazole
compounds. Anticancer Res. 27: 3693-3700, 2007a.
[PubMed]
Ohkura N, Oishi K, Sekine Y, Atsumi G, Ishida N, Matsuda J, Horie S. Comparative Study of Circadian Variation in Numbers of Peripheral Blood Cells
among Mouse Strains: Unique Feature of C3H/HeN Mice. Biol Pharm Bull. 30: 1177-1180, 2007b.
[CrossRef]
[PubMed]
Ottaway CA, Husband AJ. The influence of neuroendocrine pathways on lymphocyte migration. Immunol Today. 15: 511-517, 1994.
[CrossRef]
Pally C, Tanner M, Rizvi H, Papageorgiou C, Schuurman HJ. Tolerability profile of sodium mycophenolate (ERL080) and mycophenolate mofetil with and
without cyclosporine (Neoral) in the rat. Toxicology. 157: 207-215, 2001.
[CrossRef]
Piccione G, Fazio F, Giudice E, Grasso F, Morgante M. Nycthemeral change of some haematological parameters in horses. J Appl Biomed. 3: 123-128,
2005.
[JAB]
Reinberg AE. Concepts in chronopharmacology. Annu Rev Pharmacol Toxicol. 32: 51-66, 1992.
[CrossRef]
[PubMed]
Sani M, Sebai H, Boughattas NA, Ben-Attia M. Time-of-day dependence of neurological deficits induced by sodium nitroprusside in young mice. J
Circadian Rhythms. 9: 5, 2011.
[CrossRef]
[PubMed]
Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. The US Renal Transplant
Mycophenolate Mofetil Study Group. Transplantation. 60: 225-232, 1995.
[CrossRef]
[PubMed]
Tsutsumi H, Monnai Y, Ishii H, Tanioka Y, Tanigawa M. Diurnal variation and effects of fasting on blood constituent in minipigs. Exp Anim. 48:
247-254, 1999.
[CrossRef]
[PubMed]
Virji M, Carter JE, Lirenman DS. Single-center experience with mycophenolate mofetil in pediatric renal transplant recipients. Pediatr Transplant. 5:
293-296, 2001.
[CrossRef]
[PubMed]
Weber LT, Shipkova M, Armstrong VW, Wagner N, Schutz E, Mehls O, Zimmerhackl LB, Oellerich M, Tonhoff B. The pharmacokinetic-pharmacodynamic
relationship for total and free mycophenolic acid in pediatric renal transplant recipients: a report of the german study group on mycophenolate
mofetil therapy. J Am Soc Nephrol. 13: 759, 2002.
[PubMed]
|
BACK
|







