Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 11 (2013), No 2, p 59-70
DOI 10.2478/v10136-012-0020-0
Evolution of the circadian profile of human milk amino acids during breastfeeding
Cristina Lucia Sanchez, Javier Cubero, Javier Sanchez, Lourdes Franco, Ana Beatriz Rodriguez, Montserrat Rivero, Carmen Barriga
Address: Cristina Lucia Sanchez Lopez, Department of Physiology, Faculty of Science, University of Extremadura, Av. Elvas s/n 06006 Badajoz, Spain
crissanchez@unex.es
Received 19th June 2012.
Revised 10th October 2012.
Published online 15th October 2012.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
Acknowledgements
References
SUMMARY
Human milk is a living fluid that changes with time, composition and volume. Circadian rhythms regulate a variety of biological processes in living
organisms; and perhaps the most evident function is the sleep-wake cycle. The aim of the present study was to evaluate the circadian rhythm of breast
milk amino acids and their evolution throughout the breastfeeding period. Human breast milk samples from 77 donors were collected every 3 hours over a
24-h period. The rhythmicity of the amino acids was determined by cosinor analysis. Colostrum samples showed no circadian rhythm in most amino acids
except tryptophan. However, daily variations were observed in tryptophan and methionine at transitional phase, according to the newborn’s pattern of
intake every 3 hours regardless of whether it is day or night. During the last stage (mature milk), when breast milk has fully stabilized, most amino
acids showed a circadian rhythm. In conclusion, breast milk should be given to the baby at the same time of day it is expressed. Thus, the baby would
be adjusting its circadian pattern in harmony with his environment (day/night), which is crucial for the proper functioning and synchronization of all
systems in the human body.
KEY WORDS
amino acids; day/night; circadian rhythm; human milk; tandem mass
INTRODUCTION
Breast milk and breastfeeding have to be considered as the referent or “gold standard” of infant feeding during the first six months of life (WHO
1985). Breast milk is not a static biological fluid but, rather, it is a dynamic fluid that changes not only over the course of the period of
lactation, but also during the day, and even during the same session of nursing, and can be influenced by the mother’s own diet (Sanchez et al. 2008,
2009).
The temporal variation in its composition (inter- and intra-individual) has been widely studied. The principal focus has been on its lipid profile
(Lammi-Keefe et al. 1990, Lubetzky et al. 2006) and its protein and amino acid content (Clark et al. 1987, Shubat et al. 1989, Catarru et al. 2003,
Hernell and Lodernall 2003, Yamawaki et al. 2005, Piccione et al. 2008).
The first findings about daily variations in breast milk composition were performed to derive, within the variability of the milk itself, the most
appropriate time of day to take the analyte value such as the reference value (Hall 1979, Krebs et al. 1985, Thomas et al. 1986).
Previous studies have demonstrated that breast milk protein levels are greatest in the colostrum, and that they decline in transitional milk to a
lower level in mature milk, according to the physiological demand of the newborn. Therefore, associated changes with this content have also been
observed; in particular, breast milk protein content is higher during daylight hours than the night period (Sanchez et al. 2011). Other authors have
also reported such circadian variations in different components of breast milk, where values may differ between day and night. This is the case for
some minerals, such as calcium and magnesium, trace elements, such as zinc (Karra and Kirksey 1998), copper, and iron (Picciano and Guthrie 1976), for
some micronutrients, such as lactose, which has minimum values at 17:00 h (Viverge et al. 1986), and for the essential amino acid tryptophan which
shows a nocturnal acrophase (Cubero et al. 2005). All these compounds are crucial for the optimal growth of the newborn.
The regulation of the sleep/wake cycle is complex, and involves many neurochemical transmission systems; such as acetylcholine, dopamine,
norepinephrine, serotonin, histamine, hypocretin, and orexin, to maintain states of activity, i.e., they are promoters of wakefulness
(Murillo-Rodriguez et al. 2009). The concentration of these neurotransmitters is higher during daylight mainly in the brain stem and hypothalamic
region, and the variation may be reflected in peripheral fluids such as breast milk (Hunsley and Palmiter 2004). Other neurotransmitters such as
gamma-aminobutyric acid (GABA) and serotonin exert their neuromodulatory action on sleep in some brain regions such as the hippocampus and the raphe
nucleus, respectively, and their maximum neuroactivity occurs during the night (Reis et al. 2009).
This neural modulation is thought to be present in breast milk, thereby influencing the newborn‘s sleep/wake rhythm through precursor amino acids
whose levels may vary throughout the day depending on whether the infant is asleep or active.
In this context, the goal of the present study was to evaluate the circadian rhythm of the breast milk amino acids and their evolution throughout the
breastfeeding period, in order to elucidate the importance of these components in regulating the circadian sleep-wakefulness function.
MATERIALS AND METHODS
Subjects
Breast milk samples were collected from 77 breast-feeding women (32±5 years old, 70.8±11.6 kg, 1.65±0.06 m, and body mass index 26.1±4.3
kg/m2). Donors were recruited at the Perpetuo Socorro Hospital (Servicio Extremeño de Salud, S.E.S., Badajoz, Spain). During collection
period, the study subjects took no drugs that could affect their amino acid levels. Breastfeeding women at all breast milk stages were asked to
participate in the study. All of them received verbal and written information about the methods of the study and they signed an informed consent form
before participation. Protocols for this study were approved by the Ethical Investigation Committee from the University of Extremadura
(Spain).
Samples
Foremilk samples were collected prior to any feeding from either the left or the right breast into sterile polystyrene tubes (5 ml). Repeated samples
from each mother were collected every 3 hours over a 24-h period, regardless of the necessity for feeding, for a total of 6-8 per donor. As the focus
of this study was to characterize the circadian rhythm of amino acids in breast milk within each stage, donors were not required to provide samples
longitudinally. However, 6 women voluntarily provided milk during all the stages, whereas 22 participants provided samples during 2 stages. The
collection campaign lasted from January to December 2008. Breast milk samples were immediately aliquoted and stored at -80 °C until
processing.
Extraction of the amino acids
The technique of Yamawaki et al. (2005) was followed with certain modifications. Aliquots of 1 ml of each sample were de-fatted with 0.5 ml of diethyl
ether (Sigma-Aldrich). The supernatants were collected, discarding the fatty halo, followed by hydrolysis with 1 ml of 6N HCl (Sigma-Aldrich) for most
of amino acids, and 0.75 g of BaOH (Sigma Aldrich) and 1.75 ml of MilliQ water for tryptophan (Cubero et al. 2005). After gentle mixing, the aliquots
were allowed to stand for 22 hours at 110 °C, and then filtered through a 0.45 microm membrane filter (Millex, Millipore, USA) to remove the ash
before assay.
HPLC-ESI-MS/MS analysis
The samples were assayed using a Waters 2795 Alliance HT HPLC (Milan, Italy) coupled to a Micromass Quattro Ultima mass spectrometer (Milan, Italy)
with an ESI (Electrospray Ionization) source, together with an Agilent Zorbax Eclipse AAA C18 column (3.0 mm x 150 mm x 3.5 micron) for the amino acid
analyses. The liquid chromatography-tandem mass conditions were as follows: column temperature 80 °C; source temperature 80 °C; desolvation gas flow
650 l/h; cone gas and voltage
0 l/h and 55 V, respectively; and capillary voltage 3.50 kV (Sanchez et al. 2012).
Chronobiological analysis
A chronobiological analysis (Berger 2011) of the data was performed using the Ritme© for Windows software package. The rhythmicity of each
amino acid was studied by cosinor analysis (Halberg et al. 1967). The sinusoidal function used for the fit was:
>y(t) = M + A x cos [(2 x pi/tau) x t - phi]
where y(t) is the value of the cosine function at time t, M is the mean level of oscillation or MESOR (acronym for Midline-Estimating Statistic Of
Rhythm, the mean value about which the oscillation occurs equal to the arithmetic mean of equidistant data covering a whole number of cycles), A is
the amplitude (measure of the extent of a rhythmic change in a cycle as estimated by the sinusoidal function that best fits the data), the angular
frequency is omega = 2 x pi/tau where pi is the number pi and tau is the period (24 hours in our case), and omega is the acrophase (a phase angle
measuring the timing of the peak activity, expressed as the lag from a reference time to the crest time of the best fit sinusoidal function). A
cosinor analysis thus finds the best-fitting sinusoidal function by estimating three parameters: mesor, amplitude, and acrophase.
Using the cosinor analysis, we determined the 95% confidence intervals of the MESOR, amplitude, and acrophase. When this interval contains the value
0, one possibility - that amplitude is 0 - cannot be rejected, so that the existence of a rhythm is not statistically significant. This is equivalent
to testing whether the null hypothesis of zero amplitude is rejected at 2alpha level of 0.05.
The confidence intervals of the acrophase show whether there are significant differences between the acrophases of different variables. In particular,
if two confidence intervals overlap, the possibility that the two acrophases are equal can not be discarded.
RESULTS
Colostral stage (<5 days post-partum)
Table 1a, b summarizes the mean concentrations of each amino acid in mg/dl during the colostrum period. To facilitate understanding of the data, the
table is presented in two parts: daytime (09:00 to 21:00, Table 1a) and night-time (21:00 to 09:00, Table 1b).
Table 1a. Concentrations of amino acids (mean ± SD) during the colostral stage at the specific hours of daytime (09:00–18:00) at
which samples were taken (n = 31).
| 9:00h | 12:00h | 15:00h | 18:00h | mean | SD |
|---|
| Alanine | 63.92 | 58.83 | 78.40 | 61.45 | 55.93 | 10.39 | | Aspartic Acid | 111.52 | 105.26 | 130.55 | 109.28 | 97.75 | 18.32 | | Citrulline | 3.72 | 3.98 | 5.05 | 4.20o | 4.36 | 2.49 | | Glutamic acid | 228.41 | 219.73 | 271.05 | 232.80 | 222.38 | 36.76 | | Glycine | 34.33 | 33.24 | 42.67 | 33.60 | 31.02 | 7.70 | | Hystidine | 28.64 | 27.24 | 34.53 | 29.32 | 25.95 | 6.00 | | Leucine | 97.26 | 92.60 | 122.98 | 102.08 | 96.95 | 18.21 | | Methionine | 30.62 | 24.51 | 36.49 | 31.31 | 30.04 | 12.55 | | Ornitine | 6.51 | 6.04 | 7.69 | 6.91 | 7.48 | 2.95 | | Phenylalanine | 50.04 | 46.76 | 61.94 | 50.08 | 45.33 | 8.47 | | Proline | 123.14 | 113.19 | 143.84 | 119.82 | 114.46 | 21.72 | | Serine | 59.93 | 56.35 | 69.94 | 59.92 | 53.40 | 10.50 | | Tryptophan | 34.74 | 34.69 | 34.52 | 34.61 | 34.64 | 0.37 | | Tyrosine | 45.00 | 42.02 | 55.47 | 45.56 | 41.37 | 7.79 | | Valine | 61.14 | 57.92 | 77.87 | 64.85 | 60.37 | 12.20 | | Taurine | 73.66 | 69.93 | 67.65 | 72.15 | 71.81 | 11.90 |
Table 1b. Concentrations of amino acids (mean ± SD) during the colostral stage at the specific hours of night-time (21:00–06:00) at
which samples were taken (n = 31).
| 21:00h | 24:00h | 3:00h | 6:00h | mean | SD |
|---|
| Alanine | 59.25 | 61.62 | 50.39 | 59.67 | 57.73 | 11.72 | | Arginine | 54.49 | 56.37 | 39.20 | 66.61 | 54.17 | 13.46 | | Aspartic Acid | 102.44 | 108.61 | 76.08 | 116.32 | 100.86 | 22.59 | | Citrulline | 3.80 | 4.53 | 3.38 | 5.41 | 4.28 | 1.98 | | Glutamic acid | 210.46 | 236.81 | 194.43 | 249.83 | 222.88 | 46.86 | | Glycine | 32.05 | 32.14 | 22.02 | 39.58 | 31.45 | 9.72 | | Hystidine | 27.76 | 28.54 | 20.27 | 30.74 | 26.83 | 6.35 | | Leucine | 92.48 | 101.69 | 87.18 | 106.68 | 97.01 | 24.91 | | Methionine | 27.56 | 27.56 | 26.62 | 34.44 | 29.05 | 11.37 | | Ornitine | 7.06 | 7.21 | 5.87 | 9.22 | 7.34 | 3.32 | | Phenylalanine | 46.89 | 49.89 | 35.71 | 53.73 | 46.55 | 10.65 | | Proline | 110.52 | 121.62 | 97.13 | 131.15 | 115.11 | 25.22 | | Serine | 57.94 | 58.61 | 39.92 | 64.93 | 55.35 | 11.62 | | Tryptophan | 34.62 | 34.81 | 34.76 | 34.79 | 34.74 | 0.33 | | Tyrosine | 41.69 | 45.45 | 33.91 | 47.94 | 42.25 | 9.90 | | Valine | 58.20 | 62.82 | 53.19 | 67.49 | 60.42 | 13.99 | | Taurine | 71.52 | 75.39 | 72.27 | 70.69 | 72.46 | 11.25 |
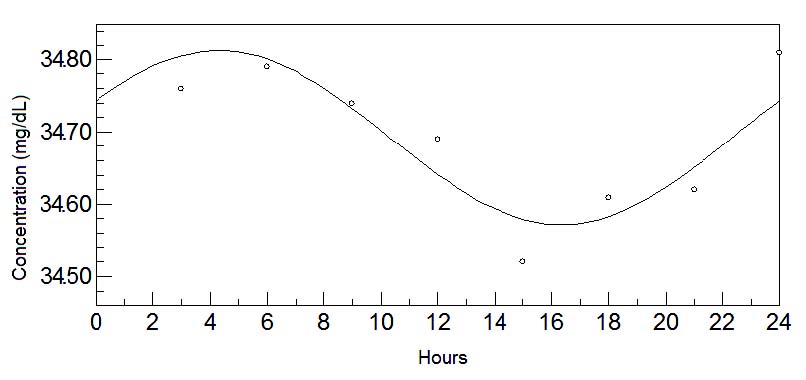
Fig. 1. 24-hours oscillations of tryptophan in colostrum. This graph depicts the fluctuations of tryptophan levels in colostrum during
24-hours. Circadian rhythm is shown by a sinusoidal curve.
These results show how the amino acids vary during this stage of lactation according to the time of day. However, the Cosinor analysis of the mean
values reveal a daily (24-hour) rhythmicity only in the essential amino acid tryptophan (Fig. 1), which perhaps exerts a marked effect on the infant
from its first feed after birth.
Transitional stage (6-15 days post-partum)
Table 2a, b presents the daytime (a) and night-time (b) concentrations of the amino acids during the transitional phase. This stage, as its name
indicates, is characterized by an intermediate composition between the colostrum and the mature milk. It occurs from days 6 to 15 of lactation.
The amino acid profile varies over the course of the day (Figs 2 and 3). In addition, the concentrations of most of the amino acids decline as nursing
advances.
The chronobiological analysis again showed the amino acid tryptophan (Fig. 2) to continue to present a circadian rhythm with acrophase during the
night (03:00 approx.). However, another essential amino acid, methionine (Fig. 3), also showed a marked circadian rhythm, with acrophase during the
daytime (18:00 approx.). Variations in concentrations at different times of day and the chronobiological data are shown in Tables 2a, b and 4,
respectively.
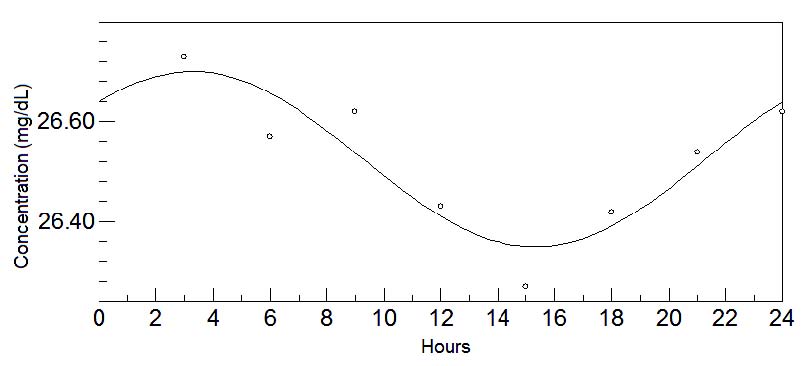
Fig. 2. 24-hours oscillations of tryptophan in transitional milk. This graph depicts the fluctuations of tryptophan levels in transitional milk
during 24-hours. Circadian rhythm is shown by a sinusoidal curve.
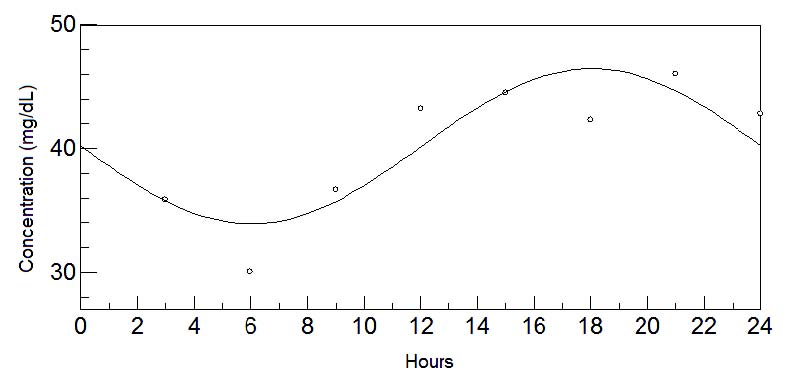
Fig. 3. 24-hours oscillations of methionine in transitional milk. This graph depicts the fluctuations of methionine levels in transitional milk
during 24-hours. Circadian rhythm is shown by a sinusoidal curve.
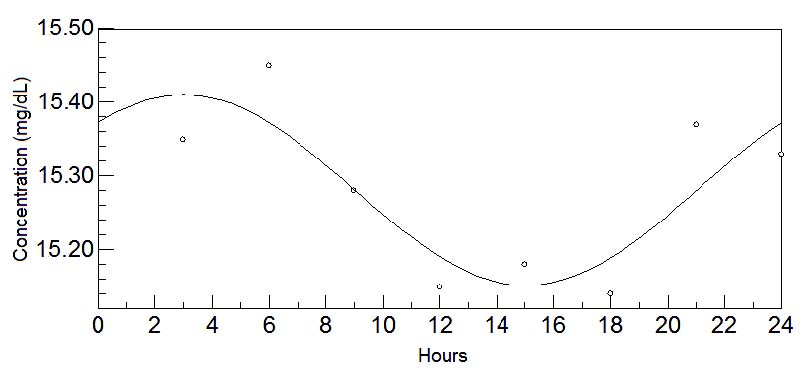
Fig. 4. 24-hours oscillations of tryptophan in mature milk. This graph depicts the fluctuations of tryptophan levels in mature milk during
24-hours. Circadian rhythm is shown by a sinusoidal curve.
Table 2a. Concentrations of amino acids (mean ± SD) during the transitional stage at the specific hours of daytime (09:00–18:00) at
which samples were taken (n = 34).
| 9:00h | 12:00h | 15:00h | 18:00h | mean | SD |
|---|
| Alanine | 51.04 | 49.58 | 48.26 | 48.01 | 49.22 | 11.85 |
|---|
| Arginine | 44.83 | 41.01 | 40.53 | 39.40 | 41.44 | 10.65 |
|---|
| Aspartic Acid | 82.73 | 86.95 | 84.33 | 83.00 | 84.25 | 17.45 |
|---|
| Citrulline | 2.73 | 3.91 | 3.33 | 3.24 | 3.30 | 1.81 |
|---|
| Glutamic acid | 170.88 | 181.21 | 175.92 | 175.00 | 175.75 | 35.00 |
|---|
| Glycine | 30.50 | 27.16 | 27.68 | 26.56 | 27.97 | 8.93 |
|---|
| Hystidine | 32.23 | 26.44 | 26.13 | 25.43 | 27.56 | 6.45 |
|---|
| Leucine | 65.99 | 69.50 | 69.92 | 67.82 | 68.31 | 14.42 |
|---|
| Methionine | 36.68 | 43.25 | 44.57 | 42.34 | 41.71 | 11.68 |
|---|
| Ornitine | 8.26 | 8.17 | 8.43 | 7.57 | 8.11 | 5.70 |
|---|
| Phenylalanine | 36.73 | 38.25 | 38.68 | 36.82 | 37.62 | 8.19 |
|---|
| Proline | 88.64 | 94.68 | 93.90 | 91.78 | 92.25 | 19.35 |
|---|
| Serine | 60.14 | 55.59 | 55.52 | 53.41 | 56.16 | 14.34 |
|---|
| Tryptophan | 26.62 | 26.43 | 26.27 | 26.42 | 26.44 | 0.54 |
|---|
| Tyrosine | 32.65 | 33.01 | 32.47 | 31.32 | 32.36 | 7.14 |
|---|
| Valine | 48.68 | 51.13 | 51.31 | 49.59 | 50.18 | 11.81 |
|---|
| Taurine | 64.93 | 75.23 | 79.33 | 75.96 | 73.86 | 19.44 |
|---|
Table 2b. Concentrations of amino acids (mean ± SD) during the transitional stage at the specific hours of night-time (21:00–06:00)
at which samples were taken (n = 34).
| 21:00h | 24:00h | 3:00h | 6:00h | mean | SD |
|---|
| Alanine | 51.12 | 44.71 | 45.57 | 42.07 | 45.87 | 8.91 | | Arginine | 41.32 | 37.86 | 39.37 | 36.83 | 38.84 | 8.25 | | Aspartic Acid | 87.73 | 82.15 | 84.69 | 83.25 | 84.45 | 15.68 | | Citrulline | 4.81 | 3.30 | 4.73 | 3.11 | 3.99 | 3.08 | | Glutamic acid | 185.94 | 172.05 | 178.42 | 178.11 | 178.63 | 37.72 | | Glycine | 27.34 | 23.62 | 24.36 | 22.87 | 24.55 | 5.52 | | Hystidine | 27.40 | 24.30 | 25.32 | 23.55 | 25.14 | 5.35 | | Leucine | 70.91 | 66.34 | 67.34 | 66.43 | 67.75 | 13.35 | | Methionine | 46.10 | 42.84 | 35.86 | 30.07 | 38.72 | 10.11 | | Ornitine | 8.80 | 6.64 | 5.79 | 5.63 | 6.72 | 3.46 | | Phenylalanine | 39.37 | 36.19 | 36.95 | 35.30 | 36.95 | 7.13 | | Proline | 98.25 | 91.51 | 89.79 | 93.10 | 93.16 | 19.48 | | Serine | 58.52 | 50.12 | 51.37 | 49.41 | 52.36 | 10.04 | | Tryptophan | 26.54 | 26.62 | 26.73 | 26.57 | 26.62 | 0.28 | | Tyrosine | 33.54 | 30.86 | 31.56 | 30.18 | 31.54 | 6.15 | | Valine | 51.87 | 47.92 | 48.60 | 46.98 | 48.84 | 9.23 | | Taurine | 78.75 | 72.31 | 71.06 | 71.55 | 73.42 | 13.53 |
Mature stage (>15 days post-partum)
The results of the assay of amino acids in the mature stage of breast milk are shown during daytime in Table 3a and night-time in Table 3b. At this
stage, most components in breast milk are stabilized for the remaining months of lactation as the chronobiological analysis shows. Besides tryptophan
and methionine (Figs 4 and 5 respectively), additional amino acids such as aspartic acid (Fig. 6), histidine (Fig. 7), phenylalanine (Fig. 8) and
tyrosine (Fig. 9), present circadian oscillations in their concentrations.
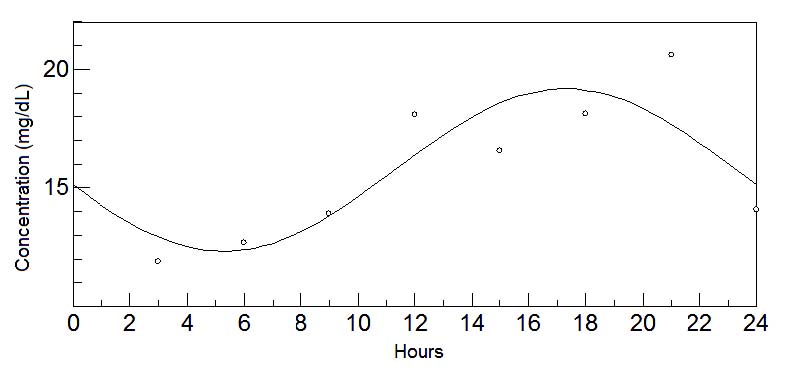
Fig. 5. 24-hours oscillations of methionine in mature milk. This graph depicts the fluctuations of methionine levels in mature milk during
24-hours. Circadian rhythm is shown by a sinusoidal curve.
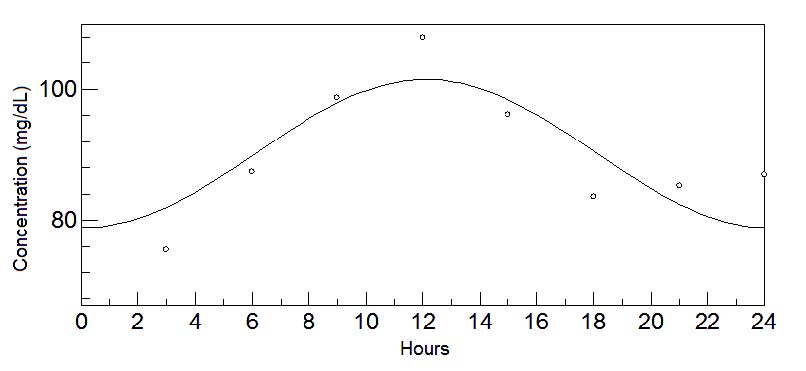
Fig. 6. 24-hours oscillations of aspartic acid in mature milk. This graph depicts the fluctuations of aspartic acid levels in mature milk
during 24-hours. Circadian rhythm is shown by a sinusoidal curve.
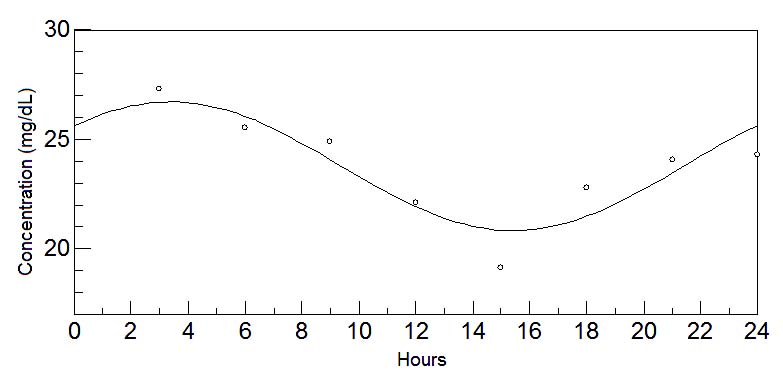
Fig. 7. 24-hours oscillations of histidine in mature milk. This graph depicts the fluctuations of histidine levels in mature milk during
24-hours. Circadian rhythm is shown by a sinusoidal curve.
Table 3a. Concentrations of amino acids (mg/dl) during the mature stage at 09:00–18:00 (n = 40).
| 9:00h | 12:00h | 15:00h | 18:00h | mean | SD |
|---|
| Alanine | 41.70 | 44.04 | 35.15 | 38.34 | 39.81 | 9.56 |
|---|
| Arginine | 38.93 | 44.46 | 34.08 | 38.16 | 38.91 | 11.76 |
|---|
| Aspartic Acid | 98.74 | 107.99 | 96.17 | 83.57 | 96.62 | 17.91 |
|---|
| Citrulline | 6.11 | 7.55 | 6.06 | 5.41 | 6.28 | 2.53 |
|---|
| Glutamic acid | 153.88 | 167.03 | 140.86 | 137.45 | 149.81 | 19.88 |
|---|
| Glycine | 26.84 | 30.21 | 28.39 | 24.65 | 27.52 | 5.65 |
|---|
| Hystidine | 24.90 | 22.12 | 19.13 | 22.81 | 22.24 | 3.82 |
|---|
| Leucine | 67.73 | 64.91 | 63.49 | 60.35 | 64.12 | 11.22 |
|---|
| Methionine | 13.93 | 18.09 | 16.57 | 18.15 | 16.69 | 4.83 |
|---|
| Ornitine | 9.04 | 9.98 | 8.69 | 8.96 | 9.17 | 2.72 |
|---|
| Phenylalanine | 37.24 | 40.27 | 37.37 | 35.53 | 37.60 | 8.87 |
|---|
| Proline | 83.71 | 92.26 | 74.06 | 70.21 | 80.06 | 16.42 |
|---|
| Serine | 48.00 | 51.24 | 41.42 | 44.27 | 46.23 | 12.58 |
|---|
| Tryptophan | 15.28 | 15.15 | 15.18 | 15.14 | 15.19 | 0.06 |
|---|
| Tyrosine | 36.27 | 36.00 | 33.34 | 29.31 | 33.73 | 5.70 |
|---|
| Valine | 54.84 | 53.44 | 52.25 | 49.80 | 52.58 | 9.09 |
|---|
| Taurine | 63.44 | 64.32 | 65.06 | 64.89 | 64.43 | 1.63 |
|---|
Table 3b. Concentrations of amino acids (mg/dl) during the mature stage at 21:00–06:00 (n = 40).
| 21:00h | 24:00h | 3:00h | 6:00h | mean | SD |
|---|
| Alanine | 42.32 | 40.60 | 38.16 | 41.03 | 40.53 | 7.56 | | Arginine | 39.49 | 39.85 | 32.69 | 36.38 | 37.11 | 9.88 | | Aspartic Acid | 85.31 | 86.88 | 75.54 | 87.40 | 83.78 | 21.11 | | Citrulline | 6.01 | 6.51 | 5.72 | 5.34 | 5.89 | 1.61 | | Glutamic acid | 154.29 | 145.53 | 139.73 | 175.02 | 153.64 | 29.54 | | Glycine | 23.18 | 25.32 | 24.89 | 26.57 | 24.99 | 3.03 | | Hystidine | 24.10 | 24.33 | 27.31 | 25.56 | 25.32 | 3.54 | | Leucine | 71.54 | 68.77 | 59.92 | 80.10 | 70.08 | 13.21 | | Methionine | 20.62 | 14.11 | 11.91 | 12.72 | 14.84 | 2.79 | | Ornitine | 9.31 | 8.36 | 9.63 | 7.79 | 8.77 | 3.02 | | Phenylalanine | 34.40 | 32.07 | 32.90 | 35.32 | 33.67 | 7.09 | | Proline | 72.54 | 73.52 | 71.83 | 95.78 | 78.42 | 18.39 | | Serine | 46.02 | 46.81 | 39.31 | 47.81 | 44.99 | 9.83 | | Tryptophan | 15.37 | 15.33 | 15.35 | 15.45 | 15.37 | 0.05 | | Tyrosine | 28.59 | 29.55 | 30.32 | 36.47 | 31.23 | 6.77 | | Valine | 58.37 | 52.13 | 49.54 | 62.20 | 55.56 | 11.41 | | Taurine | 63.99 | 64.10 | 63.71 | 64.68 | 64.12 | 2.12 |
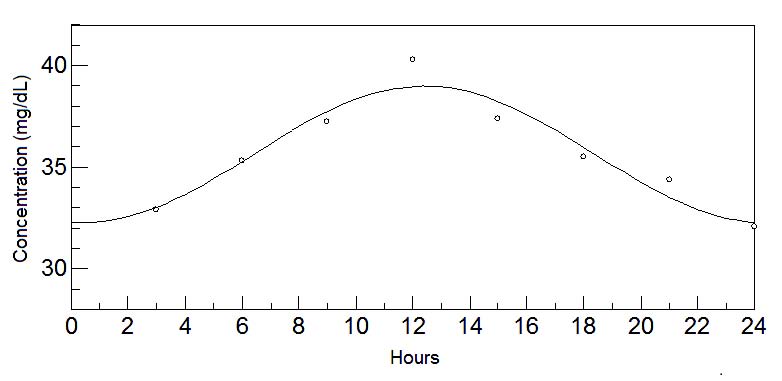
Fig. 8. 24-hours oscillations of phenylalanine in mature milk. This graph depicts the fluctuations of phenylalanine levels in mature milk
during 24-hours. Circadian rhythm is shown by a sinusoidal curve.
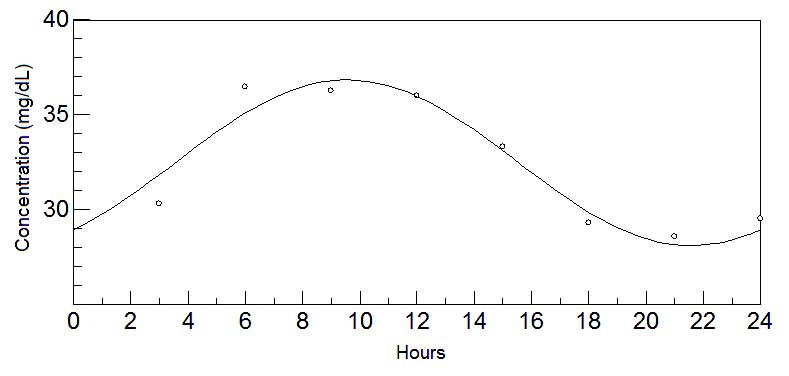
Fig. 9. 24-hours oscillations of tyrosine in mature milk. This graph depicts the fluctuations of tyrosine levels in mature milk during
24-hours. Circadian rhythm is shown by a sinusoidal curve.
Table 4 lists the chronobiological parameters MESOR, amplitude and acrophase of the amino acids which present circadian rhythms in the different
stages of lactation.
| Amino acids | COLOSTRAL MILK | TRANSITIONAL MILK | MATURE MILK |
|---|
| MESOR | Amplitude | Acrophase | MESOR | Amplitude | Acrophase | MESOR | Amplitude | Acrophase<
/th> |
|---|
| Tryptophan | 34.69±0.05 | 0.12±0.09 | 4:19±3:21* | 26.53±0.06 | 0.18±0.06 | 3.16±2:38* | 15.26±0.06<
/td> | 0.13±0.12 | 3:00±4:24* | | Methionine | – | – | – | 39.75±2.81 | 6.36±5.26 | 17:57±3:43* | 15.25±1.79 | 3.68±3.35 | 17:19±4:22* | | Aspartic acid | – | – | – | – | – | – | 89.62±5.80 | 11.27±10.84 | 12:08±4:34* | | Histidine | – | – | – | – | – | – | 23.38±1.04 | 2.89±1.94 | 3:14±2:48* | | Phenylalanine | – | – | – | – | – | – | 35.25±5.85 | 3.43±1.59 | 12:33±1:53* | | Tyrosine | – | – | – | – | – | – | 32.12±0.87 | 4.54±1.62 | 9:38±1:23* |
DISCUSSION
The results of the present study consistently characterize a circadian rhythm in some amino acids in breast milk throughout the evolution of the
breastfeeding period. These results are consistent with those reported previously by other authors (Yamawaki et al. 2005, Piccione et al. 2008).
Previously, we found that breast milk from donors in Spain showed strong daily variations in the protein content, suggesting the presence of a
regulating mechanism controlled by circadian rhythms in breast milk (Sánchez et al. 2011).
Amino acids are nitrogenous components and one of their main functions is protein anabolism, which is accelerated in newborns whose structural demand
is very high. All components analysed in the present work, except for aspartic acid and glycine, are essential for neonates and crucial for their
growth and development (Ballabriga and Carrascosa 2001).
The novel contribution of the present work is the idea that amino acids in breast milk change during the course of the day, in particular asking
whether the acrophase occurs during the stage of sleep or of wakefulness, in consonance with the neural demand of the newborns. The appropriate timing
of the acrophase is a minor consequence; to be quantified with precision, it would be necessary to study a large population affected by a less
temporal heterogeneity, i.e., collecting all the breast milk samples at the same time of day and season of the year, which was not feasible for
us.
During the colostrum period, breast milk is almost lacking circadian rhythm, reflecting the still premature secretion of the milk, and because the
infant’s sleep/wakefulness function is not fully established. Furthermore, the intervals between each feeding are very short and constant. We only
observed the beginning of a circadian rhythm for the essential amino acid tryptophan, which was confirmed throughout the subsequent stages.
During the transitional stage, in addition to tryptophan, methionine also showed a circadian rhythm (Sanchez et al. 2010). Its acrophase pattern seems
to be opposite to the tryptophan pattern, i.e., during the day, in the stage of wakefulness and with a greater likelihood of infant activity.
Finally, it is during the mature stage where the circadian rhythms of the activity-promoting neuroactive amino acids unfold: phenylalanine, an
essential amino acid; tyrosine, precursor of norepinephrine and epinephrine (Hunsley and Palmiter 2004, Mitchell and Weinshenker 2010); methionine, an
essential amino acid and precursor of acetylcholine (Sugimoto et al. 1964, Chabannes et al. 1984, Sanchez et al. 2010); and aspartic acid and glycine,
activity neurotransmitters exhibited an acrophase during wakefulness (Shubat et al. 1989).
Histidine results were unexpected because this amino acid is the histamine precursor - an excitatory neurotransmitter (Monti 1993) – and its acrophase
is unforeseen given that it occurs during the period of darkness, i.e., when there is a greater tendency to sleep. A possible reason for this is that
this component is a semi-essential amino acid at this stage of life and its endogenous levels may not be influenced by diet (Ballabriga and Carrascosa
2001).
Although the variations in our study were small, they can be significant in terms of neural response. Tryptophan showed a circadian rhythm in all
three stages of lactation (colostrum, transitional, and mature milk) and strong evidence has been noted on the action of this essential amino acid on
the neurotransmitter serotonin and the hormone melatonin, both of which are well-known sleep-inducing biogenic amines (Hajak et al. 1991, Heuther et
al. 1992).
We can summarize that breast milk is a dynamic biological fluid in that its composition is not constant, but evolves throughout the day and over the
course of lactation, just as diet evolves during the day and throughout the course of our lives (Ballabriga and Carrascosa 2001).
It could be argued again in defence of breastfeeding compared to artificial feeding because formula milks have a further handicap that has to be
considered as it does not hold the synchronization of the neuromodulatory amino acids that breast milk does. This temporal rhythm exhibited in the
components of breast milk may give to the infant a physiological advantage in development, including the sleep function, compared with artificial
feeding, given that the amino acid content is constant throughout the day.
This daily timekeeping could be driven by transcriptional/translational feedback loops, whereby rhythmic expression of “CLOCK” gene products regulates
the expression of associated genes in approximately 24-hour cycles (O’Neill et al. 2011). Recently, a metabolomics study has shown that synthesis and
degradation of nucleotides (other nitrogenous components which circadian rhythm has been already demonstrated in breast milk - Sanchez et al. 2008) in
the liver are under transcriptional circadian control (Fustin et al. 2012).
Even though some information exists in the field of chronobiology in respect to breast milk, and the chrononutrition “boom” during recent years has
increased the awareness of the importance of breastfeeding to newborn health, further research into the formation, composition, and biological effects
of human breast milk should be encouraged.
Further studies are also needed to determine the mechanisms of action of the processes responsible for these daily variations in breast milk, even
given that the alignment of internal rhythms with the outside world is well-known to be affected by the time of feeding as well as by the light/dark
cycle. Perhaps a correlation between the circadian pattern of the breast milk components and the sleeping efficiency of the mothers should be
conducted in a future to obtain a better knowledge of these biological processes.
ACKNOWLEDGEMENTS
We are grateful to the women who volunteered to patiently extract their breast milk during the 24-h period. This research was financial supported by
Ordesa Group and the University of Extremadura for the grant “II Plan de Iniciación a la Investigación, Desarrollo Tecnológico e Innovación”, awarded
to Cristina L. Sánchez López. We are also grateful to Ms. Elena Circujano for her technical help and all the medical staff of the hospital for their
kind and continuous assistance during the sampling period.
REFERENCES
Ballabriga A, Carrascosa A. Nutrition in childhood and adolescence (in Spanish), Ergon S. A., Madrid 2001.
[PubMed]
Berger J. The age of biomedicine: current trends in traditional subjects. J Appl Biomed. 9: 57-61, 2011.
[CrossRef]
[JAB]
Catarru B, Boniglia C, Scalise F, Ambruzzi AM, Sanzini E. Nitrogenous components of human milk: non-protein nitrogen, true protein and free amino
acids. Food Chem. 81: 357-362, 2003.
[CrossRef]
Chabannes B, Sarda N, Cronenberger L, Pacheco H. Diurnal variations of S-adenosyl-L-methionine and adenosine content in the rat pineal gland. Life
Sci. 35: 589-596, 1984.
[CrossRef]
Clark RM, Ross SA, Hill DW, Ferris AM. Within-day variation of taurine and other nitrogen substances in human milk. J Dairy Sci. 70: 776-780,
1987.
[CrossRef]
Cubero J, Valero V, Sanchez J, Rivero M, Parvez H, Rodriguez AB, Barriga C. The circadian rhythm of tryptophan in breast milk affects the rhythms of
6-sulfatoxymelatonin and sleep in newborn. Neuro Endocrinol Lett. 26: 657-661, 2005.
[PubMed]
Fustin JM, Doi M, Yamada H, Komatsu R, Shimba S, Okamura H. Rhythmic nucleotide synthesis in the liver: temporal segregation of metabolites. Cell
Reports. 1: 341-349, 2012.
[CrossRef]
[PubMed]
Hajak G, Huether G, Blanke J, Blomer M, Freyer C, Poeggeler B, Reimer A, Rodenbeck A, Schulz-Varszegi M, Ruther E. The influence of intravenous
L-tryptophan on plasma melatonin and sleep in men. Pharmacopsychiatry. 24: 17-20, 1991.
[CrossRef]
[PubMed]
Halberg S, Tong YL, Johnston EA. Cellular aspects from biorhythms. Springer, New York 1967.
Hall B. Uniformity of human milk. Am J Clin Nutr. 32: 304-312, 1979.
[PubMed]
Hernell O, Lodernall B. Nutritional evaluation of protein hydrolysate formulas in healthy term infants: plasma amino acids, hematology, and trace
elements. Am J Clin Nutr. 97: 224-233, 2003.
Heuther G, Hajak G, Reimer A, Poeggeler B, Blomer M, Rodenbeck A, Ruther E. The metabolic fate of infused L-tryptophan in men: possible clinical
implications of the accumulation of circulating tryptophan and tryptophan metabolites. Psychopharmacology. 109: 422-432, 1992.
[CrossRef]
Hunsley M, Palmiter R. Altered sleep latency and arousal regulation in mice lacking norepinephrine. Pharmacol Biochem Behav. 78: 765-773, 2004.
[CrossRef]
[PubMed]
Karra MV, Kirksey A. Variation in zinc, calcium, and magnesium concentrations of human milk within a 24-hour period from 1 to 6 months of lactation. J
Pediatr Gastroenterol Nutr. 7: 100-106, 1988.
[CrossRef]
[PubMed]
Krebs NF, Hambidge KM, Jacobs MA, Mylet S. Zinc in human milk: diurnal and within-feed patterns. J Pediatr Gastroenterol Nutr. 4: 227-229, 1985.
[CrossRef]
[PubMed]
Lammi-Keefe CJ, Ferris AM, Jensen RG. Changes in human milk at 0600, 1000, 1400, 1800, and 2200 h. J Pediatr Gastroenterol Nutr. 11: 83-88, 1990.
[CrossRef]
[PubMed]
Lubetzky R, Littner Y, Mimouni FB, Dollberg S, Mandel D. Circadian variations in fat content of expressed breast milk from mothers of preterm infants.
J Am Coll Nutr. 25: 151-154, 2006.
[PubMed]
Mitchell HA, Weinshenker D. Good night and good luck: Norepinephrine in sleep pharmacology. Biochem Pharmacol. 79: 801-809, 2010.
[CrossRef]
[PubMed]
Monti J. Involvement of histamine in the control of the waking state. Life Sci. 53: 1331-1338, 1993.
[CrossRef]
Murillo-Rodriguez E, Arias-Carrion O, Sanguino-Rodriguez K, Gonzalez-Arias M, Haro R. Mechanism of Sleep-Wake Cycle Modulation. CNS Neurol Disord Drug
Targets. 8: 245-253, 2009.
[CrossRef]
[PubMed]
O’Neill JS, van Ooijen G, Dixon LE, Troein C, Corellou F, Bouget FY, Reddy AB, Millar AJ. Circadian rhythms persist without transcription in a
eukaryote. Nature. 489: 554-558, 2011.
[CrossRef]
[PubMed]
Picciano MF, Guthrie HA. Copper, iron, and zinc contents of mature human milk. Am J Clin Nutr. 29: 242-254, 1976.
[PubMed]
Piccione G, Fazio F, Caola G, Refinetti R. Daily rhythmicity in nutrient content of asinine milk. Livestock Science. 116: 323-327, 2008.
[CrossRef]
Reis HJ, Guatimosim C, Paquet M, Santos M, Ribeiro FM, Kummer A, Schenatto G, Salgado JV, Vieira LB, Teixeira AL, Palotas A. Neuro-transmitters in the
central nervous system & their implication in learning and memory processes. Curr Med Chem. 16: 796-840, 2009.
[CrossRef]
[PubMed]
Sanchez CL, Rodriguez AB, Sanchez J, Gonzalez R, Rivero M, Barriga C, Cubero J. Calcium intake nutritional status in breastfeeding women (in Spanish).
Arch Latinoam Nutr. 58: 371-376, 2008.
[PubMed]
Sanchez CL, Cubero J, Sanchez J, Chanclon B, Rivero M, Rodriguez AB, Barriga C. The possible role of human milk nucleotides as sleep inducers. Nutr
Neurosci. 12: 2-8, 2009.
[CrossRef]
[PubMed]
Sanchez CL, Barriga C, Rodriguez AB, Franco L, Rivero M, Cubero J. Effects of oral administration of L-methionine on activity/rest rhythm. Acta
Physiol Hung. 97: 224-233, 2010.
[CrossRef]
[PubMed]
Sanchez CL, Hernandez A, Rodriguez AB, Rivero M, Barriga C, Cubero J. Nitrogen and protein content analysis of human milk, diurnality vs. nocturnality
(in Spanish). Nutr Hosp. 26: 511-514, 2011.
[CrossRef]
[PubMed]
Sanchez CL, Cubero J, Sanchez J, Franco L, Rodriguez AB, Rivero M, Barriga C. Screening for human milk amino acids by HPLC-ESI-MS/MS. Food Anal
Methods. 5: 312-318, 2012.
[CrossRef]
Shubat PJ, Parker D, Huxtable RJ. The effect of suckling and diurnal influences on the concentrations of free amino acids in milk. Proc West Pharmacol
Soc. 32: 73-78, 1989.
[PubMed]
Sugimoto J, Tamino K, Kadokawa C, Iida N, Morita M. L-methionine as a modulator of atrial rhythmicity in rabbit and guinea-pig. Jpn J Pharmacol. 14:
150-160, 1964.
[CrossRef]
[PubMed]
Thomas MR, Chan GM, Books LS. Comparison of macronutrient concentration of preterm human milk between two milk expression techniques and two
techniques for quantification of energy. J Pediatr Gastroenterol Nutr. 5: 597-601, 1986.
[CrossRef]
[PubMed]
Viverge D, Grimmonprez L, Cassanas G, Bardet L, Solere M. Diurnal variations and within the feed in lactose and oligosaccharides of human milk. Ann
Nutr Metab. 30: 196-209, 1986.
[CrossRef]
[PubMed]
World Health Organization. Energy and Protein Requirements. Report of a Joint FAO/WHO/ONU Expert Consultation. Technical Report Series no. 724. Geneva
1985, pp 71–80.
Yamawaki N, Yamada M, Kan-no T, Kojima T. Macronutrient, mineral and trace element composition of breast milk from Japanese women. J Trace Elem Med
Biol. 19: 171-181, 2005.
[CrossRef]
[PubMed]
|
BACK
|










