Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 11 (2013), No 3, p 173-185
DOI 10.2478/v10136-012-0023-x
Application of EPR spectroscopy to examination of free radicals in melanins from A-375 and G-361 human melanoma malignum cells
Ewa Chodurek, Magdalena Zdybel, Barbara Pilawa
Address: Ewa Chodurek, Department of Biopharmacy, School of Pharmacy and Laboratory Medicine, Medical University of Silesia in Katowice, Narcyzow 1, 41-200 Sosnowiec, Poland
echodurek@sum.edu.pl
Received 10th July 2012.
Revised 6th November 2012.
Published online 8th November 2012.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
Conclusions
Acknowledgements
References
SUMMARY
Melanins are polymorphous and multifunctional biopolymers with a relatively high concentration of free radicals. EPR spectroscopy was used to study o-semiquinone free radicals in model eumelanins synthesized from 3,4-dihydroxyphenylalanine (DOPA) and tyrosine in the presence of tyrosinase, and melanins isolated from A-375 and G-361 human melanoma malignum cells exposed to two compounds: 5,7-dimethoxycoumarin (DMC) and valproic acid (VPA). Changes were determined in the concentrations of free radicals in the individual melanins from tumour cells treated with DMC and VPA. A strong decrease in the concentrations of free radicals characterizes melanins isolated from tumour cells treated together with DMC and VPA. Slow spin-lattice relaxation processes were noted in the melanins tested with homogeneous broadened EPR spectra. The EPR technique may be useful not only for the elucidation of free radicals in melanins from A-375 and G-361 cells treated with VPA and DMC but it could also be applied to establish the relationship between melanin type and the malignancy of melanoma malignum.
KEY WORDS
free radicals; melanin; human melanoma malignum cells; 5,7-dimethoxycoumarin; valproic acid; EPR spectroscopy
INTRODUCTION
Malignant melanoma (melanoma malignum) is a skin neoplasm the incidence of which continues to rise. The extremely high malignancy of the tumour results from its rapid proliferation, early and numerous metastases and high resistance to therapy (Ibrahim and Brown 2008).
In Poland, melanoma is a relatively rare cancer. In 2006, among all cancers, it took 14th place in terms of morbidity, and 16th and 18th place as a cause of death among men and women, respectively (Wojciechowska et al. 2008). However, between 1963 and 2006, the melanoma incidence in Poland increased almost nine times among men (from 114 to 998) and six times among women (from 189 to 1103). In the same period, the mortality caused by this cancer increased from 114 to 569 in the case of men and from 58 to 485 in the case of women (Wojciechowska et al. 2008, 2010).
Melanoma malignum arises from the malignant transformation of melanocytes - the melanin producing cells - and can develop de novo or from pre-existing nevi. Over 90% of all melanoma cases occur in the skin. The most common site of the cancer in men is the trunk, especially the upper back, whereas in women, it is the lower legs (Edman and Wolfe 2000, Goldstein and Goldstein 2001, Balamurugan et al. 2011).
Melanocytes of mammals produce two types of melanin biopolymers: brown-black eumelanin and yellow-brown pheomelanin. One of the major functions of eumelanin is natural photoprotection against the destructive action of UV radiation (Ito and Wakamatsu 2003, 2008). It is supposed that epidermal eumelanin is responsible for the lower risk of UV-induced skin cancers in persons with dark skin and/or hair. In contrast, pheomelanin acts as a photosensitizer, and this activity is probably responsible for the high incidence of UV-induced skin cancers in persons with fair complexion and red or blond hair (Bennett 2008, Brenner and Hearing 2008, Tran et al. 2008).
Because of the enhanced melanogenesis in melanoma cells, melanin formation could be used as targeted therapy for the cancer (Farmer et al. 2003, Gidanian et al. 2008). For instance, co-administration of 5,7-dimethoxycoumarin (DMC) which is capable of increasing melanin synthesis (Alesiani et al. 2008, 2009) and valproic acid (VPA), one of the inhibitors of histone deacetylases, has been proposed as a new strategy for melanoma treatment (Duenas-Gonzalez et al. 2008, Chateauvieux et al. 2010, Federico and Bagella 2011).
Synthetic and natural melanins characterize paramagnetism as the result of o-semiquinone free radicals with a spin of 1/2 existence in these polymers (Pasenkiewicz-Gierula and Sealy 1986, Shima et al. 1997, Matuszczyk et al. 2004). Additionally biradicals with a spin of 1 were found in melanin samples (Pilawa et al. 2004, Kozdrowska 2006, Zdybel 2008, Najder-Kozdrowska et al. 2010). Bi-radicals exist in DOPA-melanin and its complexes with kanamycin (Kozdrowska 2006, Najder-Kozdrowska et al. 2010) and netilmicin (Zdybel 2008). Diamagnetic metal ions increase the concentrations of free radicals in melanins and a decrease is observed in the concentration of free radicals in melanin effected by paramagnetic metal ions (Matuszczyk et al. 2004, Buszman et al. 2005a, Kozdrowska 2006, Zdybel 2008, Najder-Kozdrowska et al. 2009, Zdybel et al. 2011). Reactions between the free radicals in melanin and drugs are known (Buszman et al. 2005a, Kozdrowska 2006, Zdybel 2008). Free radicals in melanin polymers and their complexes with metal ions and drugs have been examined using electron paramagnetic resonance (EPR) spectroscopy (Buszman et al. 2005a, Kozdrowska 2006, Zdybel 2008), and the EPR spectra of tumour cells containing melanin biopolymers have been analysed (Latocha et al. 2004a, b, 2005, 2006). The EPR method is useful in determining the type and concentration of paramagnetic centres in samples (Wertz and Bolton 1986, Eaton et al. 1998, Stankowski and Hilczer 2005). The distribution of free radicals in the samples and spin-lattice relaxation processes may be tested by observation of continuous microwave saturation of the EPR spectra (Wertz and Bolton 1986, Eaton et al. 1998, Stankowski and Hilczer 2005).
The aim of this study was to investigate, using the EPR technique, the free radical properties of melanins isolated from human melanoma cells (A375 and G-361 cell lines) exposed to valproic acid and 5,7-dimethoxycoumarin.
This work develops knowledge about the role of free radicals in interactions of melanin biopolymers in tumour cells with the new anticancer compounds. The modification of the amount and properties of free radicals in melanin from melanoma malignum cells by the two compounds was spectroscopically tested. The EPR spectra were continuously saturated by microwaves and their saturation was observed. The microwave saturation of the EPR lines depends on the rates of spin-lattice relaxation processes in the sample (Wertz and Bolton 1986, Stankowski and Hilczer 2005). EPR spectroscopy was applied to the examination of free radicals in whole tumour cells containing melanin (Latocha et al. 2004a, b, 2005, 2006). The influence of laser irradiation on free radicals in tumour cells has been tested by EPR (Latocha et al. 2005, 2006). The role of free radicals and singlet oxygen molecules in the photodynamic therapy of tumour cells was studied by EPR (Latocha et al. 2005, 2006). The melanins isolated from A-375 and G-361 melanoma cells interacting with 5,7-dimethoxycoumarin and valproic acid had not been previously been examined by EPR.
MATERIAL AND METHODS
Tumour cells
Human malignant melanoma cell lines A-375 and G-361 were purchased from LGC Promochem (Lomianki, Poland). The malignant G-361 cell line obtained was grown in McCoy's medium (Sigma-Aldrich) and human cancer cells A-375 were grown in the Minimum Essential Medium Eagle (MEM, Sigma-Aldrich). These media were supplemented by 10% fetal bovine serum (FBS, PAA), 100 U/ml penicillin, 100 microg/ml streptomycin (Sigma-Aldrich) and 10 mM HEPES (Sigma-Aldrich). The cultures were cultivated in the standard conditions: temp. 37 °C, and an atmosphere containing 95% air and 5% CO2. Cells were incubated with test compounds (1 mM VPA, 10 microM DMC or their combination) for 7 days.
Isolation of melanin from the human melanoma cells exposed to VPA and DMC
1 g of melanoma cells (A-375 and G-361 cell line) was mixed with 5 ml of 1% Triton X-100 (Sigma) and incubated for 1 h at room temperature (Chodurek et al. 2008, 2012). Next the sample was centrifuged (16000 x g, 15 min), the cell pellet was washed with phosphate buffer and once again centrifuged. The pellet was mixed with 5 ml of (5 mg/ml) sodium dodecyl sulfate (SDS) in Tris-HCl buffer (50 mM, pH = 7.4) with proteinase K (Sigma) to the final solution of 0.33 mg/ml. The mixture was incubated for 3 h in 37 °C. After centrifugation (16000 x g, 15 min) the melanin pigment was successively washed with 0.9% NaCl, methanol and hexane and centrifuged (16000 x g, 15 min). The melanin was dried at 37 °C and stored in a glass desiccator over PO2O5.
Preparation of synthetic melanins
Synthetic eumelanins were prepared by tyrosinasecatalyzed oxidation of 3,4-dihydroxyphenylalanine (DOPA-melanin) and tyrosine (Tyr-melanin; tyrosine-melanin). Melanin precursors were dissolved in 50 mM sodium phosphate buffer (pH 6.8) to obtain the final concentration of 2 mM, then tyrosinase 100 U/ml (Sigma, 5370 U/mg) was added and the reaction mixtures were incubated for 48 h at 37 °C with vigorous stirring and protection from light. The DOPA-melanin and Tyr-melanin pigment obtained were collected by centrifugation (5000 x g, 15 min) and washed several times with deionized water. To remove possible traces of tyrosinase, eumelanin standards were treated with SDS and methanol, NaCl, then rewashed with deionized water and dried to a constant weight at 37 °C.
EPR measurements
Free radicals in the melanin samples were examined by the use of an X-band (9.3 GHz) electron paramagnetic resonance (EPR) spectroscopy. The EPR measurements for melanin samples located in thin walled glass tubes with the external diameter of 3 mm were carried out at room temperature. The EPR spectra as the first derivative of absorption curves were recorded by a Radiopan (Poznan, Poland) spectrometer with a magnetic modulation of 100 kHz. The total microwave power (Mo) produced by klystron in the microwave bridge of this spectrometer was 70 mW. The numerical acquisition of the EPR spectra was carried out by the Rapid Scan Unit from Jagmar (Krakow, Poland). Spectroscopic programs SWAMP by Jagmar (Krakow, Poland) and LabVIEW 8.5 by National Instruments (Austin, Texas) were used to measure and to analysis of the EPR spectra.
g-Factors, amplitudes (A), integral intensities (I) and linewidths (deltaBpp) of the EPR spectra of the examined samples were obtained. g-Values were calculated from the resonance condition according to the formula (Wertz and Bolton 1986, Stankowski and Hilczer 2005):
g = hnu / microBBr
where: h - Planck constant, nu - microwave frequency, microB - Bohr magneton, Br - induction of resonance magnetic field. Microwave frequency (nu) was directly measured by a MCM101 recorder obtained from EPRAD (Poznan, Poland). The values of the resonance magnetic field (Br) was determined by the EPR spectra (Fig. 1). The apparent scalar g-factors were measured. Two different types of paramagnetic centers exist in melanins (Pilawa et al. 2004, Kozdrowska 2006, Zdybel 2008, Najder-Kozdrowska et al. 2010). The EPR measuring temperature from liquid nitrogen to room temperature performed on melanin polymers and their complexes with drugs and metal ions proved that besides o-semiquinone free radicals with spin of 1/2, biradicals with a spin of 1 exist in melanin samples. Different correlations between integral intensities and the measuring temperature were fitted for the experimental data for o-semiquinone free radicals and biradicals. The existence of two types of paramagnetic centers in melanins indicate the existence of two component lines in the resultant EPR spectra. Probably this is the main reason for their asymmetry. Deconvolution of the EPR spectra of the examined melanin samples was not carried out and the individual components were not found, because of the high level of noise in the curves.
Amplitudes (A) and linewidths (deltaBpp) were obtained from the EPR lines as is shown in Fig. 1. Integral intensities (I) as the areas under the absorption curves were calculated by double integration of the first-derivative EPR spectra.
Free radical concentrations (N) in the samples were determined. The concentration is proportional to the integral intensity (I) of the EPR line (Wertz and Bolton 1986, Eaton et al. 1998, Stankowski and Hilczer 2005). The integral intensities (I) of the EPR spectra of the tested samples were compared to the EPR spectrum of the reference - ultramarine (Iu). The second reference permanently placed in a resonance cavity - a ruby crystal (Al2O3: Cr3) was used. For each sample and for ultramarine the EPR line of a ruby crystal was detected. Amplitudes of the EPR lines of the ruby crystal located with the sample (A) and ultramarine (Au) in the resonance cavity were determined. The concentration of the free radicals (N) in the melanin samples was calculated as follow:
N = nu[(WuAu)/Iu][I/(WAm)],
where: nu - the number of paramagnetic centres in the ultramarine reference; W, Wu - the receiver gains for sample and the ultramarine; A, Au - the amplitudes of ruby signal for the sample and the ultramarine; I, Iu - the integral intensities for the sample and ultramarine, m - the mass of the sample.
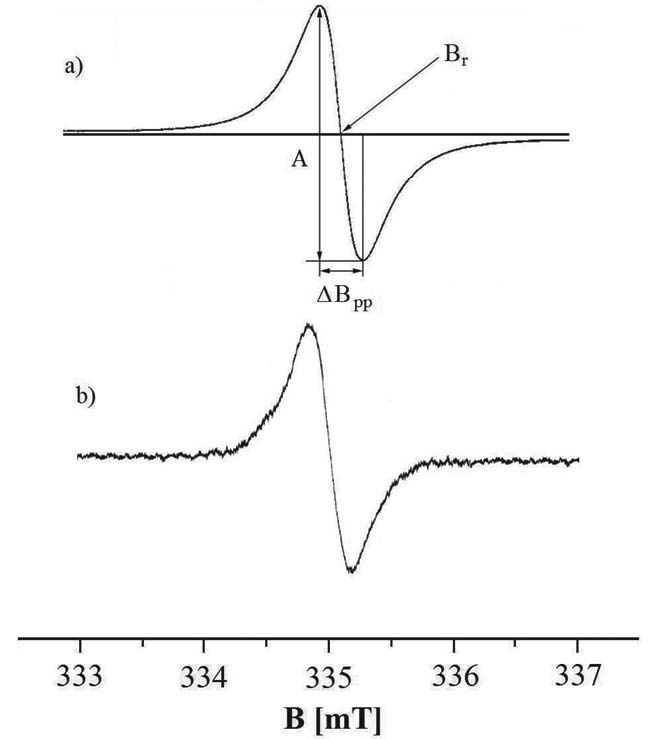
Fig. 1. Amplitude (A) and linewidth (deltaBpp) of the firstderivative EPR spectrum of DOPA-melanin (a) and tyrosine-melanin (b), and the induction of resonance magnetic field (Br). The EPR spectra were measured with microwave power of 2.2 mW.
The effect of microwave power on the EPR spectra of the melanin samples was examined. The influence of microwave power (M) in the range of 2.2-70 mW on amplitudes (A) and linewidths (deltaBpp) of EPR spectra was determined. The above mentioned correlations between amplitudes (A), linewidths (deltaBpp) and microwave power (M) give information about free radicals distribution (homogeneous or inhomogeneous) in the samples. For homogeneous distribution of free radicals in the samples, the amplitude (A) increases with increasing microwave power (M) and for the higher microwave powers its value decreases (Wertz and Bolton 1986). The linewidth (deltaBpp) of the homogeneously broadened EPR lines increases with rises in microwave power (M). For the inhomogeneous distribution of free radicals in the samples the amplitude (A) increases with increasing microwave power (M) and for the higher microwave powers its value does not change. Linewidth (deltaBpp) of the inhomogeneously broadened EPR lines is unchanged with increasing microwave power (M) (Wertz and Bolton 1986). The changes of amplitudes with increasing microwave power characterize the spin-lattice relaxation processes in the samples. The power of microwave saturation of EPR lines increases with fastening of spin-lattice relaxation processes (Wertz and Bolton 1986, Stankowski and Hilczer 2005).
RESULTS
Evaluation of the proliferation of A-375 and G-361 cell lines exposed to various concentrations of valproic acid (0.3-10 mM) and 5,7-dimethoxycoumarin (10-500 microM) was performed previously by Chodurek et al. (data not presented, paper under review) using a colorimetric test (In Vitro Toxicology Assay Kit, Sulforhodamine B Based, Sigma). On the basis of this evaluation, the concentration of 1 mM of VPA, 10 microM of DMC and their combination were chosen for further investigation.
Morphological changes in melanoma cells A-375 and G-361 after treatment with VPA and DMC are shown in Fig. 2. Human melanoma cells A-375 and G-361 (Fig. 2a) are adherent cells, that flatten and grow as a monolayer. Insignificant inhibition of growth rate was observed both in A-375 and G-361 cell culture after their treatment with a lower concentration of the compounds investigated (Fig. 2b, d). Whereas, a higher concentration of VPA and DMC (Fig. 2c, e) were evidently cytotoxic for cells and resulted in a tearing off of the cells and an increased number of cells floating in the culture medium.
Free radicals exist in all the tested samples. EPR spectra were obtained for the model synthetic melanins and for all the studied melanins isolated from tumour cells. EPR spectra of the model eumelanins - DOPA-melanin and tyrosine-melanin are shown in Fig. 1. EPR spectra of melanin isolated from A-375 cells and G-361 cells are presented in Fig. 3 and 4, respectively. All the measured EPR spectra were single asymmetric lines. Unfortunately a high level of noise characterized the experimental EPR spectra. Analyses of the lineshape by mathematical functions were not performed, because a high error rate was expected.
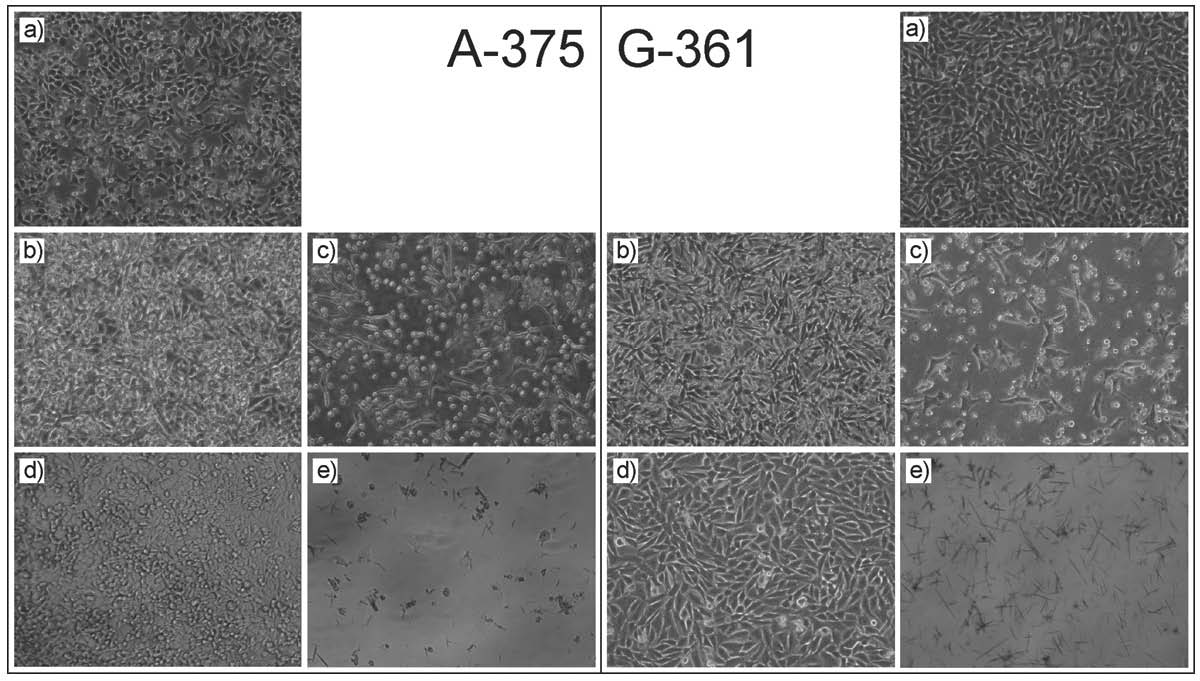
Fig. 2. A-375 and G-361 cell lines exposed to: control (a), 1 mM VPA (b), 10 mM VPA (c), 10 microM DMC (d), 500 microM DMC (e). Magnification 100x.
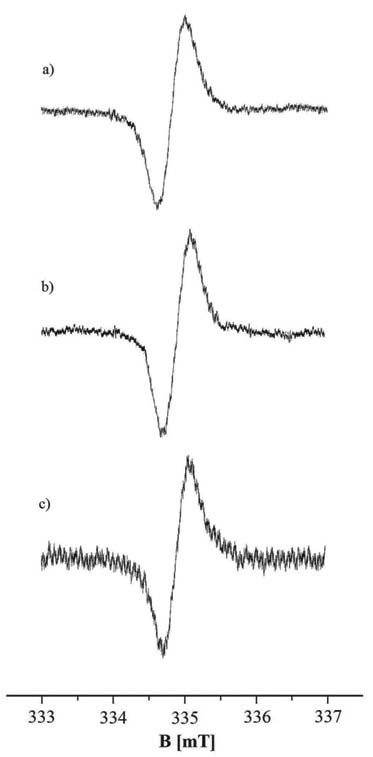
Fig. 3. EPR spectra of melanin isolated from A-375 cells treated with DMC (a), VPA (b) and both VPA and DMC (c). The EPR spectra were measured with microwave power of 2.2 mW.
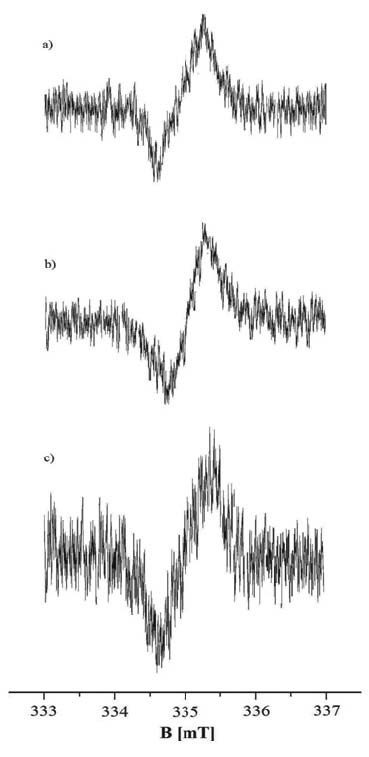
Fig. 4. EPR spectra of melanin isolated from G-361 cells treated with DMC (a), VPA (b) and both VPA and DMC (c). The EPR spectra were measured with microwave power of 2.2 mW.
g-Values, linewidths (deltaBpp) and free radical concentrations (N) in the tested synthetic and natural melanin samples are compared in Table 1. g-Values for free radicals in DOPA-melanin, tyrosine-derived melanin and melanins isolated from A-375 cells and G-361 cells treated with DMC, VPA, and both VPA and DMC, are in the range of 2.0037-2.0060 (Table 1). These g-values (Table 1) are those for o-semiquinone free radicals (Wertz and Bolton 1986).
Free radical concentrations (N) in the tested melanins are in the range of ~1019-1021 spin/gram (Table 1). The highest concentration (N) of free radicals was obtained for the synthetic DOPA-melanin (58.2 x 1020 spin/g) and natural melanin isolated from A-375 cells treated with VPA (51.4 x 1020 spin/g) (Table 1). The relatively lower concentration (N) of free radicals compared to DOPA-melanin was calculated for melanin synthesized from tyrosine (10.8 x 1020 spin/g) (Table 1). The lowest free radical concentrations (N) were obtained for melanins isolated from the two studied types (A-375 and G-361) treated with both VPA and DMC (0.6 x 1020 spin/g and 0.9 x 1020 spin/g) (Table 1).
Melanins isolated from A-375 and G-361 tumour cells treated with both DMC and VPA, relative to DOPA-melanin are characterized by a strong decrease in free radical concentration (N) (Table 1). A higher free radical concentration exists in melanin from A-375 cells treated with VPA (51.4 x 1020 spin/g) than in melanin from cells treated with DMC (24.0 x 1020 spin/g) (Table 1). A higher free radical concentration was obtained for melanin isolated from G-361 cells treated with DMC (5.4 x 1020 spin/g) than for melanin treated with VPA (2.0 x 1020 spin/g) (Table 1).
All the measured EPR spectra have high values of linewidths (deltaBpp) in the range of 0.47-0.89 mT (Table 1). The broadest (deltaBpp: 0.89 mT) EPR line was recorded for melanin isolated from G-361 cells treated with DMC (Table 1). The range of the other tested linewidths is from 0.47 mT to 0.56 mT (Table 1). The broad EPR lines indicate that strong dipolar interactions occur in the studied melanins.
Table 1. Free radicals concentrations (N), linewidths (deltaBpp) and g-factors of EPR spectra of the studied melanins.
The EPR spectra were measured with microwave power of 2.2 mW.
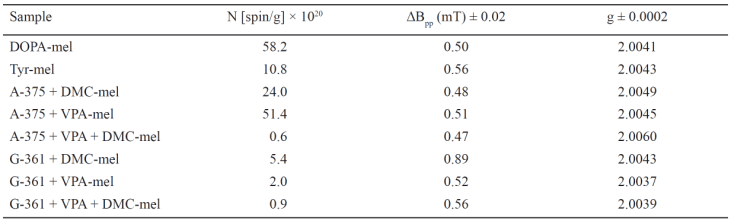
The influence of microwave power on the amplitudes (A) and linewidths (deltaBpp) of the EPR spectra of the tested synthetic melanins is shown in Fig. 5. The amplitude (A) of the EPR spectra of DOPA-melanin increases with increases in microwave power and after reaching maximum its value decreases (Fig. 5a). The amplitude (A) of the EPR spectra of melanin obtained from tyrosine increases with increases in microwave power, and reaches its maximum, but a decrease was not observed (Fig. 5a). The reaching of the maximum by the amplitudes (A) in the used microwave power range up to 70 mW indicates the slow spin-lattice relaxation processes. Faster spin-lattice relaxation processes exist in melanin synthesized from tyrosine (Fig. 5a). Increases of linewidth (deltaBpp) of the EPR spectra with increasing microwave power were observed (Fig. 5b). The changes of amplitudes (A) and linewidths (deltaBpp) of the EPR spectra of both synthetic melanins with microwave power (Fig. 5) are characteristic of homogeneously broadened lines.
The changes of amplitudes (A) and linewidths (deltaBpp) with microwave power for the EPR spectra of the examined human melanoma malignum A-375 cells treated with DMC and VPA are compared in Fig. 6. Amplitudes (A) of the EPR spectra of all the melanin samples from A-375 cells increase with microwave power, reach the maximum and decrease for the higher values of microwave power (Fig. 6a). As with synthetic melanin (Fig. 5a) slow spin lattice relaxation processes exist in melanin from A-375 cells, their EPR lines saturate in the studied range of microwave power (Fig. 6a). Amplitudes (A) of the EPR spectra of melanin obtained from A-375 cells treated with DMC and both VPA and DMC, reach the maximum earlier than amplitude (A) of the EPR lines of melanin from A-375 cells only treated with VPA (Fig. 6a), so the relatively slower spin-lattice relaxation processes exist in these melanin polymers. The linewidths (deltaBpp) of the EPR spectra of melanin isolated from A-375 cells treated with DMC, VPA and both DMC and VPA rise with increasing microwave power (Fig. 6b). The changes of amplitudes (A) and linewidths (deltaBpp) of the EPR spectra of the all melanins isolated from A-375 cells with microwave power (Fig. 6) are those known for homogeneously broadened lines.
Fig. 5. Influence of microwave power (M/Mo) on amplitude (A) (a) and linewidth (deltaBpp) (b) of EPR spectra of synthetic DOPA-melanin (○) and tyrosine-melanin (●). M, Mo - the microwave power used during the measurement of the spectrum and the total microwave power produced by klystron (70 mW), respectively.
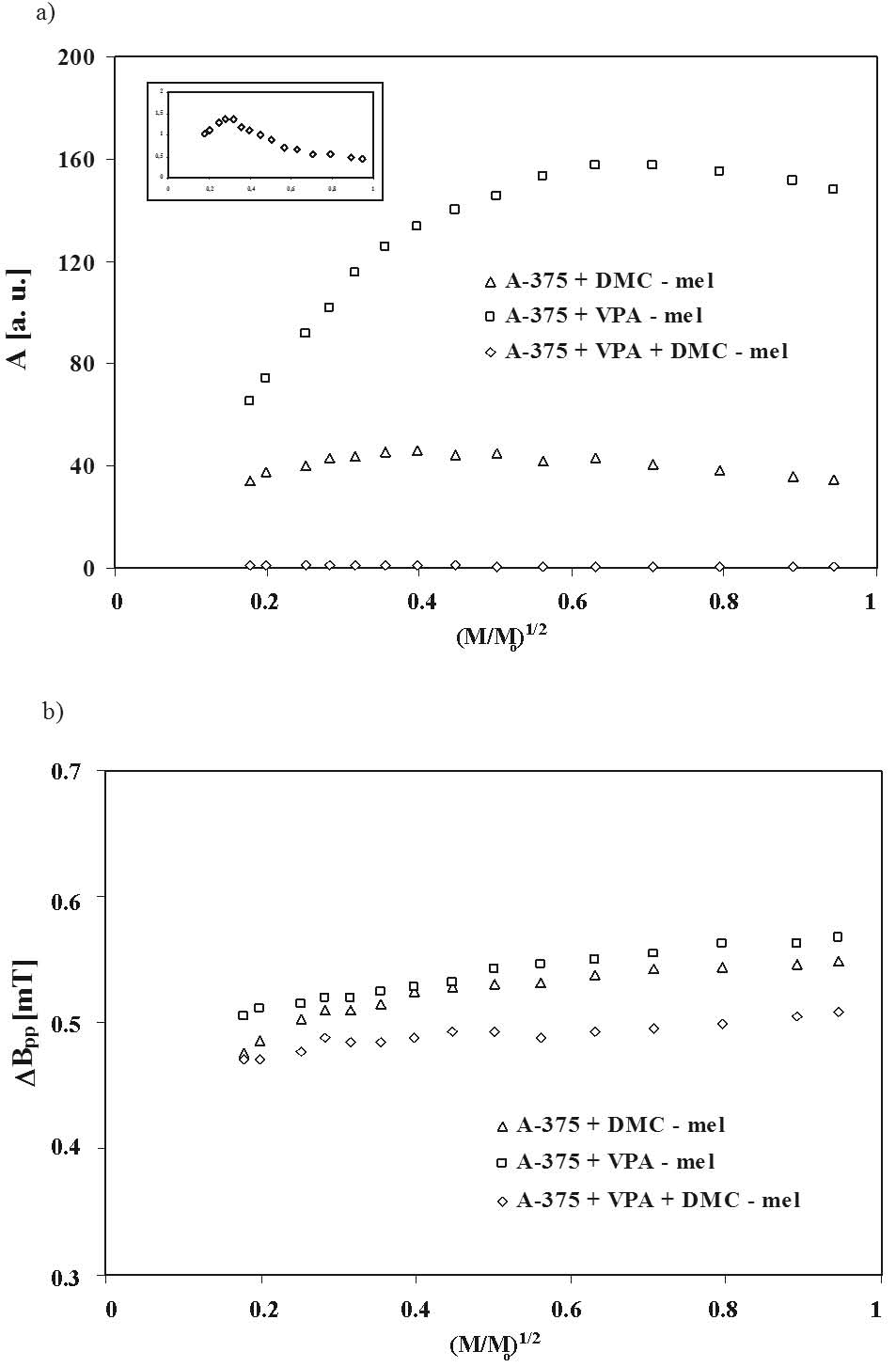
Fig. 6. Influence of microwave power (M/Mo) on amplitude (A) (a) and linewidth (deltaBpp) (b) of EPR spectra of melanin isolated from the human melanoma malignum A-375 cells treated with 10 microM DMC (delta), 1 mM VPA (□) and 1 mM VPA and 10 microM DMC (◊). M, Mo - the microwave power used during the measurement of the spectrum and the total microwave power produced by klystron (70 mW), respectively.
The correlations between amplitudes (A) and from A-375 cells (Fig. 6a) slow spin-lattice relaxation linewidths (deltaBpp) of the EPR spectra of the examined processes exist in melanin isolated from G-361 cells, human melanoma malignum G-361 cells treated with their EPR lines saturate in the range of microwave DMC and VPA, and microwave power are presented power up to 70 mW (Fig. 7a). Amplitudes (A) of the in Fig. 7, respectively. Amplitudes (A) of the EPR EPR spectra of melanin obtained from G-361 cells spectra of all the melanin samples from G-361 cells treated with VPA and both VPA and DMC, saturate decrease for the higher microwave powers (Fig. 7a). with microwave power earlier than the EPR lines of As with synthetic melanins (Fig. 5a) and melanins melanin from G-361 cells only treated with DMC (Fig. 7a), so the relatively slower spin-lattice relaxation
processes exist in the melanin from G-361 treated with VPA and both VPA and DMC, than in melanin from G-361 treated with DMC. The broadening of the EPR
spectra of melanin isolated from G-361 cells treated with DMC, VPA and both DMC and VPA with increasing of microwave power, was observed (Fig. 7b). The changes of amplitudes (A) and linewidths (deltaBpp) of the EPR spectra of all the melanins isolated from G-361 cells with microwave power (Fig. 7) are also characteristic of homogeneously broadened lines.

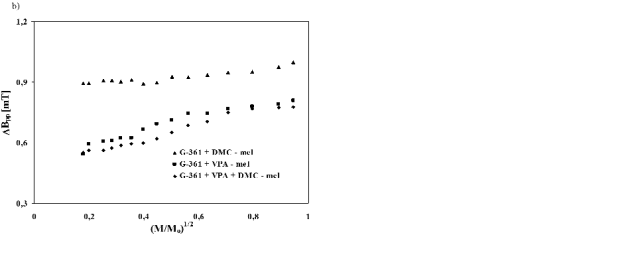
Fig. 7. Influence of microwave power (M/Mo) on amplitude (A) (a) and linewidth (deltaBpp) (b) of EPR spectra of melanin isolated from the human melanoma malignum G-361 cells treated with 10 microM DMC (▲), 1 mM VPA (■) and 1 mM VPA and 10 microM DMC (♦). M, Mo - the microwave power used during the measurement of the spectrum and the total microwave power produced by klystron (70 mW), respectively.
DISCUSSION
A large number of o-semiquinone free radicals exist in both the synthetic DOPA-melanin and tyrosine-melanin (~1021 spin/g), and in the natural melanins isolated from A-375 and G-361 human melanoma malignum tumour cells (~1019-1021 spin/g) (Table 1). The stabile and similar EPR spectra were measured for all the tested melanin samples (Figs 1, 3 and 4). The asymmetrically shaped broad EPR spectra typical of melanin were observed. The shape and parameters of the spectra changes with microwave power (Figs 5-7). The character of these changes is similar for model DOPA-melanin and melanin synthesized from tyrosine in the presence of tyrosinase (Fig. 5), and for the examined natural melanins isolated from human melanoma malignum cells (Fig. 6 and 7). EPR spectra of DOPA-melanin and melanin synthesized from tyrosine (Fig. 1), and EPR spectra of melanoma cells (Fig. 3 and 4) bring to light the fact that the same type of melanin exists in A-375 and G-361 tumour cells. The existence of eumelanin in G-361 cells was pointed out in an earlier work (Latocha et al. 2005). Similar shapes of the EPR spectra were observed for model eumelanin - DOPA-melanin and G-361 cells (Latocha et al. 2005).
Taking into account the EPR results in this work it is proposed that it is mainly melanin with a chemical structure similar to melanin synthesized from tyrosine which exists in the tested melanoma cells, both A-375 and G-361 line cells. The higher free radical concentration reveals DOPA-melanin rather than Tyr-melanin (Table 1). The free radical concentrations of the A-375 and G-361 melanoma cells treated with DMC or VPA are similar to the values in the Tyr-melanin (Table 1).
As was expected, DMC and VPA change concentrations of free radicals in melanin biopolymer in tumour cells (Table 1). The changes of free radical concentrations in cells after binding these drugs may be caused by the formation of melanin-drug complexes via unpaired electrons of free radicals. The application of unpaired electrons of melanins to the formation of the complex between melanin and the drug indicates a decrease of free radicals in melanin. Similar effects were observed for melanin complexes with kanamycin (Kozdrowska 2006), ciprofloxacin and lomefloxacin (Beberok et al. 2010).
The strong decrease of free radical concentrations characterizes melanins isolated from A-375 and G-361 tumour cells treated with both DMC and VPA (Table 1). Free radicals of melanin take part in the formation of complexes with two compounds (DMC and VPA) in these cells. The effect of the quenching of the paramagnetic properties of these tumour cells is higher than the quenching by individual compounds (Table 1). It can be expected that a greater effect will be obtained by the use of DMC and VPA together, than the application of the individual compounds. The lower amount of free radicals in melanin in this case will probably cause the relatively weaker reactions modifying the cell structures in the human organism. The higher amounts of both these compounds binding to melanins in tumour cells via o-semiquinone free radicals will interact more strongly on the pathological cells than the individual compounds. The prolongated interactions of these compounds incorporated in melanin relative to the unbounded compounds in tumour cells are expected.
The type of drug determines the resultant effect of its interactions with melanin in tumour cells. The higher free radical concentration exists in melanin from A-375 cells treated with VPA than in melanin from these cells treated with DMC (Table 1). In contrast, melanin isolated from G-361 cells treated with DMC shows a higher concentration of free radicals than when they are treated with VPA (Table 1). The interactions of the tested drugs via free radicals are different, so the biophysical and biological effect in the organism during therapy will not probably be equal.
Continuous microwave saturation (Figs 5-7) shows a homogeneous distribution in DOPA-melanin, Tyr-melanin and melanins isolated from both A-375 cells and G-361 cells. The homogeneous location of free radicals in melanins of human melanoma cells is an important result from the practical point of view. The interactions of the DMC and VPA with melanins will occur in the whole volume of these polymers in biological samples.
VPA and DMC change the chemical structures of melanin biopolymers in tumour cells. The changes may be used to check the hypothesis about the role of the individual compounds in the evolution of the tumour cells. Slow spin-lattice relaxation processes exist in DOPA-melanin, melanin synthesized from tyrosine and melanins isolated from A-375 and G-361 cells. The slower spin-lattice relaxation processes exist in DOPA-melanin than in melanin synthesized from tyrosine. The slow spin-lattice relaxation processes were also observed earlier for DOPA-melanin and natural melanins in tumour cells (Latocha et al. 2004b, Kozdrowska 2006, Zdybel 2008).
For tumour cells treated with VPA the slower spin-lattice relaxation processes characterize melanin isolated from G-361 cells than melanin isolated from A-375 cells. For tumour cells treated with DMC or both DMC and VPA the slower spin-lattice relaxation processes characterize melanin isolated from A-375 cells rather than melanin isolated from G-361 cells. The effect of other drugs and metal ions on the spin-lattice relaxation processes in melanin biopolymers has been observed (Pilawa et al. 2002, Buszman et al. 2005a, b, Kozdrowska 2006, Zdybel 2008, Beberok et al. 2010).
Electron paramagnetic resonance spectroscopy may be used to examine interactions of the antitumour compounds with free radicals existing in their melanins. The quenching of the EPR lines as the result of these interactions may be the measure of binding of the tested compound to melanin biopolymer in the tumour cells. The spectroscopic observations of spin-lattice relaxation may be used to determine the degree of change in the chemical structures after binding the drug to the melanin. The proposal in this work that electron paramagnetic resonance spectroscopy be applied to examine the effectiveness of the drug binding to melanin in human melanoma malignum tumour cells is an innovative method. The EPR method may be used in the tests for clinical applications of these potential chemopreventive and therapeutic agents for melanoma malignum.
The examination of melanin biopolymers isolated from melanoma malignum cells is important for the biomedical applications of spectroscopic methods. The electron paramagnetic resonance spectroscopy with microwaves from an X-band (9.3 GHz) is especially useful in studies of biological samples. This method is not destructive of the polymer samples relative to the chemical methods of studying free radicals,. The preparation of the melanin samples to the measurements is not complex, and major chemical procedures are not used. The interactions of the pharmaceutical compounds with melanin biopolymer may be tested with the absence of chemical treatment. The chemical structure of the examined samples is not destroyed during the EPR measurements. The low amount of the samples is enough to analyse free radicals. Electron paramagnetic resonance spectroscopy is an interesting method for the modern biomedical examination in the area of tumour science. and information about the interactions of pharmaceutics with the melanin of the tumour cells may be obtained from experiments such as this. In this work the exemplary substances for the future antitumour interactions were presented. The application of the EPR spectroscopy to the clinical use was proved.
CONCLUSIONS
Electron paramagnetic resonance (EPR) studies of the model DOPA-melanin and Tyr-melanin, and natural melanins isolated from A-375 and G-361 tumour cells pointed out that:
1. o-Semiquinone free radicals exist in both the synthetic DOPA-melanin and Tyr-melanin (~1021 spin/g), and in the natural melanins isolated from A-375 and G-361 tumour cells (~1019-1021 spin/g). The higher free radicals concentration characterizes DOPA-melanin than in Tyr-melanin.
2. DMC and VPAchange concentrations of free radicals in melanin in tumour cells. The strong decrease of free radical concentrations characterizes melanins isolated from A-375 and G-361 tumour cells treated with both DMC and VPA.
3. Higher free radical concentrations exist in melanin from A-375 cells treated with VPA than in melanin from these cells treated with DMC. In contrast, melanin isolated from G-361 cells treated with DMC shows a higher concentration of free radicals than treated with VPA.
4. For tumour cells treated with VPA the slower spin-lattice relaxation processes characterize melanin isolated from G-361 cells than melanin isolated from A-375 cells. For tumour cells treated with DMC or both DMC and VPA the slower spin-lattice relaxation processes characterize melanin isolated from A-375 cells than melanin isolated from G-361 cells.
5. EPR spectroscopy is a useful biomedical method of examination of the role of free radicals in melanins interactions with antitumour compounds.
ACKNOWLEDGEMENTS
This work was supported by SUM grant KNW-1002/P/2/0.
REFERENCES
Alesiani D, Cicconi R, Mattei M, Montesano C, Bei R, Canini A. Cell cycle arrest and differentiation induction by 5,7-dimethoxycoumarin in melanoma cell lines. Int J Oncol. 32: 425-434, 2008.
[PubMed]
Alesiani D, Cicconi R, Mattei M, Bei R, Canini A. Inhibition of Mek 1/2 kinase activity and stimulation of melanogenesis by 5,7-dimethoxycoumarin treatment of melanoma cells. Int J Oncol. 34: 1727-1735, 2009.
[PubMed]
Balamurugan A, Rees JR, Kosary C, Rim SH, Li J, Stewart SL. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 65: 69-77, 2011.
[CrossRef]
[PubMed]
Beberok A, Buszman E, Zdybel M, Pilawa B, Wrzesniok D. EPR examination of free radical properties of DOPA-melanin complexes with ciprofloxacin, lomefloxacin, norfloxacin and sparfloxacin. Chem Phys Lett. 497: 115-122, 2010.
[CrossRef]
Bennett DC. Ultraviolet wavebands and melanoma initiation. Pigment Cell Melanoma Res. 21: 520-524, 2008.
[CrossRef]
[PubMed]
Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 84: 539-549, 2008.
[CrossRef]
[PubMed]
Buszman E, Pilawa B, Zdybel M, Wrzesniok D, Grzegorczyk A, Wilczok T. EPR examination of Zn2+ and Cu2+ effect on free radicals in DOPA–melanin–netilmicin complexes. Chem Phys Lett. 403: 22-28, 2005a.
[CrossRef]
Buszman E, Pilawa B, Zdybel M, Wrzesniok D, Grzegorczyk A, Wilczok T. Paramagnetic centers in DOPA-melanin-dihydrostreptomycin complexes. Acta Phys Pol A. 108: 353-356, 2005b.
Chateauvieux S, Morceau F, Dicato M, Diederich M. Molecular and therapeutic potential and toxicity of valproic acid. J Biomed Biotechnol. 2010: 479364, 2010.
[CrossRef]
Chodurek E, Kusmierz D, Dzierzega-Lecznar A, Kurkiewicz S, Stepien K, Dzierzewicz Z. Thermochemolysis as the useful method to assess the purity of melanin isolated from the human melanoma malignum. Acta Pol Pharm. 65: 731-734, 2008.
[PubMed]
Chodurek E, Orchel A, Orchel J, Kurkiewicz S, Gawlik N, Dzierzewicz Z, Stepien K. Evaluation of melanogenesis in A-375 cells in the presence of DMSO and analysis of pyrolytic profile of isolated melanin. ScientificWorldJournal DOI:10.1100/2012/854096, 2012.
[CrossRef]
Duenas-Gonzalez A, Candelaria M, Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E, Herrera LA. Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumours. Cancer Treat Rev. 34: 206-222, 2008.
[CrossRef]
[PubMed]
Eaton GR, Eaton SS, Salikhov KM. Foundations of modern EPR. World Scientific, Singapore 1998.
Edman RL, Wolfe JT. Prevention and early detection of malignant melanoma. Am Fam Physician. 62: 2277-2285, 2000.
[PubMed]
Farmer PJ, Gidanian S, Shahandeh B, Di Bilio AJ, Tohidian N, Meyskens FL, Jr. Melanin as a target for melanoma chemotherapy: pro-oxidant effect of oxygen and metals on melanoma viability. Pigment Cell Res. 16: 273-279, 2003.
[CrossRef]
[PubMed]
Federico M, Bagella L. Histone deacetylase inhibitors in the treatment of hematological malignancies and solid tumours. J Biomed Biotechnol. 2011: 475641, 2011.
[CrossRef]
Gidanian S, Mentelle M, Meyskens FL, Jr., Farmer PJ. Melanosomal damage in normal human melanocytes induced by UVB and metal uptake - a basis for the pro-oxidant state of melanoma. Photochem Photobiol. 84: 556-564, 2008.
[CrossRef]
[PubMed]
Goldstein BG, Goldstein AO. Diagnosis and management of malignant melanoma. Am Fam Physician. 63: 1359-1368, 2001.
[PubMed]
Ibrahim SF, Brown MD. Tanning and cutaneous malignancy. Dermatol Surg. 34: 460-474, 2008.
[CrossRef]
[PubMed]
Ito S, Wakamatsu K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment Cell Res. 16: 523-531, 2003.
[CrossRef]
[PubMed]
Ito S, Wakamatsu K. Chemistry of mixed melanogenesis - pivotal roles of dopaquinone. Photochem Photobiol. 84: 582-592, 2008.
[CrossRef]
[PubMed]
Kozdrowska L. Wlasciwosci centrow paramagnetycznych kompleksow DOPA-melaniny z kanamycyna i jonami miedzi(II). Ph.D. Thesis. University of Zielona Gora, Zielona Gora 2006.
Latocha M, Pilawa B, Buszman E, Wilczok T. Identification of melanin biopolymers in cultured melanoma cells by the use of EPR spectroscopy. Pol J Med Phys Eng. 10: 119-126, 2004a.
Latocha M, Pilawa B, Chodurek E, Buszman E, Wilczok T. Paramagnetic centers in tumour cells. Appl Magn Reson. 26: 339-344, 2004b.
[CrossRef]
Latocha M, Pilawa B, Zdybel M, Wilczok T. Effect of laser radiation on free radicals in human cancer G361 cells. Acta Phys Pol A. 108: 409-412, 2005.
Latocha M, Pilawa B, Kusmierz D, Zielinska A, Nawrocka D. Changes in free radicals system of Imr-90 and C-32 cells during photodynamic therapy. Pol J Environ Stud. 15: 154-156, 2006.
Matuszczyk M, Buszman E, Pilawa B, Witoszynska T, Wilczok T. Cd2+ effect on free radicals in Cladosporium cladosporioides - melanin tested by EPR spectroscopy. Chem Phys Lett. 394: 366-371, 2004.
[CrossRef]
Najder-Kozdrowska L, Pilawa B, Wieckowski AB, Buszman E, Wrzesniok D. Influence of copper(II) ions on radical in DOPA-melanin. Appl Magn Reson. 36: 81-88, 2009.
[CrossRef]
Najder-Kozdrowska L, Pilawa B, Buszman E, Wieckowski AB, Swiatkowska L, Wrzesniok D, Wojtowicz W. Triplet states in DOPA-melanin and in its complexes with kanamycin and copper Cu(II) ions. Acta Phys Pol A. 118: 613-618, 2010.
Pasenkiewicz-Gierula M, Sealy RC. Analysis of the ESR spectrum of synthetic dopa melanin. Biochim Biophys Acta. 884: 510-516, 1986.
[CrossRef]
Pilawa B, Buszman E, Wrzesniok D, Latocha M, Wilczok T. Application of EPR spectroscopy to examination of gentamicin and kanamycin binding to DOPA-melanin. Appl Magn Reson. 23: 181-192, 2002.
[CrossRef]
Pilawa B, Latocha M, Krzyminiewski R, Kruczynski Z, Buszman E, Wilczok T. Effect of temperature on melanin EPR spectra. Phys Med. 20: 96-98, 2004.
Shima T, Sarna T, Swartz HM, Stroppolo A, Gerbasi R, Zecca L. Binding of iron to neuromelanin of human substantia nigra and synthetic melanin: an electron paramagnetic resonance spectroscopy study. Free Radic Biol Med. 23: 110-119, 1997.
[CrossRef]
Stankowski J, Hilczer W. Introduction to magnetic resonance spectroscopy. Wydawnictwo Naukowe PWN, Warszawa 2005.
Tran TT, Schulman J, Fisher DE. UV and pigmentation: molecular mechanisms and social controversies. Pigment Cell Melanoma Res. 21: 509-516, 2008.
[CrossRef]
[PubMed]
Wertz JE, Bolton JR. Electron spin resonance: elementary theory and practical applications. Chapman and Hall, London 1986.
Wojciechowska U, Didkowska J, Zatonski WA. Cancer in Poland in 2006. Centr Onkol Inst M Sklodowska-Curie, Warszawa 2008.
Wojciechowska U, Didkowska J, Zatonski WA. Cancer in Poland - five-year survival rates by regions. Centr Onkol Inst M Sklodowska-Curie, Warszawa 2010.
Zdybel M. Zlozony uklad centrow paramagnetycznych kompleksow DOPA-melaniny z netilmicyna, jonami cynku(II) i miedzi(II). Ph.D. Thesis. Medical University of Silesia, Katowice 2008.
Zdybel M, Chodurek E, Pilawa B. EPR studies of DOPA-melanin complexes with Fe(III). Appl Magn Reson. 40: 113-123, 2011.
[CrossRef]
|
BACK
|











