Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 11 (2013), No 2, p 93-103
DOI 10.2478/v10136-012-0033-8
Alpha-tomatine activates cell cycle checkpoints in the absence of DNA damage in human leukemic MOLT-4 cells
Jana Kudelova, Martina Seifrtova, Lenka Sucha, Pavel Tomsik, Radim Havelek, Martina Rezacova
Address: Martina Rezacova, Department of Med. Biochemistry, Medical Faculty in Hradec Kralove, Simkova 870, 500 38 Hradec Kralove 1, Czech Republic
rezacovam@lfhk.cuni.cz
Received 18th October 2012.
Revised 20th December 2012.
Published online 8th January 2013.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
Acknowledgement
References
SUMMARY
Alpha-tomatine is a major glycoalkaloid found in the roots, leaves, stems and fruit of tomatoes Lycopersicon esculentum. Recently,
alpha-tomatine has been recognized as a potential anticancer drug. In the present study, we identified the signaling cascades involved in the
antitumor effect of alpha-tomatine on MOLT-4 leukemic cells. Alpha-tomatine inhibited the proliferation and decreased the viability of MOLT-4 cells in
a dose-dependent manner. An increase in the activity of caspases 9 and 3/7 was not observed. However, an increase in the amount of p53 and its
phosphorylation on serine 15, as well as an increased amount of mitochondrial protein PUMA was detected 4 and 24 h after exposure to alpha-tomatine at
a concentration of 1–3 micromol/l. Inhibition of the proliferation of MOLT-4 cells by alpha-tomatine is also associated with an increase in
p21WAF1/CIP1 and the activation of Chk2. The comet assay did not detect significant amounts of single or double DNA strand breaks in cells
treated with alpha-tomatine at concentrations of 0.1-9 mmol/l. Our results thus contribute to the understanding of the anticancer action of
alpha-tomatine.
KEY WORDS
alpha-tomatine; DNA damage; p53; PUMA; leukemia
Abbreviations:
Ann, Annexin V; DSB, double strand break of DNA; EC50, concentration causing the effect in 50% of the cells; PI, propidium iodide
INTRODUCTION
Alpha-tomatine is a major glycoalkaloid found in the roots, leaves, stems and fruit of tomatoes Lycopersicon esculentum. During the maturation
of tomatoes, the content of alpha-tomatine decreases - an immature green tomato contains up to 500 mg of alpha-tomatine per kg of fresh fruit weight,
but as the tomato ripens, alpha-tomatine is degraded and its content in red tomatoes is around 5 mg/kg of fresh fruit weight (Friedman 2002). The
molecule of alphatomatine consists of tomatidine (aglycon) attached by an O-glycosidic bond to the tetrasaccharide moiety of beta-lycotetraose
(consisting of D-galactose, D-xylose and 2 molecules of D-glucose) (Shih et al. 2009).
Simultaneously with the first isolation and purification of tomatine (the mixture of two distinct alkaloids, alpha-tomatine and dehydrotomatine) from
tomatoes by Fontaine et al. in 1948, the antifungal effect of tomatine against Candida albicans was demonstrated (Ma and Fontaine, 1948). Even
before this, the antimicrobial and antifungal effect of alphatomatine was reported by the same group (Irving et al. 1946). Experiments with rats and
mice demonstrated the anti-inflammatory activity of tomatine connected with several unusual properties which had never been described for any
anti-inflammatory substance in general (Filderman and Kovacs 1969). Currently it is known that the anti-inflammatory action of tomatine is mediated by
the inhibition of NF-kappaB and JNK signaling (Chiu and Lin 2008, Shih et al. 2009, Shieh et al. 2011).
Alpha-tomatine has low oral and local toxicity. Whereas chronic oral application does not cause any systemic changes or abnormalities, the parenteral
administration of a few dozen milligrams per kg is fatal in rodents. After intravenous injection, hypotension, an increase in respiratory rate and
depth, and hemolysis occur (Wilson et al. 1961). In addition, a toxic effect on rat heart cells was described; the mechanism of action is similar to
that of heart glycosides (Bergers and Alink 1980).
Herbal compounds such as alkaloids, phenolics, polyphenols, quinones and terpenoids have been shown to be very effective alternatives to synthetic
therapeutics (Berger 2011). Recently, it has been discovered that alpha-tomatine exhibits an antiproliferative effect on tumor cell lines derived from
human prostate carcinoma (Lee et al. 2011), human colon (HT29) and liver (HepG2) carcinoma (Lee et al. 2004), and lung carcinoma (Sheih et al. 2011).
The exact underlying mechanisms of the anticancer action, however, are far from clear. Programmed cell death, which serves as a protective process by
eliminating cells that are abnormal and potentially dangerous (Fuchs and Steller 2011), could be potentiated by alpha-tomatine.
For our study, we chose the MOLT-4 cell line derived from human T-lymphoblastic leukemia. These cells express specific surface markers: CD1+
(49%), CD41 (55%), CD51 (72%) and CD71 (77%). MOLT-4 cells also contain wild-type protein p53. Their high
sensitivity to ionizing radiation and cytostatic drugs make them a useful model for studying the molecular response to DNA damage (Tichy et al.
2007).
In the present study, we identified the signaling cascades involved in the antitumor effect of alphatomatine on MOLT-4 leukemic cells and demonstrated
that, despite the activation of p53 and Chk2, direct DNA fragmentation is not involved in the cytostatic action of alpha-tomatine.
MATERIAL AND METHODS
Cell cultures and culture conditions
Human leukemic T-lymphocytes (MOLT-4 cell line) were obtained from the American Type Culture Collection ATCC (Manassas, USA) and maintained in
Iscove's modified Dulbecco's medium (Sigma, USA). The medium was supplemented with 20% fetal calf serum and all cells were cultured at 37 °C in a
humidified atmosphere of 5% CO2. Cell lines in the maximal range of up to 20 passages were used for the experiment.
Cytostatic treatment
Alpha-tomatine: A stock solution of alpha-tomatine (Santa Cruz Biotechnology, California, USA) was prepared by dissolving 2 mg of the substance in 1
ml of DMSO and 4 ml of MOLT-4 culture medium (final concentration c = 387 micromol/l). A new stock solution was prepared every day and stored at a
temperature of 8 °C. For the experiments, the stock solution was diluted with the culture medium to final concentrations of 0.1-9 micromol/l.
Irradiation: The cells were irradiated in culture flasks at room temperature using a 60Co gamma-ray source (Chisotron, Chirana, Czech Rep.)
at a distance of 1 m from the source and at a photon dose rate of 1 Gy/min.
Mitoxantrone: A stock solution of mitoxantrone (Sigma, USA) was prepared by dissolving 1.3 mg of the substance in 2.5 ml of distilled water (final
concentration c = 1 mmol/l). For experiments the stock solution was diluted to a final concentration of 5 nmol/l.
Proliferation and viability
Cell proliferation and viability were determined 4, 24, and 72 hours after treatment with 1, 2, 3 and 4 micromol/l alpha-tomatine. The cultures were
divided every second day by dilution to a concentration of 2 x 105 cells/ml. Cell membrane integrity was determined using the Trypan blue
exclusion technique - mixing of 50 microl Trypan blue and 50 microl of cell suspension. The cell counts were carried out using a Burker
chamber.
Western Blotting
Whole-cell lysates (Cell Lysis Buffer, Cell Signaling Technology, Inc, USA) were prepared 4, 24 and 72 hours after the application of 1, 2 and 3
micromol/l of alpha-tomatine, and quantification of the protein content was performed using BCA assay (Sigma-Aldrich, USA). The lysates (20 microg
purified protein) were loaded into each lane of a polyacrylamide gel. After electrophoretic separation, the proteins were transferred to a PVDF
membrane (Bio-Rad, USA). Non-specific binding of the membranes was blocked for 1 hour in Tris-buffered saline containing 0.05% Tween 20 and 5% non-fat
dry milk. The membranes were washed twice with TBST, each time for 5 minutes, and once with TBS, again for 5 minutes.
Incubation with primary antibody (p53 - Exbio, Czech Republic; beta-actin, p21WAF1/Cip1 - Sigma-Aldrich, USA; p53_serine15 -
Calbiochem-Merck, USA; Chk2, Chk2_threonin68, PUMA - Cell Signaling, Boston, MA, USA) was performed at 4 °C overnight. The following day the
membranes were washed five-times with TBST, each time for 5 minutes, and once with TBS, again for 10 minutes, and then incubated with appropriate
secondary antibody (DakoCytomation, Denmark) for one hour at room temperature. Band detection was performed using a chemiluminiscence detection kit
(Roche, Switzerland). To ensure equal protein loading, each membrane was reprobed and beta-actin was detected. Bands were quantified using an ImagePro
5.1 computer image analysis system (Media Cybernetics, Bethesda, MD, USA).
Flow-cytometry
Apoptosis was determined by flow cytometry using APOPTESTTM-FITC kit (DakoCytomation, Denmark) according to the manufacturer's
instructions. The APOPTESTTM-FITC kit employs the property of fluorescein isothiocyanate (FITC) conjugated to Annexin V (Ann-FITC) to bind
to phosphatidylserine in the presence of Ca2+, and the property of propidium iodide (PI) to enter cells with damaged cell membranes and to
bind to DNA. Measurement was performed immediately using a CyAnTM ADP (Beckman Coulter, Miami, FL, USA) flow cytometer. Listmode data were
analyzed using Summit v4.3 software (Beckman Coulter, USA).
For the analysis of cell cycle distribution, the cells were washed with ice cold PBS and fixed with 70% ethanol. They were then incubated for 5 min at
room temperature in buffer (192 ml 0.2 mol/l Na2HPO4+ 8 ml 0.1 mol/l citric acid, pH 7.8) and stained with propidium iodide (PI)
in Vindelov's solution for 60 minutes at 37 °C. The DNA content was determined by means of a BD FACS Aria III flow cytometer (Becton, Dickinson
and Company, New Jersey, USA) using a 13 mW Coherent® SapphireTM solid-state laser with excitation at 488 nm; total emission
above 560 nm was recorded. List mode data were analyzed using BD FACS Diva 6.1.3. software (Becton, Dickinson and Company, New Jersey, USA).
Activity of caspases
The induction of programmed cell death was determined by monitoring the activities of caspase 3/7 and caspase 9 by Caspase-Glo Assays (Promega,
Madison, WI, USA) 4 and 24 hours after treatment with 1, 2 and 3 μmol/l of alpha-tomatine. The assays provide proluminogenic substrates in an
optimized buffer system. The addition of Caspase-Glo Reagents results in cell lysis, followed by caspase cleavage of the substrate and the generation
of a luminescent signal. A total of 1 x 104 cells were seeded per well using a 96-well-plate format (Sigma, St. Louis, MO, USA). After
treatment, Caspase-Glo Assays reagents were added to each well (50 microl/well) and incubated for 30 minutes before luminescence was measured using a
Tecan Infinite M200 spectrometer (Tecan Group, Mannedorf, Switzerland).
Comet assay
DNA damage was measured using alkaline and neutral versions of comet assay as described previously (Olive at Banáth 1993, Collins et al. 1996,
Calini et al. 2002). Briefly, cells embedded in 1% agarose (Sigma) on microscope slides were lysed in 10 mmol/l Tris-buffered 2.5 mol/l NaCl (pH 10.0;
Penta, Prague, Czech Republic) containing 1% Triton X 100 (Merck) and 100 mmol/l EDTA (Penta) for 1 hour at 4 °C. In alkaline conditions (NaOH,
EDTA), the electrophoresis was carried out at 40 V, 300 mA, for 30 minutes at 4 °C after 40 minutes of unwinding. The electrophoresis in the
neutral conditions (90 mmol/l Tris, 90 mmol/l boric acid, 2 mmol/l EDTA, pH 8.0) was performed at 29 V, 6 mA, for 40 minutes at 4 °C after washing
in borate buffer. DNA damage was analyzed by the comet module of Lucia 6.20 image analysis (Laboratory Imaging, Prague, Czech Republic) after the
cells were stained with ethidium bromide (Sigma). The percentage of DNA in the comet tail was measured. At least fifty cells per slide were
analyzed.
Statistics
The statistical data for comet assay results were calculated with STATISTICA 10.0 software (StatSoft CR, Czech Republic). Differences within the
groups were evaluated by the Kruskal-Wallis test. All experimental data were expressed as median and 25th and 75th percentiles.
Other results were evaluated with descriptive statistics, and the charts were made using Microsoft Office Excel (Microsoft Inc., Redmond, WA, USA).
The values were expressed as arithmetic means with standard deviation (SD) unless otherwise indicated. Significant differences between groups were
analyzed using the Student t-test. Experimental data were evaluated at the significance level 2alpha=0.05.
RESULTS
Proliferation and viability
The effect of alpha-tomatine was assessed by comparing cell proliferation and viability in the MOLT-4 leukemic cell line exposed to alpha-tomatine in
concentrations of 1, 2, 3 and 4 microimol/l for 4, 24 and 72 h with the untreated control cell line (Fig. 1). The application of 1 and 2 micromol/l
alpha tomatine had no pronounced effect on cell viability and slightly inhibited proliferation within 24 h of treatment. After incubation with
alpha-tomatine at the concentration of 3 micromol/l, the number of cells decreased initially (to 360 x 103 after 4 hours and remained at
about 400 x 103 for 24 hours). Proliferation recovered after 72 hours and the number of cells increased to 1355 x 103 (control -
1700 x 103). The changes in viability after exposure to the concentration of 3 micromol/l were only slight - 93% of the cells were viable
after 4 hours, 82% after 24 hours, and 87% after 72 hours. The treatment with 4 micromol/l alpha-tomatine led to a significant decrease in viability
(after 4 hours only 29% of cells remained viable; after 24 hours, 12%; and after 72 hours, 14%) and a rapid decrease in the number of cells (after 4
hours 20 x 103, compared to 500 x 103 in control).
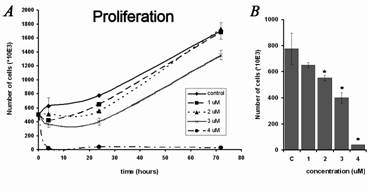
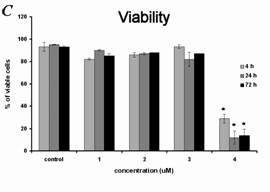
Fig. 1. Effect of alpha-tomatine (1-4 micromol/l) on the proliferation and viability of human MOLT-4 leukemic cells. Results are shown as mean
± SD from three experiments. * Statistically significant as compared with controls. (A) Changes in the number of cells during the 3 days
after treatment. (B) Details of changes in the number of cells after 24 hours of treatment, during the period of maximal effect of tomatine at
concentrations below 4 micromol/l. (C) Changes in the viability of cells during the 3 days after treatment. The percentage of viable cells was
determined using Trypan blue exclusion staining.
Changes in p53, p21 and Chk2, and cell cycle
First, we detected changes in the levels of the proteins p53 and p21WAF1/CIP1. The amount of tumor suppressor protein p53 increased after 4
and 24 hours. The exposure of MOLT-4 cells to alpha-tomatine also induced phosphorylation of p53 on serine 15 after the same periods of incubation. An
inhibitor of cyclindependent kinases, p21WAF1/CIP1 increased after 4 h (Fig. 2). These changes in p21WAF1/CIP1 disappeared
after 24 h. After 72 hours, we did not detect any changes in the p53-p21 pathway, and the amount of p53 and p21WAF1/CIP1 was the same as in
the control group (data not shown).
To further elucidate the mechanisms inhibiting proliferation, we determined the changes in Chk2, particularly the phosphorylation of threonine 68.
After 4 and 24 hours, there was a concentration-dependent increase in the level of Chk2 phosphorylated on threonine 68, while the overall amount of
Chk2 did not change significantly (Fig. 2). No changes in Chk2 phosphorylation after 72 hours were observed.
To determine cell cycle distribution and possible cell cycle arrest after alpha-tomatine treatment, we employed cell cycle analysis of DNA content
using flow cytometry. The treatment of MOLT-4 cells with 3 micromol/l alpha-tomatine resulted in cell cycle arrest and in an accumulation of cells in
the G1 phase, accompanied by a decrease in cells in the G2 phase. 24 hours after the application of 3 micromol/l alpha-tomatine, 60% of cells were in
the G1 phase, while in untreated control samples, the proportion was 52% (Fig. 3).
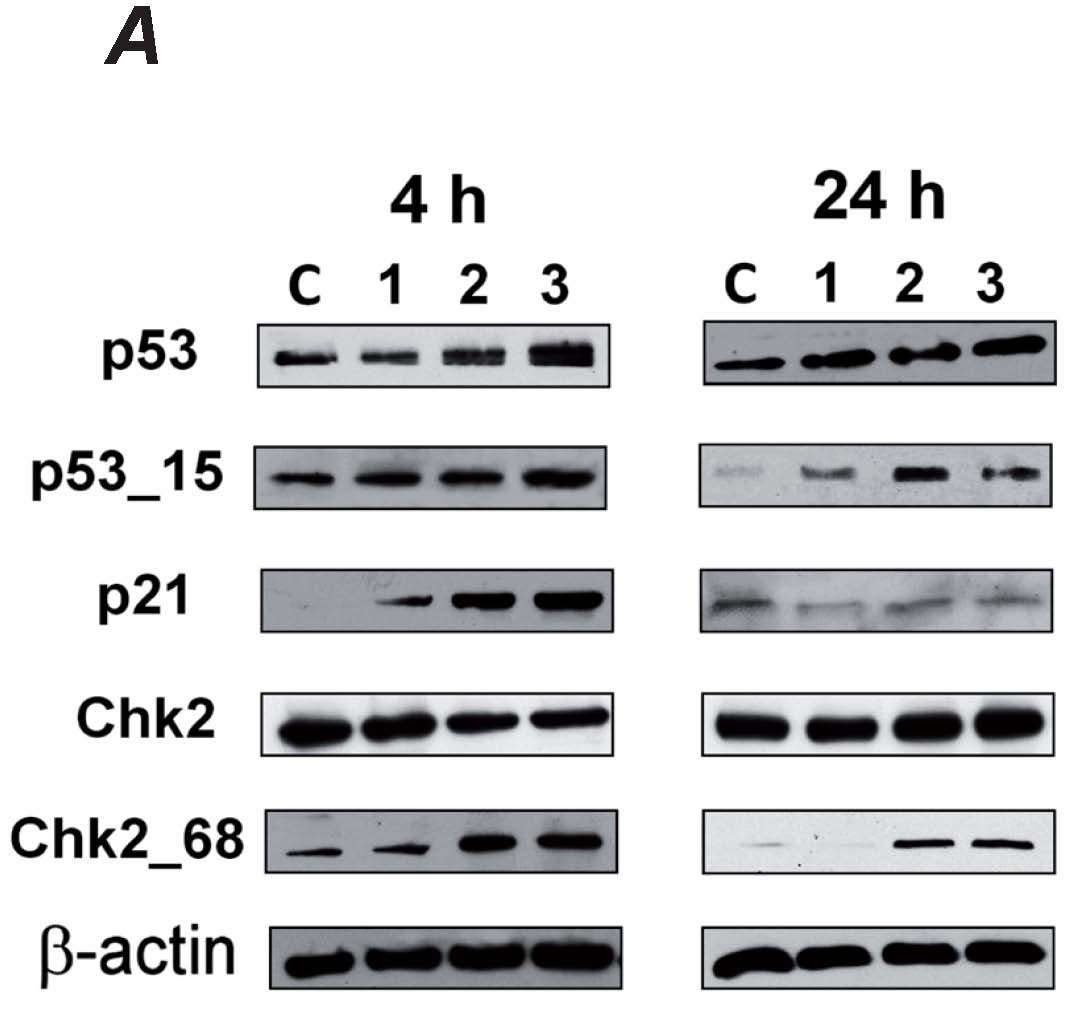

Fig. 2. Induction and activation of p53, p21WAF1/Cip1 and Chk2 in MOLT-4 cells exposed to 1, 2 and 3 micromol/l alpha-tomatine 4 and 24
hours after the application of the drug detected by western blot (A). To ensure equal protein loading, membranes were reincubated with
beta-actin. Data were quantified densitometrically and are expressed as the mean ± SD from two independent experiments. The values of the
integrated optical density were expressed as a percentage of beta-actin. Untreated control; p53_15 - p53 phosphorylated on serine 15; Chk2_68 - Chk2
phosphorylated on threonine 68 (B).
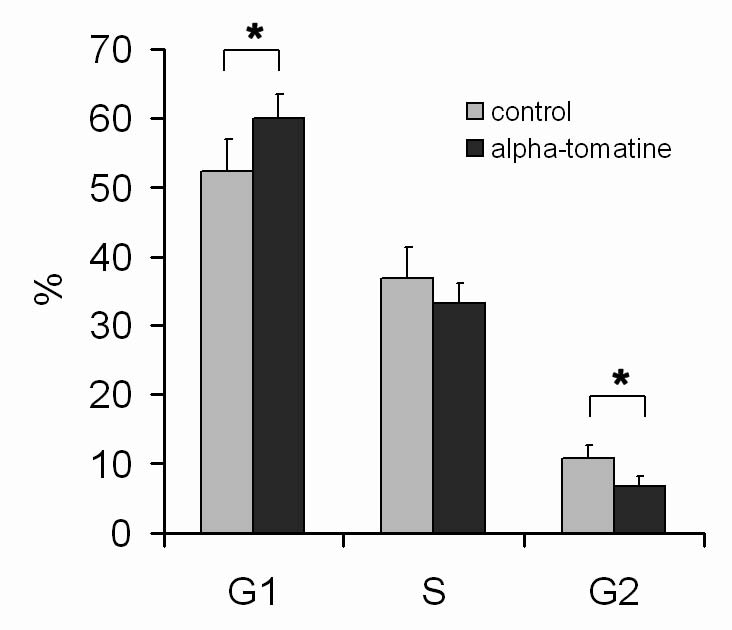
Fig. 3. Analysis of the cell cycle of MOLT-4 cells 24 h after the application of 3 micromol/l alpha-tomatine - percentage of cells in G1, S,
and G2/M phase. Results are shown as mean ± SD from five measurements from three independent experiments. * Statistically significant as
compared with controls.
DNA fragmentation – comet assay
The most common reason for an increase in the amount of p53 is cellular stress related to DNA damage. Therefore, using comet assay, we examined
whether alpha-tomatine induces breaks in the DNA. Our results show that in untreated control groups MOLT-4 cells were intact. Using the alkaline
version of comet assay, we evaluated the amount of single strand breaks in cells. No significant DNA damage was found in cells treated with 0.1-9
micromol/l of alpha-tomatine for 4 h (Fig. 4A-C, G). The neutral version of comet assay was used to evaluate the number of double strand breaks (DSB)
in cells. Again, no significant amount of DSB was found in cells treated with 0.1-9 micromol/l of alpha-tomatine for 4 h (Fig. 4D-F, H). Furthermore,
neither significant phosphorylation of H2AX nor the formation of DNA damage-associated foci were observed after exposure to 3 micromol/l
alpha-tomatine for 4 or 24 h (data not shown).
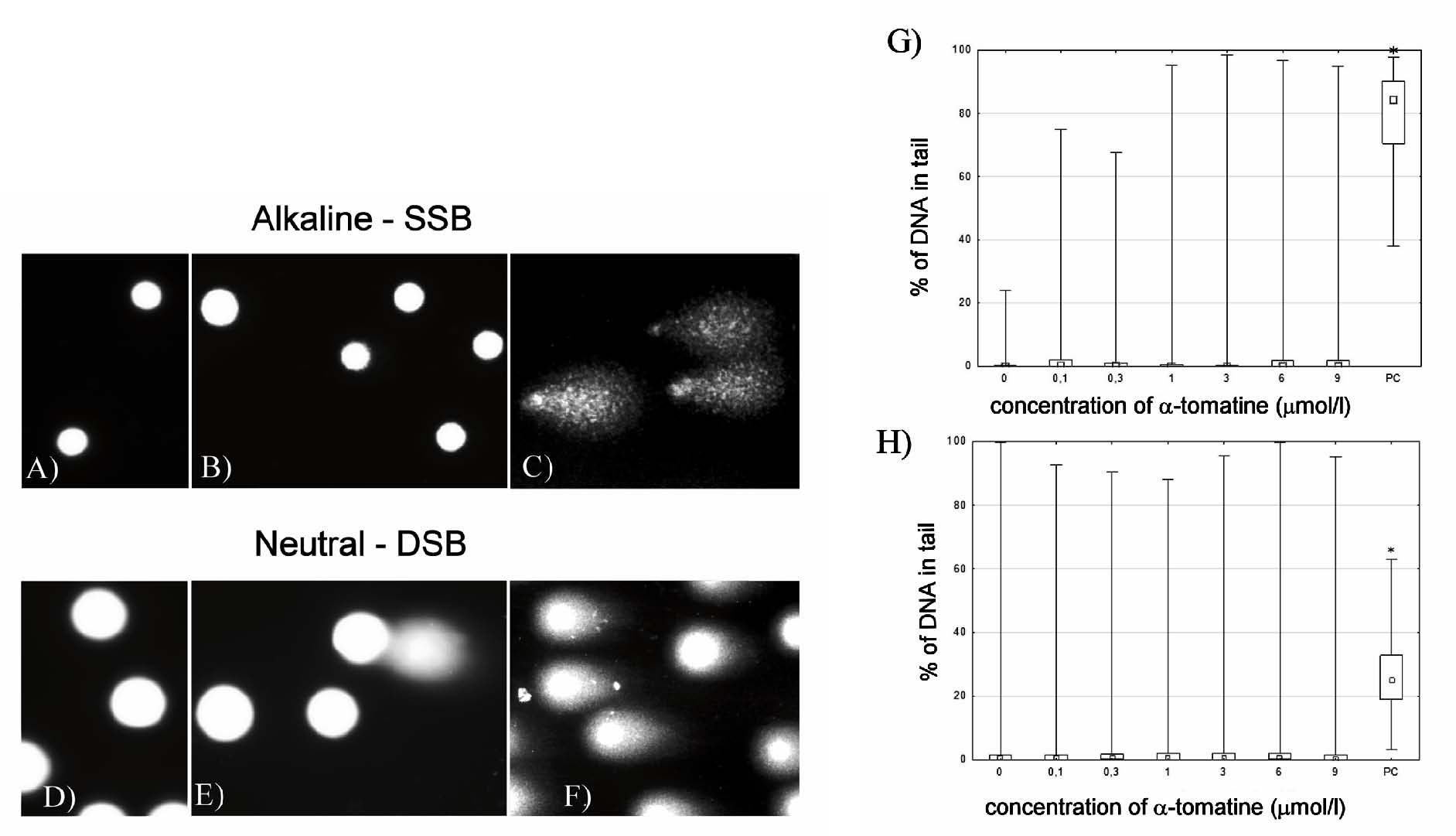
Fig. 4. Detection of DNA damage by comet assay in MOLT-4 cells exposed to 0.1-9 micromol/l alpha-tomatine. (A-C) Digital camera
photograph of the alkaline version of comet assay. Control MOLT-4 cells without exposure to alpha-tomatine (A), cells treated with 3 micromol/l
of alpha-tomatine (B), and 1.5% H2O2-treated cells (positive control) (C). (D-E) Digital camera photograph
of the neutral version of comet assay. Control MOLT-4 cells without exposure to alpha-tomatine (D), cells treated with 3 micromol/l of
alpha-tomatine (E), and cells after exposure to 20 Gy of irradiation (positive control) (F). (G) The dependence of DNA
single-strand breaks on the concentration of alpha-tomatine after 4 hours of exposure. PC - positive control (1.5% H2O2).
(H) The dependence of DNA double-strand breaks on the concentration of alpha-tomatine after 4 hours of exposure. PC - positive control (20 Gy
gamma radiation). * Statistically significant as compared with controls.
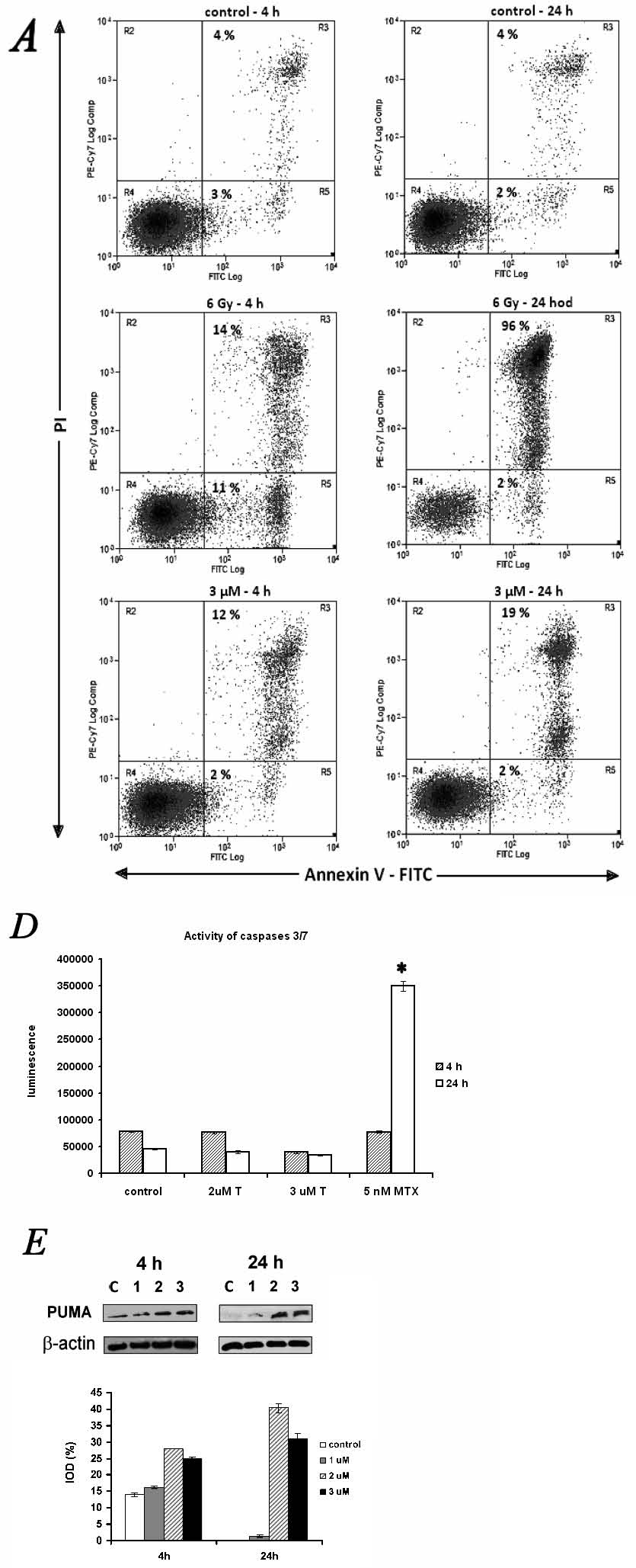
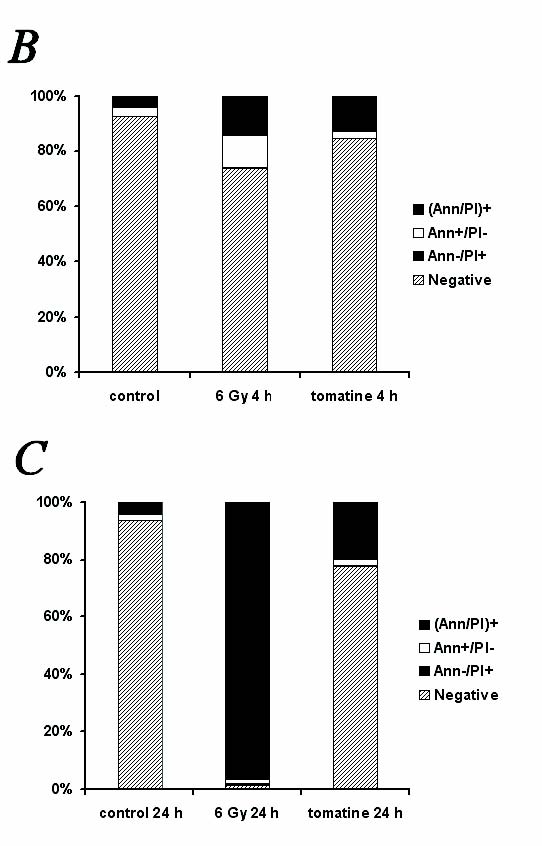
Fig. 5. Effect of alpha-tomatine on the induction of programmed cell death in human MOLT-4 leukemic cells. (A-C) Programmed cell death
induction was determined by Annexin V and propidium iodide staining. Representative dotplots of one of three experiments (A). Quantification of
programmed cell death 4 h (B) and 24 h (C) after the application of 3 micromol/l of alpha-tomatine. Irradiation by 6 Gy of gamma
radiation was used as a positive control. Ann - Annexine V; PI - propidium iodide. (D) Activity of caspases 3/7 was determined 4 and 24 h after
exposure to 2 and 3 micromol/l of alpha-tomatine. 5 nmol/l of mitoxantrone was used as a positive control. T - alpha-tomatine; MTX - mitoxantrone.
(E) Changes in proapoptotic PUMA protein detected by western blot followed by densitometry 4 and 24 h after exposure to 1, 2 and 3 micromol/l
of alpha-tomatine. To ensure equal protein loading, membranes were reincubated with beta-actin. The densities of protein bands were quantified and
expressed as a percentage of the integrated optical density related to beta-actin. Results are shown as mean ± SD from three independent
experiments.
Mechanisms of cell death
The analysis of apoptosis induction in the MOLT-4 cells was performed after a 4h- and 24h-long incubation with 3 micromol/l alpha-tomatine, using
Annexin V (Ann) binding to phosphatidylserine on the cell surface of apoptotic cells. Propidium iodide (PI) was used as a marker for cell membrane
permeability. To investigate the effect of alpha-tomatine, a positive control of gamma-irradiated cells (6 Gy) was used. The treatment with a single
dose of 3 micromol/l alpha tomatine did not lead to an increase in the number of early apoptotic cells (Ann+/PI-), but to a quantitative increase in
the number of late apoptotic/necrotic cells (Ann+/PI+) after 4 h (12%) and 24 h (19%) - in comparison with the control group (4%). The cells affected
by 6 Gy of gamma-radiation were found to be in the stage of early apoptosis (11% of Ann+/PI- cells), transformed to late apoptosis/necrosis (96%) 24 h
later (Fig. 5A-C). Next, we evaluated the activation of caspases 9 and 3/7. A significant increase in the activity of these caspases after exposure to
3 micromol/l alpha-tomatine was not observed up to 24 h post-treatment (Fig. 5D). However, an increase in the amount of PUMA protein from the bcl-2
family was detected 4 and 24 h after exposure to 1-3 micromol/l alpha-tomatine (Fig. 5E).
DISCUSSION
Recently, alpha-tomatine has been recognized as a potential anticancer drug. However, several recent studies report different antiproliferative
mechanisms; thus, the underlying mechanisms are far from clear. In human prostatic adenocarcinoma PC-3 cells, alpha-tomatine induced programmed cell
death, which is not associated with cell cycle arrest and inhibits NF-κB nuclear translocation (Lee et al. 2011). In NCI-H460 human non-small
cell lung cancer cells, alphatomatine inactivated the FAK/PI3K/Akt signaling pathway and reduced the DNA-binding activity of NF-kappaB (Shieh et al.
2011). In MCF-7 (breast) cancer cells, it suppressed the protein kinase alpha/ERK/NFkappaB pathway, which led to the downregulation of the
12-O-tetradecanoylphorbol-13-acetate-induced activation of metalloprotease 2/9 and thereby inhibited the migration and invasion of the cells
(Shi et al. 2012). The study by Lee at al. reporting antiproliferative effects on human colon (HT29) and liver (HepG2) cancer cells did not identify a
specific mechanism. They show that the effect of alphatomatine was more pronounced on liver cells than on colon cells (Lee et al. 2004). PC-3 cells
appear to be particularly susceptible to inhibition, when compared with breast (MDA-MB-321) or gastric (KATOIII) cancer cells, or normal cells (Choi
et al. 2012). Interestingly, the removal of one, two or three sugar molecules from alpha-tomatine results in a decrease in antitumor activity. The
aglycon tomatidine had the lowest activity (Lee et al. 2004, Friedman et al. 2009, Choi et al. 2012).
After treatment with 4 micromol/l alpha-tomatine, we detected an abrupt decrease in cell number already after 4 hours, which was accompanied by a
significant decrease in cell viability. After treatments with lower alpha-tomatine concentrations (1-3 micromol/l), we observed only a transient
inhibition of proliferation. The proliferation and viability of cells treated with 1-3 micromol/l of alpha-tomatine recovered after 72 hours. The
study by Lee et al. demonstrates that concentrations of alpha-tomatine effective on PC-3 cells are in a similar range: PC-3 cells treated with 2.5 and
5.0 micromol/l alpha-tomatine showed a decrease in viability and the EC50 value at 24 h post treatment was estimated at 1.67±0.3
micromol/l. Similarly, the cell viability of NCI-H460 cells significantly decreased after 24 h treatment with alpha-tomatine at concentrations between
2-4 micromol/l. Non-cytotoxic concentrations reduced cell invasion and migration (Shieh et al. 2011). In a recent study by Chao et al., the
EC50 values at 24 h post treatment for the HL60 and K526 leukemic cell lines were 1.92 and 1.51 micromol/l respectively (Chao et al.
2012).
How exactly alpha-tomatine induces cell death remains an unanswered question. The treatment of PC-3 prostate cancer cells with 2 micromol/l
alphatomatine for 24 hours resulted in an increase in the number of early apoptotic cells as well as late apoptotic cells, and the activation of
caspase-3, caspase-8 and caspase-9 was confirmed (Lee et al. 2011). In contrast, in HL-60 and K562 leukemic cells, alpha-tomatine induced
caspase-independent programmed cell death – caspase-3, -8, -9 were not activated (Chao et al. 2012). Our data support the findings of Chao et
al. In our study on MOLT-4 leukemic cells, the 3 micromol/l concentration of alphatomatine induced a slight decrease in cell viability 24 h after
application of the drug. Also, after 24 hours, 20 % of the cells bound Annexin V and propidium iodide, indicating late apoptosis/necrosis. We did not
detect a significant increase in the activity of caspases 9 and 3/7. However, an increase in the amount of mitochondrial protein PUMA was detected 4
and 24 h after exposure to 1-3 micromol/l alpha-tomatine.
PUMA protein belongs to the group of proapoptotic factors from the Bcl-2 family, characterized by the presence of only the BH3 domain. The role of
these BH3-onlies is to mediate apoptotic signalization dependent on cell integrity. BH3-onlies bind to anti-apoptotic Bcl-2-like factors and enable
oligomerization of Bax-like pro-apoptotic factors and permeabilization of the mitochondrial membrane. In undamaged cells, these factors are absent or
exist in an inactive form (Bogner et al. 2010). The treatment with alpha-tomatine led to an increase in PUMA protein level after 4 and 24 hours. This
fact correlates with the observed increase in p53, because one of the p53 roles is to increase the transcription of PUMA. Our study is the first to
report changes in BH3-onlies induced by alphatomatine; however, our findings are consistent with recent findings that alpha-tomatine causes a decrease
in membrane potential, the upregulation of pro-apoptotic Bax-like protein Bak, and the release of programmed cell death-inducing factor (AIF) from
mitochondria (Chao et al. 2012).
Transcription of the PUMA gene (BBC3) can be activated by protein p53 (Lakin and Jackons 1999, Erlacher et al. 2005). Human p53 is a tumor
suppressor phosphoprotein; its most investigated role is the ability to act as a specific activator of gene transcription. In this way, p53 is
involved in regulation of the cell cycle, programmed cell death, senescence, DNA repair, cell differentiation, and angiogenesis (Sionov and Haupt
1999). The p53 in normal cells is degraded by the proteasome 26S. DNA damage and other stressors induce post-translational modifications of p53,
mainly phosphorylations, participating in its stabilization and activation (Bai and Zhu 2006, Lavin and Gueven 2006). Our experimental data showed a
dose-dependent increase in p53 and in its phosphorylation on serine 15 after 4 and 24 hours of exposure to alpha-tomatine. Phosphorylation of serine
15 on the N-terminal domain of p53 can be mediated directly by ATM kinase or through Chk1/Chk2 kinase, and it inhibits the interaction of p53 with its
negative regulator oncoprotein Mdm2 (Shieh et al. 1997, Yap et al. 2004).
P53 readily reacts to genotoxic stressors, mainly to the induction of single and double strand breaks. However, the examination by comet assay did not
detect significant amounts of single and double DNA strand breaks in cells treated with 0.1-9 mmol/l of alpha-tomatine. The increase in p53 must
therefore be due to another type of stress. Different forms of stress, such as DNAdamage (ultraviolet or ionizing radaition, chemical agents),
oxidative stress, hypoxia, ribonucleotide depletion, and deregulated oncogene expression induce an increase in the amount of p53 (Prives and Hall
1999). Alpha-tomatine interacts with cholesterol and can cause damage to the integrity of biomembranes (Keukens et al. 1995, 1996), which may be
responsible for the observed p53 increase.
Our study also showed a dose-dependent increase in p21 after 4 hours as well as the activation of Chk2 (phosphorylated at threonine 68) after 4 and 24
hours. Both of these proteins are involved in cell cycle regulation. Chk2 is a protein responsible for regulation of the cell cycle and controls the
checkpoints in G1/S and G2/M transition by the inhibition of Cdc25A and Cdc25C (Bartek and Lukas 2003). The gene CDNK1A encoding protein
p21WAF1/CIP1 (wild type activated fragment 1, cyclin-dependent kinase inhibitor protein 1) is another target of p53.
P21WAF1/CIP1 triggers mainly cell cycle arrest in the G1/S phase, but also participates in cell cycle arrest at the G2/M checkpoint (Taylor
and Stark 2001, Cazzalini et al. 2010). A previous study revealed that alpha-tomatine did not affect cell cycle distribution (Chao et al. 2012);
however, the increased amounts of p21 and phosphorylated Chk2 (threonine 68) correlate well with the transient decrease in cell proliferation,
indicating an overall slow-down of the cycle.
We may conclude that alpha-tomatine does not cause direct DNA damage. It induces caspase-independent cell death associated with an increase in p53 and
the BH3only protein PUMA. The inhibition of proliferation by alpha-tomatine is associated with an increase in p21WAF1/CIP1 level and the
activation of Chk2. Alpha-tomatine is a potential antitumor agent; however, the mechanism of its anticancer effect requires additional study.
ACKNOWLEDGEMENT
We wish to thank Dr. Jaroslav Pejchal from the Department of Radiobiology, Faculty of Military Health Sciences, University of Defence for his valuable
help with image analysis, and Ms. Nadezda Mazankova and Ms. Bozena Janska for their skilful technical assistance. This study was supported by the
program PRVOUK P37/01 of Charles University in Prague.
REFERENCES
Bai L, Zhu WG. P53: Structure, unction and therapeutic applications. J. Cancer Mol. 2: 141-153, 2006.
Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 3: 421-429, 2003.
[CrossRef]
Berger J. The age of biomedicine: current trends in traditional subjects. J Appl Biomed 9: 57-61, 2011.
[CrossRef]
[JAB]
Bergers WW, Alink GM. Toxic effect of the glycoalkaloids solanine and tomatine on cultured neonatal rat heart cells. Toxicol Lett. 6: 29-32, 1980.
[CrossRef]
Bogner C, Leber B, Andrews DW. Apoptosis: embedded in membranes. Curr Opin Cell Biol. 22: 845-851, 2010.
[CrossRef]
[PubMed]
Calini V, Urani C, Camatini M. Comet assay evaluation of DNA single- and double-strand breaks induction and repair in C3H10T1/2 cells. Cell Biol
Toxicol. 18: 369-379, 2002.
[CrossRef]
[PubMed]
Cazzalini O, Scovassi AI, Savio M, Stivala LA, Prosperi E. Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat
Res. 704: 12–20, 2010.
[CrossRef]
[PubMed]
Chao MW, Chen CH, Chang YL, Teng CM, Pan SL. Alpha-Tomatine-Mediated Anti-Cancer Activity In Vitro and In Vivo through Cell Cycle- and
Caspase-Independent Pathways. PLoS One. 7: e44093, 2012.
[CrossRef]
[PubMed]
Chiu FL, Lin JK. Tomatidine inhibits iNOS and COX-2 through suppression of NF-kappaB and JNK pathways in LPS-stimulated mouse macrophages. FEBS Lett.
582: 2407-2412, 2008.
[CrossRef]
[PubMed]
Choi SH, Ahn JB, Kozukue N, Kim HJ, Nishitani Y, Zhang L, Mizuno M, Levin CE, Friedman M. Structure-activity relationships of alpha-, beta(1)-,
gamma-, and delta-tomatine and tomatidine against human breast (MDA-MB-231), gastric (KATO-III), and prostate (PC3) cancer cells. J Agric Food Chem.
60: 3891–
3899, 2012.
[CrossRef]
Collins AR, Dusinska M, Gedik CM, Stetina R. Oxidative damage to DNA: do we have a reliable biomarker? Environ Health Perspect. 104(Suppl 3): 465-469,
1996.
[PubMed]
Erlacher M, Michalak EM, Kelly PN, Labi V, Niederegger H, Coultas L, Adams JM, Strasser A. Villunger A. BH3-only proteins Puma and Bim are rate
limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood. 106: 4131-4138, 2005.
[CrossRef]
[PubMed]
Filderman RB, Kovacs BA. Anti-inflammatory activity of the steroid alkaloid glycoside, toatine. Br J Pharmacol. 37: 748-755, 1969.
[CrossRef]
[PubMed]
Fontaine TD, Irwing GW, Ma R, Poole JB, Doolittle SP. Isolation and partial characterization of crystalline tomatine, an antibiotic agent from the
tomato plant. Arch Biochem. 18: 467-475, 1948.
[PubMed]
Friedman M. Tomato glycoalkaloids: role in the plant and in the diet. J Agric Food Chem. 50: 5751-5780, 2002.
[CrossRef]
[PubMed]
Friedman M, Levin CE, Lee SU, Kim HJ, Lee IS, Byun JO, Kozukue N. Tomatine-containing green tomato extracts inhibit growth of human breast, colon,
liver, and stomach cancer cells. J Agric Food Chem. 57: 5727–5733, 2009.
[CrossRef]
[PubMed]
Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 147: 742-758, 2011.
[CrossRef]
[PubMed]
Irwing GW, Fontaine TD, Doolittle SP. Partial Antibiotic Spectrum of Tomatin, an Antibiotic Agent from the Tomato Plant. J Bacteriol. 52: 601-607,
1946.
Keukens EA, de Vrije T, van den Boom C, de Waard P, Plasman HH, Thiel F, Chupin V, Jongen WM, de Kruijff B. Molecular basis of glycoalkaloid induced
membrane disruption. Biochim Biophys Acta. 1240: 216-228, 1995.
[CrossRef]
Keukens EA, de Vrije T, Jansen LA, de Boer H, Janssen M, de Kroon AI, Jongen WM, de Kruijff B. Glycoalkaloids selectively permeabilize cholesterol
containing biomembranes. Biochim Biophys Acta. 1279: 243-250, 1996.
[CrossRef]
Lakin ND, Jackson SP. Regulation of p53 in response to DNA damage. Oncogene. 18: 7644-7655, 1999.
[CrossRef]
[PubMed]
Lane DP. Cancer. P53, guardian of the genome. Nature. 358: 15-16, 1992.
[CrossRef]
[PubMed]
Lavin MF, Gueven N. The complexity of p53 stabilization and activation. Cell Death Differ. 13: 941-950, 2006.
[CrossRef]
[PubMed]
Lee KR, Kozukue N, Han JS, Park JH, Chang EY, Baek EJ, Chang JS, Friedman M. Glycoalkaloids and metabolites inhibit the growth of human colon (HT29)
and liver (HepG2) cancer cells. J Agric Food Chem. 19: 2832-2839, 2004.
[CrossRef]
[PubMed]
Lee ST, Wong PF, Cheah SC, Mustafa MR. Alpha-tomatine induces apoptosis and inhibits nuclear factor-kappa B activation on human prostatic
adenocarcinoma PC-3 cells. PLoS One. 6: e18915, 2011.
[CrossRef]
[PubMed]
Ma R, Fontaine TD. In vitro antibiotic activity of crystalline tomatine toward Candida albicans; antagonistic effect of rutin and quercetin.
Arch Biochem. 16: 399-402, 1948.
[PubMed]
Olive PL, Banath JP. Detection of DNA double-strand breaks through the cell cycle after exposure to X-rays, bleomycin, etoposide and 125IdUrd. Int J
Radiat Biol. 64: 349-358, 1993.
[CrossRef]
[PubMed]
Prives C, Hall PA. The p53 pathway. J. Pathol. 187: 112–126, 1999.
[CrossRef]
Shi MD, Shih YW, Lee YS, Cheng YF, Tsai LY. Suppression of 12-O-Tetradecanoylphorbol-13-Acetate-Induced MCF-7 Breast Adenocarcinoma Cells
Invasion/Migration by alpha-Tomatine Through Activating PKC?/ERK/NF-alphaB-Dependent MMP-2/MMP-9 Expressions. Cell Biochem Biophys. 2012 Nov 1. [Epub
ahead of print].
[CrossRef]
Shieh JM, Cheng TH, Shi MD, Wu PF, Chen Y, Ko SC, Shih YW. Alpha-Tomatine Suppresses Invasion and Migration of Human Non-Small Cell Lung Cancer
NCI-H460 Cells through Inactivating FAK/PI3K/Akt Signaling Pathway and Reducing Binding Activity of NF-kappa B. Cell Biochem Biophys. 60: 297-310,
2011.
[CrossRef]
[PubMed]
Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage induced phosporylation of p53 alleviates inhibition by MDM2. Cell. 91: 325-334, 1997.
[CrossRef]
Shih YW, Shieh JM, Wu PF, Lee YC, Chen YZ, Chiang TA. Alpha-tomatine inactivates PI3K/Akt and ERK signaling pathways in human lung adenocarcinoma A549
cells: effect on metastasis. Food Chem Toxicol. 47: 1985-1995, 2009.
[CrossRef]
[PubMed]
Sionov RV, Haupt Y. The cellular response to p53: the decision between life and death. Oncogene. 18: 6145-6157, 1999.
[CrossRef]
[PubMed]
Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 20: 1803-1815, 2001.
[CrossRef]
[PubMed]
Tichy A, Zaskodova D, Rezacova M, Vavrova J, Vokurkova D, Pejchal J, Vilasova Z, Cerman J, Osterreicher J. Gamma-radiation-induced ATM-dependent
signalling in human T-lymphocyte leukemic cells, MOLT-4. Acta Biochim Pol. 54: 281-287, 2007.
[PubMed]
Wilson RH, Poley GW, De Eds F. Some pharmacologic and toxicologic properties of tomatine and its derivatives. Toxicol Appl Pharmacol. 3: 39-48,
1961.
[CrossRef]
Yap DBS, Hsieh J-K, Zhong S, Heath V, Gusterson B, Crook T, Lu X. Ser392 phosphorylation regulates the oncogenic function of mutant p53.
Cancer Rest. 64: 4749–4754, 2004.
[CrossRef]
[PubMed]
|
BACK
|









