Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 11 (2013), No 3, p 153-162
DOI 10.2478/v10136-012-0039-2
Human colostrum melatonin exhibits a day-night variation and modulates the activity of colostral phagocytes
Adenilda Cristina Honorio-Franca, Cristiane Castro Pernet Hara, Joao Victor Silva Ormonde, Gabriel Triches Nunes, Eduardo Luzia Franca
Address: Eduardo Luzia Franca, Instituto de Ciencias Biologicas e da Saude, UFMT, Barra do Garcas, MT, Rodovia BR070 KM 5 s/no, Barra do Garcas, MT, Brazil
denifran@terra.com.br
Received 10th July 2012.
Revised 12th February 2013.
Published online 14th February 2013.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
Acknowledgements
References
SUMMARY
Some studies report that hormone melatonin can be found in human milk, but the daily variation in colostrum melatonin is not available. This study verified the effects of milk collection time (diurnal/nocturnal) on colostral melatonin levels and the ability of this hormone to modulate colostral phagocyte activity. Colostrum samples were collected from 30 mothers during the day and night, for a total of 60 samples. We determined melatonin levels in colostrum and superoxide release and bacterial killing by colostral phagocytes. Melatonin levels were higher in colostrum samples collected at night. Phagocytes in nocturnal colostrum samples increased spontaneous superoxide release. In diurnal colostrum samples, mononuclear (MN) phagocytes increased superoxide release when exposed to enteropathogenic Escherichia coli (EPEC), but not polymorphonuclear (PMN) phagocytes. Phagocytes exposed to both EPEC and melatonin had higher superoxide release, independent of phagocyte type and colostrum collection period. Phagocytosis rate was higher in colostrum samples collected at night. In diurnal samples, EPEC killing by MN phagocytes was lower than by PMN phagocytes. Phagocytosis increased significantly in the presence of melatonin in both MN and PMN cells, irrespective of colostrum collection period. In response to melatonin, MN phagocytes from both diurnal and nocturnal samples increased bactericidal activity, whereas colostral PMN phagocytes increased it only in diurnal samples. The melatonin increased in the intracellular Ca2+ levels. The highest intracellular Ca2+ release were found in MN phagocytes diurnal samples. These results confirm that melatonin levels in human colostrum follow a day-night variation and increase phagocytic activity of colostral cells against bacteria.
KEY WORDS
melatonin; phagocyte; colostrum; breastfeeding; intracellular calcium
INTRODUCTION
Human milk is important not only for nutrition but also for the immunological defense of infants. It has an appropriate balance of nutrients, provided in highly digestible and bioavailable form (Lawrence and Lawrence 2005). Even colostrum, the first lacteal Human milk is important not only for nutrition but secretion produced, contains the nutrients needed for also for the immunological defense of infants. It growing infants and provides adequate amounts of has an appropriate balance of nutrients, provided in lipids, carbohydrates, proteins, cells and hormones (American Dietetic Association 2009).
Studies have report that hormone melatonin in human milk exhibits a pronounced circadian rhythm, reaching high levels at night but undetectable
amounts during the day. This suggests that melatonin fluctuation indicates time of day to breastfed infants (Illnerova et al. 1993).
Melatonin production in newborns does not display a circadian rhythm, nor is it driven by environmental lighting (Ogasawara et al. 1991). At birth, newborns are essentially non-circadian, probably because input/ output pathways are undeveloped, i.e., immature temporal controlis revealed by the lack of consolidated sleep and rhythms in hormonal and body temperature variations (Swaab et al. 1990). Fluctuations in milk content increase progressively up to 3 months postpartum, producing a rhythm that is assumed to stabilize the circadian system of the newborn while other controlling mechanisms such as the rest-activity cycle of adults are not fully developed (Attanasio et al. 1986, Rivkees and Hao 2000).
Some studies report that cellular, immunological and biochemical components of human milk follow a circadian rhythm. Chronobiological variations of milk are already observed in the first month postpartum and may represent an additional breastfeeding mechanism to improve newborn adaptation to environmental conditions, an endogenous force that promotes the establishment of biological rhythms in humans (Franca et al. 2010). The action of colostral melatonin on a newborn’s intestine is scarcely known. However, it should be more thoroughly investigated because infants are vulnerable to a number of pathogens such as enteropathogenic Escherichia coli (EPEC).
Experimental, clinical and epidemiological studies have demonstrated that human colostrum and milk protect children against several potentially pathogenic agents (Honorio-Franca et al. 1997, 2001, Franca-Botelho et al. 2006, Franca et al. 2011a, 2011b, Franca et al. 2012). However, the action mechanism of these agents is only partially understood. Both soluble components and cells are believed to be involved in defense responses and most likely act together (Honorio-Franca et al. 1997, Field 2005, Hanson 2007).
In addition to hormones, soluble bioactive components and anti-infectious factors, human colostrum contains large amounts of viable leukocytes (109 cells/mL in the first days of lactation), especially macrophages and neutrophils (Islam et al. 2006). Colostrum phagocytes, immunocompetent cells that are easily recovered in a non-stimulated state, traverse the mammary epithelium, which remains highly permeable in the first few days after delivery (Filteau 2003). These cells produce free radicals, exhibit phagocytic and bactericidal activity and are known to be the first line of host defense against bacterial infections (Honorio-Franca et al. 1997, 2001, Franca-Botelho et al. 2006, Franca et al. 2011a).
Colostral melatonin might be important not only for synchronization of infant rhythm, but it likely also contributes to the protective effects of milk (Kennaway et al. 1996, Franca et al. 2010). This pineal hormone is known to have some putative protective effects on ischemia reperfusion injury (Korkmaz et al. 2009), local (Hooton et al. 1991) and chronic (Carlson and Hanson 1994) inflammation, in addition to increasing antibody response (Cassone 1992), antigen presentation by macrophages (Zaidam et al. 1994) and phagocytosis by macrophages (Silva et al. 2006). In the present work, we show the effects colostrum collection time (diurnal/nocturnal) on melatonin levels and its capacity to modulate colostrum phagocyte activity.
MATERIAL AND METHODS
After delivery, colostrum samples was collected from clinically healthy women, 18-35 years of age, at the Health System Program of Barra do Garcas, Mato Grosso, Brazil (N=30). All the mothers had given birth to healthy term babies through vaginal delivery. Two colostrum samples were collected in sterile plastic tubes between 48 and 72 hours postatpartum at 12:00 h (diurnal period) and 24:00 h (nocturnal period) from each mother, according to Franca et al (2010), with a total of 60 samples. This study was approved by the institutional Research Ethics Committee, and all the subjects gave informed written consent before entering the experimental protocol.
Obtaining supernatant from human colostrum
About 8 ml of colostrum from each woman was collected in sterile plastic tubes between 48 and 72 hours postpartum in two periods of time. The samples were obtained by centrifugation (10 min, 160 x g, 4 °C), the upper fat layer was discarded, and the aqueous supernatant stored at -80 °C to isolate posterior melatonin hormone.
Melatonin hormone dosage by immunoenzymatic method
Melatonin was extracted by affinity chromatoraphy, concentrated in speed-vacuum and determined by ELISA kit from Immuno-Biological Laboratories (IBL, Hamburg). Reaction rates were measured by absorbance plate-reading spectrophotometer with a 405 nm filter. The results were calculated according to the standard curve and shown in pg/ml.
Separation of colostral cells
Colostrum from each woman was collected in sterile plastic tubes. The samples were centrifuged (160 x g, 4 °C) for 10 min, which separated colostrum into three different phases: cell pellet, an intermediate aqueous phase, and a lipid-containing supernatant by Honorio-Franca et al (1997). Cells were separated by a Ficoll-Paque gradient (Pharmacia, Upsala, Sweden). This procedure generated 95% pure polymorphonuclear and 98% pure mononuclear cell preparations as analyzed by light microscopy. Purified neutrophils and macrophages were resuspended independently in serum-free 199 medium at a final concentration of 2 x 106 cells/ml.
Treatment of colostral phagocytes with melatonin hormone
To assess the effect of melatonin on superoxide anion release, MN or PMN phagocytes (2 x 106 cells/ml) were incubated with with 10 microl of Phorbol Myristate Acetate (PMA - Sigma St. Louis, USA, final concentration at 10-7M) or 50 microl of melatonin (Sigma St. Louis, USA, final concentration at 10-7M; Franca et al. 2009) for 1 h at 37 °C. To investigate the effects of melatonin on phagocytic, microbicidal activity and intracellular Ca2+ release, mononuclear phagocytes (2 x 106 cells/ml) were incubated with 50 microl of melatonin (Sigma St. Louis, USA, final concentration at 10-7M; Franca et al. 2009) for 1 h at 37 °C. The phagocytes were then washed once with 199 medium at 4 °C and immediately used in the assays developed to measure superoxide release, phagocytosis, bactericidal activity and intracellular Ca2+ release.
E. coli strain
The enterophatogenic Escherichia coli (EPEC) used was isolated from stools of an infant with acute diarrhea (serotype 0111:H–, LA+, eae+, EAF+, bfp+). This material was prepared and adjusted to 107 bacteria/ml, as previously described (Honorio-França et al. 1997).
Release of superoxide anion
Superoxide release was determined by cytochrome C (Sigma, St. Louis, USA) reduction (Pick and Mizel 1981, Honorio-Franca et al. 1997). Briefly, MN or PMN phagocytes and bacteria were mixed and incubated for 30 min for phagocytosis. Cells were then resuspended in PBS containing 2.6 mM CaCl2, 2 mM MgCl2, and cytochrome C (Sigma, St. Louis, USA; 2 mg/ml). The suspensions (100 microl) were incubated for 60 min at 37 °C on culture plates. The reaction rates were measured by absorbance at 550 nm and the results were expressed as nmol/O2-. All the experiments were performed in duplicate or triplicate.
Bactericidal assay
Phagocytosis and Microbicidal activity were evaluated by the acridine orange method described by Bellinati-Pires et al. (1995). Equal volumes of bacteria and cell suspensions were mixed and incubated at 37 °C for 30 min under continuous shaking. Phagocytosis was stopped by incubation in ice. To eliminate extracellular bacteria, the suspensions were centrifuged twice (160 x g, 10 min, 4 °C). Cells were resuspended in serum-free 199 medium and centrifuged. The supernatant was discarded and the sediment dyed with 200 microl of acridine orange (Sigma, St. Louis, USA; 14.4 g/l) for 1 min. The sediment was resuspended in cold 199 medium, washed twice and observed under immunofluorescence microscope at 400x and 1000x magnification.
The phagocytosis index was calculated by counting the number of cells ingesting at least 3 bacteria in a pool of 100 cells. To determine the bactericidal index, we stained the slides with acridine orange and counted 100 cells with phagocytized bacteria. The bactericidal index is calculated as the ratio between orange-stained (dead) and green-stained (alive) bacteria x 100 (Franca et al. 2011a). All the experiments were performed in duplicate or triplicate.
Intracellular Ca2+ release determined by fluorescence and flow cytometry
We performed fluorescence staining at the FACS Calibur (BD San Jose USA) to assess intracellular Ca2+ release in colostrum phagocytes (Fagundes et al. 2012). Cells were loaded with the fluorescent radiometric calcium indicator Fluo-3-Acetoxymethyl (Fluo-3-AM - Sigma St. Louis, USA). Cell suspensions, pre-treated or not with 50 microl of melatonin (Sigma, final concentration of 10-7M; Franca et al. 2009), mixed and incubated at 37 °C for 30 min under continuous stirring. Suspensions were centrifuged twice (160 x g, 10 min, 4 °C) and resuspended in PBS containing BSA (5 mg/ml). This suspension was incubated with 5 microl of Fluo-3 (1 microg/ml) for 30 min at 37 °C. After incubation, cells were washed twice in PBS containing BSA (5 mg/ml; 160 x g, 10 min, 4 °C) and then analyzed by flow cytometry (FACS Calibur system - BD, San Jose, USA). Calibration and sensitivity were routinely checked using CaliBRITE 3 Beads (BD Cat. No 340486 USA). Fluo-3 was detected at 530/30 nm filter for intracellular Ca2+. The rate of intracellular Ca2+ release was expressed in geometric mean fluorescence intensity of Fluo-3. Data shown in the figures correspond to one of several trials performed.
Statistics
Data are expressed as the mean ± standard deviation. The statistically significant difference was evaluated using the analysis of variance (ANOVA) for superoxide release anion, phagocytosis, bactericidal index and intracellular Ca2+ release in the presence or absence of melatonin and the difference was compared at the significance level at 2alpha=0.05.
RESULTS
Melatonin concentration in colostrum
Melatonin concentration in human colostrum was higher in nocturnal than diurnal colostrum samples (Fig. 1).
Effect of melatonin on the superoxide release of colostrum phagocytes
In nocturnal colostrum samples, both MN and PMN phagocytes increased spontaneous superoxide release compared to diurnal sample phagocytes. In PMA-stimulated cells, melatonin stimulated similar rates of superoxide release. Melatoninstimulated MN phagocytes had higher superoxide release than melatonin-stimulated PMN phagocytes. When exposed to EPEC, superoxide release by MN phagocytes from diurnal samples increased (statistically significant). MN and PMN phagocytes in nocturnal samples had higher superoxide release when incubated with EPEC than non-incubated phagocytes (statistically significant). In diurnal samples, PMN phagocytes had lower superoxide release in the presence of EPEC than MN phagocytes. Both types of phagocytes, from diurnal and nocturnal samples, had higher superoxide release when exposed to EPEC and melatonin (Table 1).
Effect of melatonin on the phagocytosis of colostrum phagocytes
The phagocytosis index was higher in nocturnal samples. MN and PMN cells from nocturnal samples had similar phagocytosis rates, which were not affected by the presence of melatonin. MN phagocytes from the diurnal samples had lower phagocytosis rates when exposed to EPEC than PMN phagocytes under the same conditions. In response to melatonin, colostral MN phagocytes exhibited increased phagocytic activity, reaching similar values to those of PMN phagocytes. Phagocytosis by both MN and PMN phagocytes increased in the presence of melatonin, in both diurnal and nocturnal samples (Fig. 2).
Effect of melatonin on the bactericidal activity of colostrum phagocytes
In general, colostral phagocytes exhibited some bactericidal activity against EPEC in both diurnal and nocturnal colostrum samples. In response to melatonin, MN phagocytes from both diurnal and nocturnal samples displayed increased bactericidal activity. When stimulated with melatonin, the bactericidal activity of PMN phagocytes from diurnal samples increased. In nocturnal samples, PMN phagocytes had lower bactericidal activity in response to melatonin stimulation (Fig. 3).
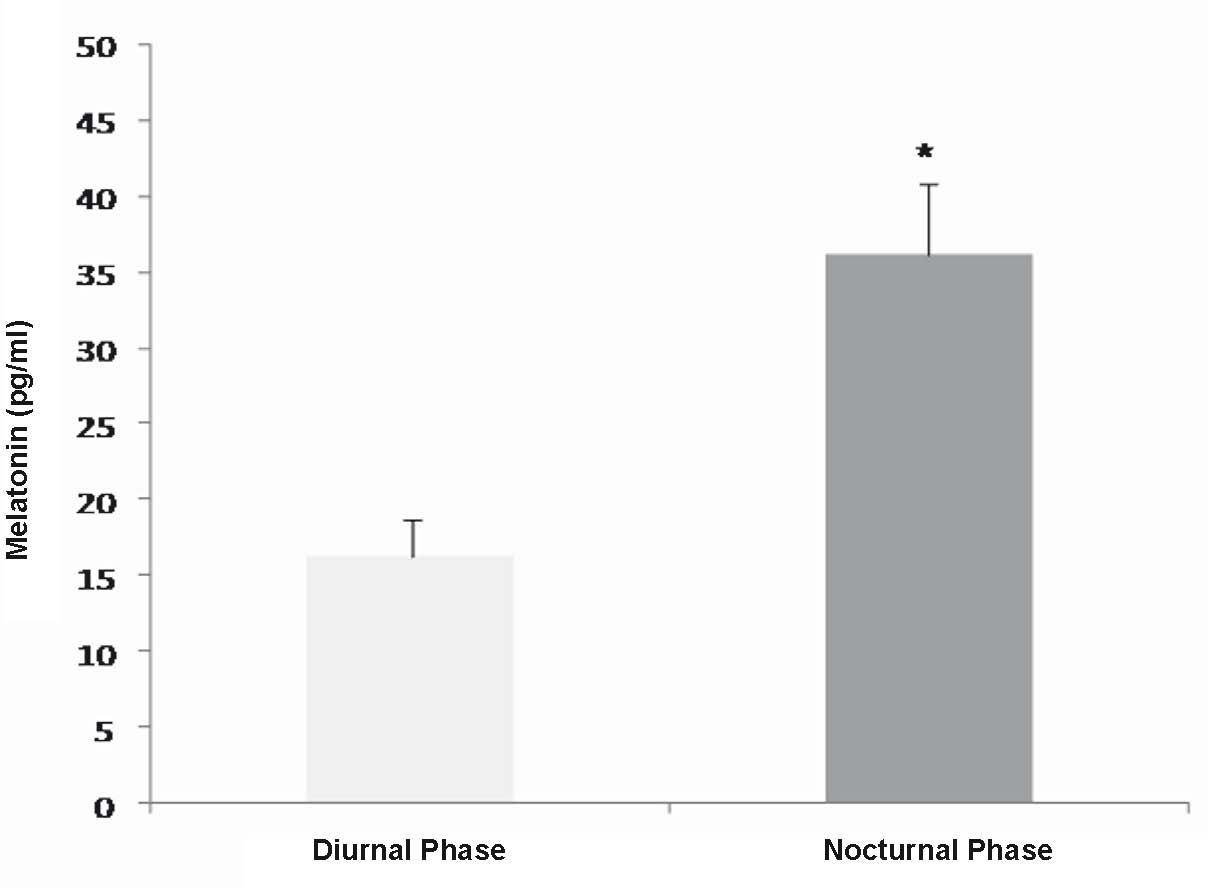
Fig. 1. Daily fluctuation of melatonin concetration (pg/ml) in colostrum supernatant of healthy puerpearae. Seven colostrum samples of diferents mothers. * Statistically significant as compared with the diurnal findings.
Table 1. Superoxide release by colostrum phagocytes.
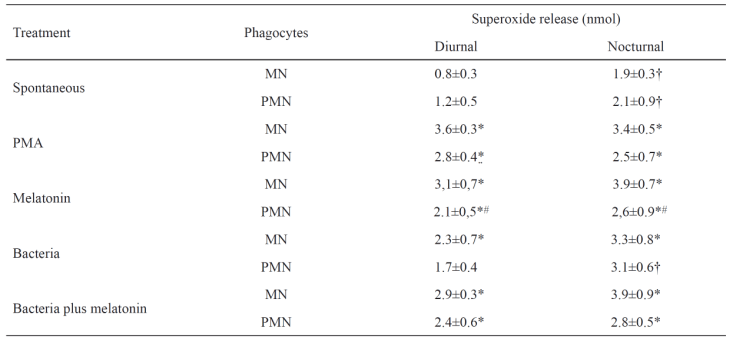
Phagocytes stimulated with melatonin in the presence or not of bacteria. In controls assays, cells were pre-incubated with PBS or PMA (positive control). N=10 in each treatment. PMN, polymorphonuclears; MN, mononuclears; * statistically significant as compared treatment (PMA, Melatonin, Bacteria and Bacteria plus melatonin) with the spontaneous release within collection period and kind of cells; † statistically significant as compared collection period within each treatment and kind of cell; # statistically significant as compared kind of cells within each treatment and collection period.
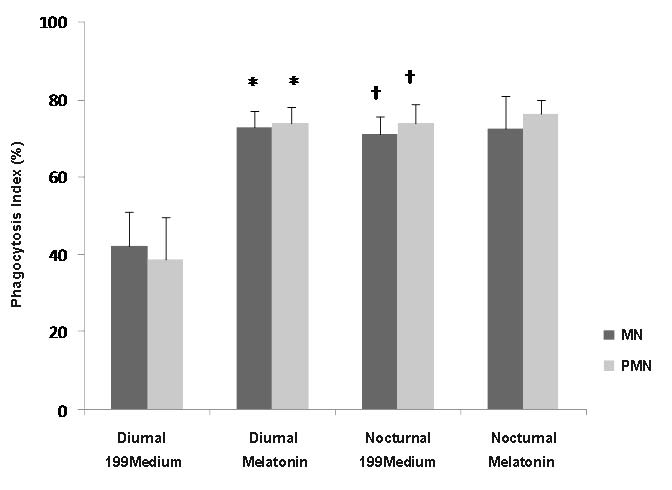
Fig. 2. Bacterial phagocytosis by colostrum cells. Bacterial phagocytosis by cells from colostrum was determined with the acridine orange method. N=10 in each treatment; * statistically significant versus 199medium; † indicates statistically significant as compared collection period differences within each treatment and kind of cells. Other symbols as in Table 1.
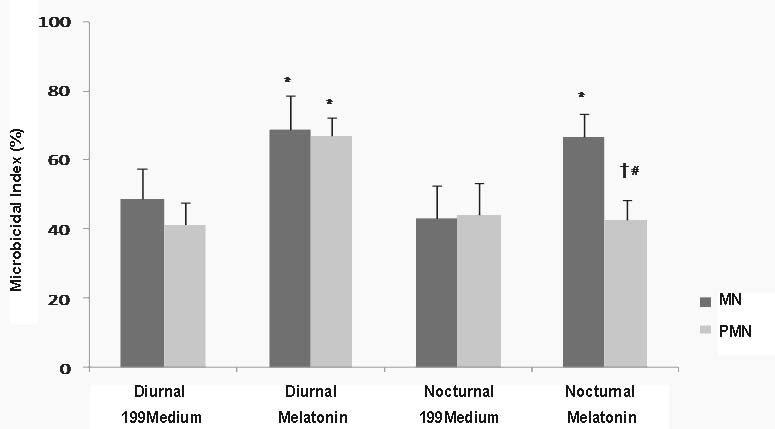
Fig. 3. Bacterial elimination index. Bactericidal activity by phagocytes from colostrum was determined with the acridine orange method. N=10 in each treatment; * indicates statistically significant as compared 199 medium with melatonin within collection period and kind of cells; † indicates statistically significant as compared collection period within each treatment and kind of cells (ANOVA); # indicates statistically significant as compared with kind of cells within each treatment and collection period. Other symbols as in Table 1.
Effect of melatonin on the Intracellular Ca2+ release of colostrum phagocytes
The incubation of colostrum phagocytes with melatonin revealed that the cells had increased in the intracellular Ca2+ levels in both kind of cells and collection time (Fig. 4). Table 2 shows the rate of Fluo-3 to assess intracellular Ca2+ release by fluorescence intensity of colostral phagocyte treated with melatonin. The highest intracellular Ca2+ release were found in MN phagocytes diurnal samples. Compared to untreated cells, diurnal PMN phagocytes had lower intracellular Ca2+ release (Table 2).
Table 2. Intracellular Ca2+ release by mononuclear and polymorphonuclear colostrum phagocytes in the presence of melatonin indicated by fluorescence intensity.

Five experiments with cells of different individuals. * Statistically significant versus the group without melatonin. # Statistically significant as compared the kind of cells and collection period. Other symbols as in Table 1.
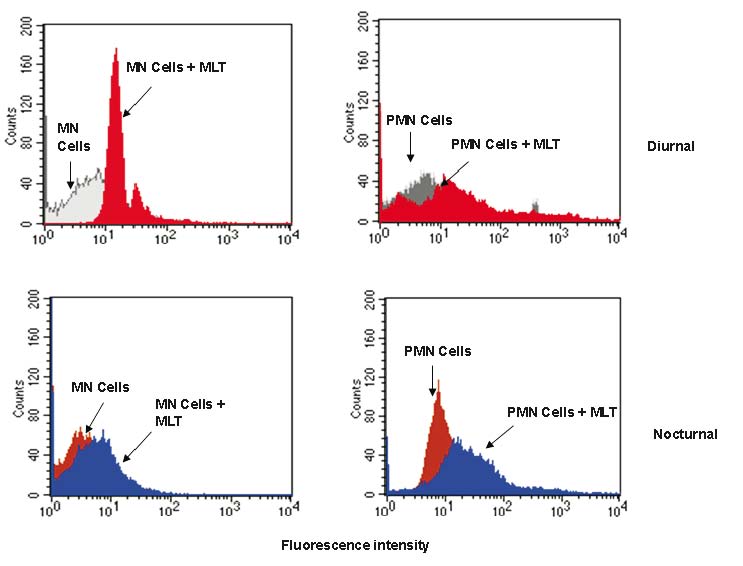
Fig. 4. Mononuclear colostrum phagocytes and polymorphonuclear colostrum phagocytes stimulated with melatonin were staining with Fluo-3 to assess intracellular Ca2+ release. Immunofluorescence analysis was then carried out by flow cytometry (FACScalibur, Becton Dickinson, USA). Symbols as in Table 1.
DISCUSSION
The nutritional and immunological value of milk is undisputed. The complexity of human milk makes this secretion the ideal food source for babies for at least the first 6 months of their life. This early nutrition is an important environmental input that can exert lifelong effects on child metabolism and development. Food and its composition represent very important environmental influences on human health. These factors can change the progression of diseases as well as longevity (Berger 2013). The immune system is influenced by nutrition and some nutrients can be directly regulate signalling pathways, while some dietary factors have a regulatory influence on hormones that, in turn, modulate the immune system (Raqib and Cravioto 2009). Colostrum, the first milk produced by mammals after parturition, has been thoroughly studied in recent years. For nursing babies, it represents an important source of hormones such as melatonin, which is released during the night by the pineal gland (Ilnerova et al. 1993). In the present study, we confirm the fluctuation of melatonin levels in colostrum and its immunostimulatory effect on colostral phagocyte activity.
Newborns have low melatonin levels, which increase progressively up to the 3rd month of age, when a characteristic circadian rhythm is detectable (Attanasio et al. 1986). Accordingly, some authors propose that a primary role of milk melatonin is to stabilize the newborn circadian rhythm (Ilnerova et al. 1993) while other biological phenomena such as rest-activity or sleep-wake cycles have yet to be firmly established. The present study found that colostral melatonin levels is similar to melatonin concentration in human peripheral blood, suggesting that this hormone is present at the same levels in different body liquids, probably exercising the same biological functions. Melatonin concentrations in colostrum were higher at night than during the day, confirming previous studies that compared diurnal and nocturnal melatonin concentration in mother’s milk in different lactation periods (Ilnerova et al. 1993).
Melatonin contained in milk is likely derived from blood and plays an important role in newborn synchronization with the mother's rhythm (Ilnerova et al. 1993, Franca et al. 2010). Milk melatonin may provide information on day/night cycles to the child until this rhythm is established (Attanasio et al. 1986). The action of melatonin in the newborn's intestine may be associated to prevention of gastrointestinal mucosa ulceration by antioxidant action, reduction of hydrochloric acid secretion, and immune system stimulation, fostering epithelial regeneration and increased microcirculation (Bubenik 2002).
The melatonin contained in maternal milk can contribute to protecting the gastrointestinal mucosa of newborns, and the daily fluctuations of this exogenous hormone over a 24 h period, due to breastfeeding, might contribute to the synchronization between the child's rhythms and those of its mother (Attanasio et al. 1986).
Neurohormonal control is significantly responsible for driving the immunomodulatotory effects (Besedovsky and del Rey 1996). Melatonin has beneficial free radical scavenging actions beyond its stimulatory effects on the diverse cytosolic antioxidant enzyme systems (Klepac et al. 2005, Hanson, 2007, Sudnikovich et al. 2007, Pandi-Perumal et al. 2008, Franca et al. 2011b). Many studies have reported that melatonin strongly stimulates immune cells (Skwarlo-Sonta 2002, Pawlak et al. 2005, Franca et al. 2009, Honorio-França et al. 2009). The present study found that melatonin stimulates the cellular oxidative metabolism of colostrum phagocytes. This was evidenced by the increase in superoxide release in the presence of melatonin, which was similar to that obtained in the positive control composed of PMA-induced cells. Bacteria are known to increase superoxide production, and here we show that melatonin not only potentiates free radical production in the absence of microorganisms, but also in the presence of bacteria. Free radical generation has been considered an important body protection mechanism during infectious processes, mainly intestinal infections (Rodriguez et al. 2004, Franca et al. 2011a, b).
We found that melatonin in vitro increases EPEC phagocytosis by colostral cells, provoking time dependent bactericidal activity. This is a remarkable result because human colostral PMN phagocytes, which express the non-functional CD89-IgA receptor lacking gamma chain association (Honorio-Franca et al. 2001), have hitherto been considered to have insignificant bactericidal activity (Honorio-Franca et al. 1997).
In the present study we showed that PMN cells are able kill bacteria only in the diurnal period and in the presence of melatonin, whereas colostral MN phagocytes increase phagocytosis and bactericidal activity in the presence of melatonin independently of time of day. In experimental studies showed that changes in phagocytic characteristics are a part of the circadian system of the immune system (Berger and Slapnickova 2003) and studies in vitro have reported that colostral phagocyte activity is dependent on sampling period, with the different phagocyte types exhibiting specific activity patterns and chronobiological profiles, which change over the lactation phases (Franca et al. 2010).
The microbicidal activity promoted by melatonin and the resulting oxidation products may have important clinical implications (Silva et al. 2006). The superoxide may come from a change of responsiveness to intracellular Ca2+ level and to phosphorylation events during oxidative metabolism (Carrichon et al. 2011). In the study we observed that melatonin present direct effects on colostrum phagocytes. Here we show that melatonin is capable of significantly raising colostrum phagocytes intracellular calcium, and this activity is dependent on sampling period, with the different phagocyte types. Melatonin have been reported elevates intracellular calcium in human platelet by inositol 1,4,5-trisphosphate independent mechanism of the PLC-IP3 axis (Kumari and Dash 2011). Ours results supported that melatonin increase the superoxide by colostrum phagocytes and change the intracellular Ca2+ level, which facilitate the microbicidal activity of cells considering the differences between the cells and time of day.
In conclusion, the chronobiological pattern of melatonin in human colostrum suggests the existence of an auxiliary physiological defence mechanism against infections in breastfed children and the present study confirm the circadian rhythm of human colostral melatonin. In addition, we show that this hormone has a time-dependent stimulatory effect on phagocytosis and killing activity by colostral phagocytes against enteropathogenic E. coli. Thus, colostral melatonin likely improves colostrum's ability to protect children against EPEC and other infections, in addition to promoting newborn adaptation to environmental changes, contributing to the establishment of biological rhythms through breastfeeding.
ACKNOWLEDGEMENTS
This research received grants from Fundacao de Amparo a Pesquisa de Mato Grosso (FAPEMAT No 299032/2010) and Conselho Nacional de Pesquisa (CNPq No 475826/2010-8; No 475739/2011-6).
The authors declare no conflict of interest and non-financial competing interests.
REFERENCES
American Dietetic Association (ADA). Position of the American Dietetic Association: Promoting and Supporting Breastfeeding. J Am Diet Assoc. 109: 1926-1942, 2009.
[CrossRef]
[PubMed]
Attanasio A, Rager K, Gupta D. Ontogeny of circadian rhythmicity for melatonin, serotonin, and N-acetylserotonin in humans. J Pineal Res. 3: 251-256, 1986.
[CrossRef]
[PubMed]
Bellinati-Pires R, Salgado MM, Hypolito IP, Grumach AS, Carneiro-Sampaio MMS. Application of a fluorochrome-lysostaphin assay to the detection of phagocytic and bactericidal disturbances in human neutrophils and monocytes. J Investig Allergol Clin Immunol. 5: 337-342, 1995.
[PubMed]
Berger J. Influence of food restriction on mammalian immunity. J Appl Biomed. 11: 1-5, 2013.
[CrossRef]
[JAB]
Berger J, Slapnickova M. Circadian structure of rat neutrophil phagocytosis. Comp Clin Pathol. 12: 84-89, 2003.
[CrossRef]
Besedovsky HO, del Rey A. Immune-neuro-endocrine interactions: Facts and hypotheses. Endocr Rev. 17: 64-102, 1996.
[PubMed]
Bubenik GA. Gastrointestinal Melatonin: Localization, Function, and Clinical Relevance. Dig Dis Sci. 47: 2336-2348, 2002.
[CrossRef]
[PubMed]
Carlson B, Hanson LA. Immunologic effects of breast-feeding on the infant. In: Ogra P, Strober W, McGhee JR, Bienestock J (eds.). Handbook of Mucosal Immunology. London: Academic Press, pp. 653-666, 1994.
Carrichon L, Picciocchi A, Debeurme F, Defendi F, Beaumel S, Jesaitis A J, Dagher MC, Stasia MJ. Characterization of superoxide overproduction by the D-Loop Nox4-Nox2 cytochrome b558 in phagocytes - Differential sensitivity to calcium and phosphorylation events. Bioch Biophys Acta. 1808: 78-90, 2011.
[CrossRef]
[PubMed]
Cassone VM. The pineal gland influences rat circadian activity rhythms in constant light. J Biol Rhythms. 7: 27-40, 1992.
[CrossRef]
[PubMed]
Fagundes DLG, Franca EL, Hara CCP, Honorio-Franca AC. Immunomodulatory effects of poly (ethylene glycol) microspheres adsorbed with cortisol on activity of colostrum phagocytes. Int J Pharmacol. 8: 510-518, 2012.
[CrossRef]
Field CJ. The immunological components of human milk and their effect on immune development in infants. J Nutr. 135: 1-4, 2005.
[PubMed]
Filteau S. The influence of mastitis on antibody transfer to infants through breast milk. Vaccine. 24: 3377-3381, 2003.
[CrossRef]
Franca EL, Feliciano ND, Silva KA, Ferrari CKB, Honorio-Franca AC. Modulatory role of melatonin on superoxide release by spleen macrophages isolated from alloxan-induced diabetic rats. Bratisl Med J. 110: 517-522, 2009.
[PubMed]
Franca EL, Nicomedes TR, Calderon IMP, Honorio-Franca AC. Time-dependent alterations of soluble and cellular components in human milk. Biol Rhythm Res. 41: 333-347, 2010.
[CrossRef]
Franca EL, Bitencourt RV, Fujimori M, Morais TC, Calderon IMP, Honorio-Franca AC. Human colostral phagocytes eliminate enterotoxigenic Escherichia coli opsonized by colostrums supernatant. J Microbiol Immunol Infec. 44: 1-7, 2011a.
[CrossRef]
[PubMed]
Franca EL, Morceli G, Fagundes DLG, Rugde MVC, Calderon IMP, Honorio-Franca AC. Secretory IgA-Fcalpha Receptor interaction modulating phagocytosis and microbicidal activity by phagocytes in human colostrum of diabetics. APMIS. 119: 710-719, 2011b.
[CrossRef]
[PubMed]
Franca EL, Calderon IMP, Vieira EL, Morceli G, Honorio-Franca AC. Transfer of maternal immunity to newborns of diabetic mothers. Clin Develop Immunol. 2012: ID: 928187, 2012.
[CrossRef]
Franca-Botelho AC, Honorio-Franca AC, Franca EL, Gomes MA, Costa-Cruz JM. Phagocytosis of Giardia lamblia trophozoites by human colostral leukocytes. Acta Paediatr. 95: 438-443, 2006.
[CrossRef]
[PubMed]
Hanson LA. Feeding and infant development breast-feeding and immune fuction. Proc Nutrit Soc. 66: 384-396, 2007.
[CrossRef]
[PubMed]
Honorio-Franca AC, Carvalho MP, Isaac L, Trabulsi LR, Carneiro-Sampaio MMS. Colostral mononuclear phagocytes are able to kill Enteropathogenic Escherichia coli opsonized with colostral IgA. Scand J Immunol. 46: 59-66, 1997.
[CrossRef]
[PubMed]
Honorio-Franca AC, Launay P, Carneiro-Sampaio MMS, Monteiro RC. Colostral neutrophils express Fc alpha receptors (CD89) lacking gamma chain association and mediate noninflammatory properties of secretory IgA. J Leukoc Biol. 69: 289-296, 2001.
[PubMed]
Honorio-Franca AC, Silva KA, Feliciano ND, Calderon IMP, Rudge MVC, Franca EL. Melatonin effects on macrophage in diabetic rats and the maternal hyperglycemic implications for newborn rats. Int J Diabet Metabol. 17: 87-92, 2009.
Hooton JWL, Pabst HF, Spady DW, Paetkau V. Human colostrum contains an activity that inhibits the production of IL2. Clin Exp Immunol. 86: 520-524, 1991.
[CrossRef]
[PubMed]
Illnerova H, Buresova M, Presl J. Melatonin rhythm in human milk. J Clin Endocrinol Metab. 77: 838-841, 1993.
[CrossRef]
[PubMed]
Islam N, Ahmed L, Khan NI, Huque S, Begun A, Yunus ABM. Immune components (IgA, IgM, IgG immune cells) of colostrum of Bangladeshi mothers. Pediatr Int. 48: 543-548, 2006.
[CrossRef]
[PubMed]
Kennaway DJ, Goble FC, Stamp GE. Factors influencing the development of melatonin rhythmicity in human. J Clin Endocrinol Metab. 81: 1525-1532, 1996.
[CrossRef]
[PubMed]
Klepac N, Rudes Z, Klepac R. Effects of melatonin on plasma oxidative stress in rats with streptozotocin induced diabetes. Biomed Pharmacother. 60: 32-35, 2005.
[CrossRef]
[PubMed]
Korkmaz A, Topal T, Tan DX, Reiter RJ. Role of melatonin in metabolic regulation. Rev Endocr Metab Disord. 10: 261-270, 2009.
[CrossRef]
[PubMed]
Kumari S, Dash D. Melatonin elevates intracellular free calcium in human plaquets by inositol 1,4,5-triphosphate independent mechanism. FEBS Lett. 585: 2345-2351, 2011.
[CrossRef]
[PubMed]
Lawrence RA, Lawrence RM. Breastfeeding: A Guide for the Medical Profession. St. Louis, MO: Mosby-Year Book, pp. 105-170, 2005.
Ogasawara T, Adachi N, Nishijima M. Melatonin levels in maternal plasma before and during delivery, and in fetal and neonatal plasma. Nip Sanka Fuj Gak Zass. 43: 335-341, 1991.
[PubMed]
Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJM, Zisapel N. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 85: 335-353, 2008.
[CrossRef]
[PubMed]
Pawlak J, Singh J, Lea RW, Skwarlo-Sonta K. Effect of melatonin on phagocytic activity and intracellular free calcium concentration in testicular macrophages from normal and streptozotocin-induced diabetic rats. Mol Cell Biochem. 275: 207-213, 2005.
[CrossRef]
[PubMed]
Pick E, Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 46: 211-226, 1981.
[CrossRef]
Raqib R, Cravioto A. Nutrition, immunology, and genetics: future perspectives. Nutr Rev. 67: S227-S236, 2009.
[CrossRef]
[PubMed]
Rivkees AS, Hao H. Developing circadian rhythmicity. Semin Perinatol. 24: 232-242, 2000.
[CrossRef]
[PubMed]
Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 36: 1-9, 2004.
[CrossRef]
[PubMed]
Silva SO, Carvalho SRQ, Ximenes VF, Okada SS, Campa A. Melatonin and its kynurenin-like oxidation products affect the microbicidal activity of neutrophils. Microb Infect. 8420: 425, 2006.
Skwarlo-Sonta K. Bi-directional communication between pineal gland and immune system. J Physiol. 543(Suppl. 1): 6S-7S, 2002.
Sudnikovich EJ, Maksimchik YZ, Zabrodskaya SV, Kubyshin VL, Lapshina EA, Bryszewska M. Melatonin attenuates metabolic disorders due to streptoxotocin-induced diabetes rats. Eur J Pharmacol. 569: 180-187, 2007.
[CrossRef]
[PubMed]
Swaab DF, Hofman MA, Honnebier MB. Development of vasopressin neurons in the human suprachiasmatic nucleus in relation to birth. Dev Brain Res. 52: 289-293, 1990.
[CrossRef]
Zaidam R, Geoffriau M, Brun J, Taillard J, Bureau C, Chazot G, Claustrat B. Melatonin is able to influence its secretion in human: description of a phase-response curve. Neuroendocrinol. 60: 105-112, 1994.
[CrossRef]
|
BACK
|







