Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 11 (2013), No 3, p 163-172
DOI 10.2478/v10136-009-012-0022-y
Effects of glucocorticoids on cytochrome P450 1A1 (CYP1A1) expression in isolated human placental trophoblast
Lucie Stejskalova, Radim Vrzal, Alice Rulcova, Zdenek Dvorak, Petr Pavek
Address: Petr Pavek, Department of Pharmacology and Toxicology, Faculty of Pharmacy, Charles University in Prague, Heyrovskeho 1203, 500 05 Hradec
Kralove, Czech Republic
pavek@faf.cuni.cz
Received 14th August 2012.
Revised 10th October 2012.
Published online 18th October 2012.
Full text article (pdf)
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
Acknowledgements
References
SUMMARY
Antenatal glucocorticoid administration is used in cases of fetuses at risk to be born prematurely to enhance fetal pulmonary surfactant production and
prevent infant respiratory distress syndrome. The CYP1A1 is the most important xenobiotic-metabolizing cytochrome P450 enzyme in the human placenta.
Importantly, CYP1A1 generates reactive species and its placental activity is elevated in smoking women. CYP1A1 expression is mainly controlled by aryl
hydrocarbon receptor (AHR) ligands. Glucocorticoid co-regulation of CYP1A1 has been described in various cell types but has not been systematically
examined in the human placental trophoblast.
We studied the effects of the glucocorticoids dexamethasone and betamethasone on inducibility of CYP1A1 and other AHR target genes
CYP1A2, CYP1B1, UGT1A1 (UDP-glucuronosyltransferase 1A1) and BCRP (Breast cancer resistance protein) by prototype AHR ligand
-methylcholanthrene (3MC) in isolated human placental trophoblast culture.
We show that glucocorticoids alone had no effect on activity and protein/mRNA expression of CYP1A1 and little effect on mRNA expression of other
AHR target genes. However, glucocorticoids significantly stimulated CYP1A1 mRNA, but not CYP1A2, CYP1B1, UGT1A1 and BCRP
mRNAs, induction mediated by the AHR ligand. Consistently, glucocorticoids significantly augmented 7-ethoxyresorufin-O-deethylation (EROD)
enzymatic activity in primary human placental trophoblast. Dexamethasone did not influence AHR and ARNT (Aryl hydrocarbon receptor nuclear
translocator) mRNAs, suggesting that this phenomenon is not due to AHR or ARNT up-regulation by glucocorticoids in human trophoblast.
In conclusion, our data suggest that glucocorticoids have no effect on AHR target genes expression per se, but they may potentiate CYP1A1
induction in human term placental trophoblast.
KEY WORDS
trophoblast; aryl hydrocarbon receptor; CYP1A1; cytochrome P450; glucocorticoids; placenta
INTRODUCTION
Glucocorticoids are steroid hormones that are essential for development and maturation of fetal organs before birth. Glucocorticoids dexamethasone and
betamethasone are transplacentally administered before 35 weeks of pregnancy in fetuses destined to be born prematurely for increasing fetal pulmonary
surfactant production and preventing respiratory distress syndrome. Antenatal administration of synthetic glucocorticoids is also used for reducing
other pregnancy-associated complications, such as intrahepatic cholestasis, intraventricular hemorrhage, necrotizing enterocolitis and systematic
infection in the first hours of newborn life (Ballard and Ballard 1995, Roberts and Dalziel 2006, Hallman et al. 2010). The effects of glucocorticoids
in target cells are mediated by glucocorticoid receptor (GR). Recently, we and others have described expression and localization of GR in the human
placental syncytiotrophoblast and cytotrophoblast (Zhu and Lee 2005, Pavek et al. 2007) and in rodent placentas (Waddell et al. 1998).
Aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor which belongs to the bHLH/PAS (basic-Helix-Loop-Helix/Per-Arnt-Sim) family.
AHR is ubiquitously expressed, with the highest levels in lung, placenta, spleen and ovary (Dolwick et al. 1993, Yamamoto et al. 2004, Stejskalova et
al. 2011a). AHR controls the expression of a diverse set of genes (the so-called AHR gene battery) involved in biotransformation and transport
of both xenobiotic and endogenous compounds, such as the phase I enzymes, the cytochrome P450 enzymes CYP1A1, CYP1A2, CYP1B1; the phase II conjugation
enzymes UGT1A1 (UDP-glucuronosyltransferase 1A1), UGT1A6 and ALDH3A1 (Aldehyde dehydrogenase 3A1); and drug transporters such as BCRP (Breast cancer
resistance protein, ABCG2 gene) (Cygalova et al. 2008, Iqbal et al. 2012). Importantly, BCRP efflux transporter is highly expressed in placental
trophoblast, where it is involved in protection of the fetus against xenobiotics (Staud and Pavek 2005, Cygalova et al. 2008).
Numerous toxic compounds are AHR ligands and inducers of AHR target genes including polycyclic aromatic hydrocarbons (PAHs) and halogenated aromatic
hydrocarbons (HAHs). PAHs, such as benzo[a]pyrene, 3-methylcholanthrene (3MC) and pyrene, are produced during burning of fossil fuel and
garbage, and are components of cigarette smoke and grilled meat - see review (Stejskalova et al. 2011b). Thus, pregnant women are unavoidably exposed
to AHR ligands during their pregnancies. CYP1A1, a dominant target gene of AHR, is the only xenobiotic-metabolizing enzyme of the cytochrome P450
superfamily for which significant expression, catalytic activity and inducibility have been conclusively demonstrated in the placental trophoblast
throughout human pregnancy (Hakkola et al. 1996a, b, 1997, Stejskalova and Pavek 2011). Recently, we reported that only CYP1A1 mRNA, but not
other AHR target genes, is significantly up-regulated via AHR in isolated human placental cytotrophoblast culture (Stejskalova et al. 2011b). CYP1A1 is
involved in biotransformation of carcinogens and procarcinogens, such as PAHs and heterocyclic amines, and it generates reactive species that
afterwards may form adducts with DNA (Stejskalova and Pavek 2011).
Importantly, elevated CYP1A1 placental activity (determined as arylhydrocarbon hydroxylase activity) has been observed in smoking women with adverse
birth outcomes such as intrauterine growth retardation (Okey et al. 1997).
In addition to AHR ligands, both synthetic and endogenous glucocorticoids have been shown to be involved in transcription regulation of the
CYP1A1 gene. Although a role of GR in modulating CYP1A1 and CYP1A2 genes induction has been proven in both rodent and human
hepatic cells, the precise molecular mechanism is not yet fully understood (Monostory et al. 2005, Sonneveld et al. 2007, Dvorak et al. 2008, Vrzal et
al. 2009). The effect of glucocorticoids on CYP1A1 and others in the AHR gene battery has not been systematically studied in human placental
trophoblast. A sole report had shown that antepartum glucocorticoid therapy slightly suppresses EROD activity in placental samples (Paakki et al.
2000).
In this work, we aimed to study effects of the clinically relevant glucocorticoids dexamethasone (DEX) and betamethasone (BETA) on inducibility of the
AHR target gene battery by the prototype AHR ligand 3-methylcholanthrene (3MC) in human placental trophoblast cultures.
MATERIALS AND METHODS
Chemicals
Dimethyl sulfoxide (DMSO), 3-methylcholanthrene (3MC), dexamethasone (DEX), betamethasone (BETA), and cell culture media were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The final concentration of DMSO in culture media was 0.1% (v/v) in all experiments.
Human cytotrophoblast isolation and preparation of primary trophoblast cultures
Human placentas were obtained from 8 pregnancies, mostly after vaginal delivery between 34 and 39 weeks of gestation - see Table 1 (Nelson and Burton,
2010). The study was approved by the Ethics Committee of the Charles University in Prague. The study was performed in accordance with The Code of
Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and informed consent was obtained from all the women
involved. The isolation and culture of cytotrophoblasts from term human placentas had been originally reported by Kliman et al., and the modified
protocol described by Meyer zu Schwabedissen and coworkers (Kliman et al. 1986, Meyer Zu Schwabedissen et al. 2005, Pospechova et al. 2009) was used in
the study. Briefly, after mechanical and enzymatic dissection of human term placental tissue, cells were separated on density Percoll gradient. Cells
between the 40% and 50% Percoll bands were collected and plated at density 5x106 cells/plate (60 mm diameter). The cells were grown in M199
medium supplemented with 20% fetal bovine serum (FBS) (PAA, Pasching, Austria), 100 units/ml penicillin/streptomycin, L-glutamine (3 mM), and 5 ng/ml
epidermal growth factor (EGF) in a 5% CO2 humidified atmosphere at 37 °C. The cells were maintained in culture for 3 days before treatment
with glucocorticoids and 3MC to form syncytium. Human primary cytotrophoblast cells fuse into syncytium (syncytiotrophoblast) during cultivation in
this condition, which is the crucial component of the human placental barrier. The viability and syncytialization of trophoblast cultures were checked
by measuring human chorionic gonadotropin production (Pospechova et al. 2009).
Trophoblast cultures TR-H (21, 22, 24, 31, 34, 35) were isolated from the term placentas of Caucasian non-smokers, and TR-H (31) was from epileptic
women. TR-G (37) trophoblast cells were isolated from the placenta at gestational age 34 weeks of a non-smoking.
Real-time qRT-PCR analyses of CYP1A1, CYP1A2, CYP1B1, UGT1A1, BCRP, AHR, ARNT and AHRR mRNAs expression in primary trophoblast culture
The human placental trophoblast cells were cultured in M199 medium supplemented with 20% FBS, 100 units/ml penicillin/streptomycin, L-glutamine (3 mM),
and 5 ng/ml EGF in a 5% CO2 humidified atmosphere at 37 °C. Cells were treated for a period of 24 h with the AHR ligand 3MC (5 microM), the
glucocorticoids DEX (50 nM) or BETA (100 nM, 200 nM) alone or in combination with 3MC. After the treatment, the total RNA was isolated, reverse
transcribed and qRT-PCR was performed as we have described previously (Pospechova et al. 2009). Commercial TaqMan qRT-PCR detection systems were used
for CYP1A1, CYP1B1, CYP1A2, UGT1A1, AHR, ARNT (Aryl hydrocarbon receptor nuclear translocator) and AHRR (Aryl hydrocarbon receptor
repressor) genes expression analysis (Generi-Biotech, a. s., Hradec Kralove, Czech Republic). The expression of the target genes have been normalized
against total HPRT (hypoxanthine phosphoribosyltransferase) housekeeping gene expression. All samples were run in triplicates simultaneously
with negative controls as described in our previous report (Pospechova et al. 2009). These concentrations of dexamethasone and betamethasone used in
the work correlate with therapeutic plasma concentration of the glucocorticoids.
Western blotting analysis
For Western blotting experiments, cells were treated as described in section Real-time qRT-PCR analyses... Sample preparation and immunodetection of
CYP1A1 in total placental trophoblast cell lysates were performed according to a Western blotting protocol described previously (Vrzal et al.
2009).
7-ethoxyresorufin-O-deethylation (EROD) enzyme assay
7-Ethoxyresorufin-O-deethylation (EROD) activity was determined in a monolayer of trophoblast cells as we reported in our previous report (Vrzal et al.
2009). Briefly, the trophoblast cells (1x106 cells) were seeded on 24-well dishes in M199 medium supplemented with 20% FBS, 100 units/ml
penicillin/streptomycin, L-glutamine (3 mM), and 5 ng/ml EGF in a 5% CO2 humidified atmosphere at 37 °C. Cells were treated with 3MC (5
microM), DEX (50 nM) or BETA (100 nM) alone or together with 3MC, and DMSO as control. After 24 h or 48 h, the cells were washed twice with phosphate
buffer solution (0.01 M), containing NaCl, KCl, Na2HPO4, NaH2PO4, and incubated with Opti-MEM medium
(Gibco) containing 8 microM 7-ethoxyresorufin (a CYP1 substrate) and 10 microM dicumarol (Sigma-Aldrich) to prevent further metabolism of resorufin.
After 30 min of incubation at 37 °C, an aliquot of 75 microl of the medium was mixed with 100 microl of methanol and the amount of resorufin formed and
released into the extracellular matrix was quantified. The fluorescence of resorufin was measured at 535 nm excitation and 590 nm emission wavelengths
using a Tecan INFINITY M-200 Luminometer (Tecan Group, Mannedorf, Switzerland). Experiments were performed in triplicates.
Statistical analyses
All bars in the accompanying figures indicate means ± standard deviations (SD). Differences between the groups were compared using Student’s paired
two-tailed t-test or ANOVA followed by Tukey’s post hoc test. All statistical analyses were performed using GraphPad Prism 4 Software
(GraphPad Software Inc., San Diego, CA) at the significance level 2alpha=0.05.
RESULTS
Effect of glucocorticoid treatment on induction of CYP1A1 and BCRP mRNAs in human placental trophoblast culture
Glucocorticoid treatment of human trophoblast did not significantly affect the level of CYP1A1 mRNA (Table 1). We found that the expression of
CYP1A1 mRNA was significantly (Student’s paired two-tailed t-test, but not by ANOVA followed by Tukey’s post hoc test)
up-regulated by 3MC (fold induction ranges from 47 to 23,000) (Table 1). Importantly, the presence of glucocorticoids significantly potentiated the
CYP1A1 mRNA induction by 3MC (statistically significant; Student’s paired two-tailed t-test) in trophoblast cultures (Table 1).
Next we analyzed the effect of the glucocorticoids and 3MC on BCRP transporter gene mRNA expression. We did not observe statistically
significant (analyzed employing Student’s paired two-tailed t-test and ANOVA followed by Tukey’s post hoc test) induction of BCRP
mRNA by 3MC (Table 2). Glucocorticoids alone (DEX and BETA) did not significantly affect the expression of BCRP mRNA. Finally, BCRP mRNA expression was
not significantly augmented by DEX relative to 3MC-treated samples, although we observed up-regulation of the mRNA level after co-treatment with DEX in
some trophoblast preparations (Table 2).
Table 1. CYP1A1 mRNA fold induction normalized to a HPRT housekeeping gene and related to vehicle-treated cells (set to be 1) after 24
h-glucocorticoid treatment in the absence or presence of the prototype AHR ligand 3-methylcholanthrene (3MC, 5 microM) in primary human trophoblast
cultures.
| CYP1A1 mRNA fold
induction |
| Treatment |
Trophoblast
preparation |
TR-H
(21) |
TR-H
(22) |
TR-H
(24) |
TR-H
(34) |
TR-H
(35) |
TR-H
(31) | |
statistical
significance |
|
|
|
|
|
| | glucocorticoid |
N.S |
1.3 |
2.2 |
4.4 |
4.1 |
4.0 |
4.4 | | 3MC 5 microM |
* |
151.1 |
489.7 |
5507.4 |
2209.8 |
2457.1 |
1048.8 | | 3MC/glucocorticoid |
* |
380.2 |
2228.1 |
5084.5 |
4071.0 |
4231.7 |
3895.2 | |
* to 3MC-treated |
|
|
|
|
|
|
* * Statistical significance to vehicle-treated controls (set to be 1) in non-smokers TR-H21, TR-H22, TR-H24, TR-H31 analyzed
employing Student's paired t-test. N.S. - not statistically significant.
Table 2. BCRP mRNA fold induction normalized to a HPRT housekeeping mRNA expression and related to vehicle-treated
cells (set to be 1) after 24 h-glucocorticoid treatment in the absence or presence of the prototype AHR ligand
3-methylcholanthrene (3MC, micro5 M) in primary human trophoblast cultures.
|
BCRP mRNA fold
induction | | Treatment |
Trophoblast
preparation |
TR-H
(21) |
TR-H
(22) |
TR-H
(24) |
TR-H
(34) |
TR-H
(35) |
TR-H
(31) | |
statistical
significance |
|
|
|
|
|
| | glucocorticoid |
N.S |
1.5 |
1.9 |
4.2 |
0.8 |
8.2 |
5.3 | | 3MC 5 microM |
N.S. |
1.6 |
2.8 |
5.7 |
1.7 |
13,6 |
1.9 | | 3MC/glucocorticoid |
N.S. |
0.4 |
3.7 |
6.6 |
1.8 |
36.2 |
8.9 | |
N.S. to 3MC-treated |
|
|
|
|
|
|
Symbols as in Table 1.
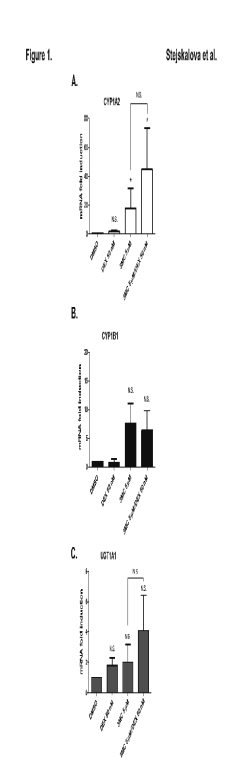
Fig. 1. Effect of dexamethasone (DEX; 50 nM) and 3-methylcholanthrene (3MC; 5 microM) on CYP1A2 (A), CYP1B1 (B) and UGT1A1 (C)
mRNAs expression in human placental trophoblast cultures. Trophoblast cultures were treated with DEX, 3MC, combination of DEX and 3MC, and/or with
DMSO as control, for an interval of 24 h. Target genes’ mRNA expression was evaluated using qRT-PCR. The expression of tested genes in control
(DMSO-treated) cells was set to 1 and the data were expressed as the mean ± SD of 4 independent trophoblast preparations [n = 4; TR-H (21), TR-H
(22), TR-H (24), TR-H (34)]. Symbols as in Table 1.
Effect of DEX on induction of other AHR target genes CYP1A2, CYP1B1 and UGT1A1 mRNAs in human placental trophoblast cultures
Next we analyzed the effects of DEX (50 nM), 3MC (5 microM), and their combination (DEX with 3MC) on mRNAs induction of the other AHR target genes
CYP1A2, CYP1B1, and UGT1A1 in 4 human trophoblast cultures [TR-H (21, 22, 24, 34)] isolated from non-smoking women.
We found that DEX had no statistically significant effects on basal expression of CYP1A2, CYP1B1 and UGT1A1 mRNAs (Fig. 1A, B, C). 3MC
significantly induced CYP1A2 mRNA (p<0.05, Fig. 1A), although the overall expression of CYP1A2 mRNA was very low and close to the
detection limit of the qRT-PCR method used. In subsequent experiments, we found that 3MC did not significantly affect the expression of CYP1B1
and UGT1A1 mRNAs (Fig. 1B, C).
Co-treatment using 3MC with DEX did not result in statistically significant up-regulation of CYP1A2 mRNA expression when compared to 3MC-treated
cells (Fig. 1A). Similarly, DEX did not significantly potentiate CYP1B1 and UGT1A1 mRNA induction by 3MC (Fig. 1B, C).
Effect of DEX on induction of AHR, ARNT and AHRR mRNAs in human placental trophoblast cultures
In the next experiments, we studied the effects of 3MC and DEX on the expression of AHR, ARNT and AHRR mRNAs. We observed that DEX and
3MC alone did not significantly affect the levels of AHR, ARNT and AHRR mRNAs. Combination of DEX and 3MC also did not significantly
change the level of AHR, ARNT and AHRR mRNAs in the studied trophoblast preparations (Fig. 2A, B, C).
Enzymatic activity of CYP1A1 in trophoblast cultures after treatment with DEX, 3MC or their combination analyzed by EROD assay
Next, we examined the effect of 3MC (5 microM) and glucocorticoids DEX (50 nM) and BETA (100 nM) on EROD activity in isolated trophoblast cultures. In
both time intervals examined (24 h and 48 h), 3MC caused induction of CYP1A1 EROD activity (3-fold after 24 h and 3.5-fold after 48 h of treatment)
(Fig. 3A). DEX and BETA alone did not significantly influence the EROD activity (Fig. 3A). Consistent with the data presented in Table 1, co-treatment
using 3MC with DEX significantly augmented EROD activities by about 1.6-fold relative 3MC-treated samples in both time intervals (Fig. 3A). BETA also
significantly increased (1.2-fold, statistically significant) the 3MC-mediated induction of EROD activity in trophoblast cells (Fig. 3A).
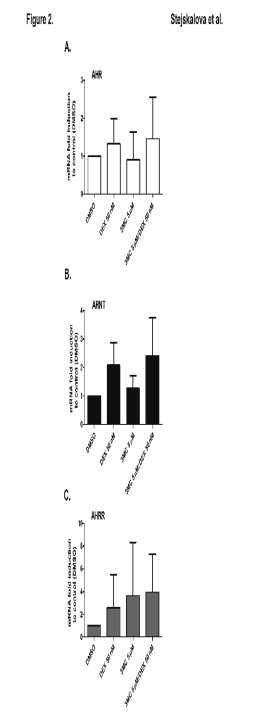
Fig. 2. Effect of dexamethasone (DEX; 50 nM) and
3-methylcholanthrene (3MC; 5 microM) on AHR (A), ARNT
(B) and AHRR (C) mRNAs expression in human
placental trophoblast cultures. Trophoblast cultures were
treated with DEX, 3MC, combination of DEX and 3MC,
and/or with DMSO for an interval of 24 h. mRNAs were
then evaluated using qRT-PCR. The expression of tested
genes in control (DMSO-treated) cells was set to 1 and the
data were expressed as the mean ± SD of 5 independent
isolated trophoblast preparations [n = 5; TR-H (21), TR-H (22), TR-H (24), TR-H (25), TR-H (34)].
We also analyzed CYP1A1 protein expression in selected human trophoblast cultures TR-H (24), TR-H (34) and TR-G (37) using Western blotting. CYP1A1
protein expression was significantly induced by 3MC in all trophoblast cultures used (Fig. 3B). However, DEX had no obvious effect on CYP1A1 protein
expression in cultures treated simultaneously with 3MC in comparison with 3MC-treated cells without glucocorticoids [Fig. 3B, representative experiment
in TR-G (37)]. This discrepant observation may be the consequence of the low quantitative sensitivity of Western blotting method.
DISCUSSION
In this study, we described for the first time the effects of glucocorticoids on expression and induction of AHR target genes in isolated human
placental trophoblast. In particular, we concentrated on expression and activity of CYP1A1 enzyme, which is the most important xenobiotic-metabolizing
enzyme of the human placental barrier formed by continuous trophoblast layer. In addition, we evaluated the effect of glucocorticoid receptor
activation on AHR-mediated regulation of BCRP transporter gene, which significantly contributes to the placental barrier function and to
protection of the fetus against xenobiotics (Cygalova et al. 2008, Iqbal et al. 2012).
We showed that glucocorticoids per se had no significant effect on mRNA expression of the AHR target genes CYP1A1, CYP1A2, CYP1B1, UGT1A1 and
BCRP. In addition, the basal level of CYP1A1 protein and basal CYP1A1 enzymatic activity were also not significantly affected by
glucocorticoids. In contrast, we report herein that induction of CYP1A1 by prototype AHR ligand 3MC was augmented by glucocorticoids in human
placental trophoblast cultures of the term placentas. Expression of other AHR target genes CYP1A2, CYP1B1, UGT1A1 and BCRP was not
influenced by glucocorticoids in human trophoblast cultures. We observed the similar phenomenon of DEX on AHR ligand-induced CYP1A1 in BeWo
cells, but not in JEG-3 choriocarcinoma cell line (data will be presented elsewhere). These results correlate with an earlier study in the BeWo
placental choriocarcinoma cell line, where DEX increased EROD activity after treatment with the AHR ligand beta-naphthoflavone (Avery et al.
2003).
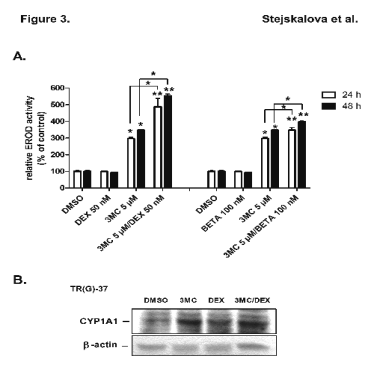
Fig. 3. (A) Effects of dexamethasone (DEX; 50 nM), betamethasone (BETA; 100 nM) and 3-methylcholanthrene (3MC;
5 microM) on 7-ethoxyresorufin-O-deethylation (EROD) activity in human placental trophoblast culture. Trophoblast cultures
TR-H (24) and TR-H (34) was treated with 3MC, DEX, BETA, 3MC and DEX, or 3MC and BETA. CYP1A1 activity
represented by EROD activity was measured by fluorescence spectrometry as described in the Methods. Experiments were
performed in two independent preparations in triplicates. Data from trophoblast TH-H(34) are presented. (B) Expression of
CYP1A1 protein in a representative human placental trophoblast culture TR-G (37). Trophoblast culture was treated for
24 h with DEX (50 nM), 3MC (5 microM), combination of DEX and 3MC, and/or DMSO as vehicle and in control wells. Total
cellular fractions were isolated and Western blot analysis of CYP1A1 proteins was performed with primary goat polyclonal
antibody against CYP1A1 (G-18, Santa Cruz Biotechnology). Symbols as in Table 1.
In the next experiments, we studied CYP1A1 enzymatic activity in human trophoblast culture after exposure to glucocorticoids together with AHR ligand.
Although EROD activity is specific for CYP1 enzymes, CYP1A2 and CYP1B1 mRNA expression is negligible when compared to CYP1A1 in
full-term placental trophoblast and CYP1B1 mRNA is not inducible in placental cells (Fig. 1B) (Hakkola et al. 1997). Therefore, we could suppose
that EROD activity represents specific activity of CYP1A1 in placental trophoblast in our experiments. Consistently with qRT-PCR experiments, we
confirmed that DEX is able to potentiate activity of CYP1A1 induced by the AHR ligand 3MC in primary human trophoblast cultures (Table 1, Fig. 3).
Since the effects of glucocorticoids on AHR target genes expression had not heretofore been studied in normal placental trophoblast either in
vitro or in vivo, we can compare our current results in human placental trophoblast with data we obtained in primary human hepatocytes and
hepatoma cells. In primary culture of human hepatocytes, we and others have observed that DEX did not significantly affect 3MC- and TCDD-mediated
induction of CYP1A1 mRNA expression (Monostory et al. 2005, Vrzal et al. 2009). However, DEX caused statistically significant up-regulation of
AHR ligand-dependent CYP1A2 mRNA induction (Vrzal et al. 2009). Similar to the data in the case of placental trophoblast, basal CYP1A1
and CYP1A2 mRNA expression was not affected by DEX in primary human hepatocytes (Vrzal et al. 2009). Synergistic effect of DEX and AHR/Ahr
ligands on Cyp1a1 mRNA induction was also demonstrated in primary culture of rat hepatocytes (Monostory et al. 2005) and in rat hepatocarcinoma
cell line H4IIe, where this effect was proposed to be dependent on a post-transcriptional process (Lai et al. 2004, Sonneveld et al. 2007). Sonneveld
and his colleagues also found that DEX potentiated the TCDD-mediated induction of other Ahr-dependent genes Cyp1b1 and Cyp1a2 in rat
H4IIe cell line (Sonneveld et al. 2007).
In the next experiment, we found that placental AHR, ARNT and AHRR mRNA expression was not significantly affected by DEX alone or by DEX
in combination with 3MC in primary human trophoblast cultures (Fig. 2). Therefore we cannot explain the synergistic effect of AHR and GR co-activation
on CYP1A1 expression via AHR and ARNT up-regulation or AHRR repressor down-regulation after treatment of primary human
trophoblast with DEX. Therefore our ongoing experiments are focused on the effects of glucocorticoids on coactivators and corepressors regulation by
glucocorticoids in the placental trophoblast. In addition, we examine whether glucocorticoids affect translocation and DNA binding of AHR/ARNT complex
into promoter response elements in the placental trophoblast.
In conclusion, we herein provide evidence that there exists a functional cross-talk between cellular signaling of AHR and GR receptors in
transcriptional regulation of the CYP1A1 gene in human placental trophoblast culture. This finding can have important clinical and toxicological
implications since numerous women are treated with glucocorticoids during pregnancy for various indications (Lunghi et al. 2010, Marciniak et al. 2011)
and most pregnant women are exposed to AHR ligands, either from environmental pollution or diet. These xenobiotic are usually metabolized by CYP1A1
into reactive compounds and procarcinogens that can form DNA adducts in the placenta and the fetus. Consequently, we can suppose that the fetus is at
higher risk of adverse birth outcomes if placental CYP1A1 activity is up-regulated by AHR ligands and the expression is stimulated by
glucocorticoids.
We therefore believe that prospective clinical trials are needed to study the association between glucocorticoid therapy during pregnancy, placental
CYP1A1 expression and pregnancy outcome. Ongoing experiments in our laboratory are aimed to extend the study in further primary trophoblast
preparations from term and preterm placentas, to elucidate the mechanistic nature of the phenomenon and bring clear evidence that the DEX effect is
mediated via stimulation of CYP1A1 gene transcriptional regulation in the placental trophoblast.
ACKNOWLEDGEMENTS
This work was supported by grants CZ.1.05/2.1.00/01.0030, GACR 303/12/G163, GACR 503/10/0579 and GACR 304/10/0149.
REFERENCES
Avery ML, Meek CE, Audus KL. The presence of inducible cytochrome P450 types 1A1 and 1A2 in the BeWo cell line. Placenta. 24: 45-52, 2003.
[CrossRef]
[PubMed]
Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol. 173: 254-262, 1995.
[CrossRef]
Cygalova L, Ceckova M, Pavek P, Staud F. Role of breast cancer resistance protein (Bcrp/Abcg2) in fetal protection during gestation in rat. Toxicol
Lett. 178: 176-180, 2008.
[CrossRef]
[PubMed]
Dolwick KM, Schmidt JV, Carver LA, Swanson HI, Bradfield CA. Cloning and expression of a human Ah receptor cDNA. Mol Pharmacol. 44: 911-917, 1993.
[PubMed]
Dvorak Z, Vrzal R, Pavek P, Ulrichova J. An evidence for regulatory cross-talk between aryl hydrocarbon receptor and glucocorticoid receptor in HepG2
cells. Physiol Res. 57: 427-435, 2008.
[PubMed]
Hakkola J, Pasanen M, Hukkanen J, Pelkonen O, Maenpaa J, Edwards RJ, Boobis AR, Raunio H. Expression of xenobiotic-metabolizing cytochrome P450 forms
in human full-term placenta. Biochem Pharmacol. 51: 403-411, 1996a.
[CrossRef]
Hakkola J, Raunio H, Purkunen R, Pelkonen O, Saarikoski S, Cresteil T, Pasanen M. Detection of cytochrome P450 gene expression in human placenta in
first trimester of pregnancy. Biochem Pharmacol. 52: 379-383, 1996b.
[CrossRef]
Hakkola J, Pasanen M, Pelkonen O, Hukkanen J, Evisalmi S, Anttila S, Rane A, Mantyla M, Purkunen R, Saarikoski S, Tooming M, Raunio H. Expression of
CYP1B1 in human adult and fetal tissues and differential inducibility of CYP1B1 and CYP1A1 by Ah receptor ligands in human placenta and cultured cells.
Carcinogenesis. 18: 391-397, 1997.
[CrossRef]
[PubMed]
Hallman M, Peltoniemi O, Kari MA Enhancing functional maturity before preterm birth. Neonatology. 97: 373-378, 2010.
[CrossRef]
[PubMed]
Iqbal M, Audette MC, Petropoulos S, Gibb W, Matthews SG. Placental drug transporters and their role in fetal protection. Placenta. 33: 137-142,
2012.
[CrossRef]
[PubMed]
Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF, 3rd. Purification, characterization, and in vitro differentiation of
cytotrophoblasts from human term placentae. Endocrinology. 118: 1567-1582, 1986.
[CrossRef]
[PubMed]
Lai KP, Wong MH, Wong CK. Modulation of AhR-mediated CYP1A1 mRNA and EROD activities by 17beta-estradiol and dexamethasone in TCDD-induced H411E cells.
Toxicol Sci. 78: 41-49, 2004.
[CrossRef]
[PubMed]
Lunghi L, Pavan B, Biondi C, Paolillo R, Valerio A, Vesce F, Patella A. Use of glucocorticoids in pregnancy. Curr Pharm Des. 16: 3616-3637, 2010.
[CrossRef]
[PubMed]
Marciniak B, Patro-Malysza J, Poniedzialek-Czajkowska E, Kimber-Trojnar Z, Leszczynska-Gorzelak B, Oleszczuk J. Glucocorticoids in pregnancy. Curr
Pharm Biotechnol. 12: 750-757, 2011.
[PubMed]
Meyer Zu Schwabedissen HE, Grube M, Heydrich B, Linnemann K, Fusch C, Kroemer HK, Jedlitschky G. Expression, localization, and function of MRP5
(ABCC5), a transporter for cyclic nucleotides, in human placenta and cultured human trophoblasts: effects of gestational age and cellular
differentiation. Am J Pathol. 166: 39-48, 2005.
[CrossRef]
Monostory K, Kohalmy K, Prough RA, Kobori L, Vereczkey L. The effect of synthetic glucocorticoid, dexamethasone on CYP1A1 inducibility in adult rat and
human hepatocytes. FEBS Lett. 579: 229-235, 2005.
[CrossRef]
[PubMed]
Nelson DM, Burton GJ. A technical note to improve the reporting of studies of the human placenta. Placenta. 32: 195-196, 2010.
[CrossRef]
[PubMed]
Okey AB, Giannone JV, Smart W, Wong JM, Manchester DK, Parker NB, Feeley MM, Grant DL, Gilman A. Binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin to AH
receptor in placentas from normal versus abnormal pregnancy outcomes. Chemosphere. 34: 1535-1547, 1997.
[CrossRef]
Paakki P, Kirkinen P, Helin H, Pelkonen O, Raunio H, Pasanen M. Antepartum glucocorticoid therapy suppresses human placental xenobiotic and steroid
metabolizing enzymes. Placenta. 21: 241-246, 2000.
[CrossRef]
[PubMed]
Pavek P, Cerveny L, Svecova L, Brysch M, Libra A, Vrzal R, Nachtigal P, Staud F, Ulrichova J, Fendrich Z, Dvorak Z. Examination of Glucocorticoid
receptor alpha-mediated transcriptional regulation of P-glycoprotein, CYP3A4, and CYP2C9 genes in placental trophoblast cell lines. Placenta. 28:
1004-1011, 2007.
[CrossRef]
[PubMed]
Pospechova K, Rozehnal V, Stejskalova L, Vrzal R, Pospisilova N, Jamborova G, May K, Siegmund W, Dvorak Z, Nachtigal P, Semecky V, Pavek P. Expression
and activity of vitamin D receptor in the human placenta and in choriocarcinoma BeWo and JEG-3 cell lines. Mol Cell Endocrinol. 299: 178-187, 2009.
[CrossRef]
[PubMed]
Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev.
19(3):CD004454, 2006.
[PubMed]
Sonneveld E, Jonas A, Meijer OC, Brouwer A, van der Burg B. Glucocorticoid-enhanced expression of dioxin target genes through regulation of the rat
aryl hydrocarbon receptor. Toxicol Sci. 99: 455-469, 2007.
[CrossRef]
[PubMed]
Staud F, Pavek P. Breast cancer resistance protein (BCRP/ABCG2). Int J Biochem Cell Biol. 37: 720-725, 2005.
[CrossRef]
[PubMed]
Stejskalova L, Pavek P. The function of cytochrome P450 1A1 enzyme (CYP1A1) and aryl hydrocarbon receptor (AhR) in the placenta. Curr Pharm Biotechnol.
12: 715-730, 2011.
[PubMed]
Stejskalova L, Dvorak Z, Pavek P. Endogenous and exogenous ligands of aryl hydrocarbon receptor: current state of art. Curr Drug Metab. 12: 198-212,
2011a.
[CrossRef]
[PubMed]
Stejskalova L, Vecerova L, Perez LM, Vrzal R, Dvorak Z, Nachtigal P, Pavek P. Aryl hydrocarbon receptor (AHR) and aryl hydrocarbon nuclear translocator
(ARNT) expression in human and rat placentas and transcription activity in human trophoblast cultures. Toxicol Sci. 123: 26-36, 2011b.
[CrossRef]
[PubMed]
Vrzal R, Stejskalova L, Monostory K, Maurel P, Bachleda P, Pavek P, Dvorak Z. Dexamethasone controls aryl hydrocarbon receptor (AhR)-mediated CYP1A1
and CYP1A2 expression and activity in primary cultures of human hepatocytes. Chem Biol Interact. 179: 288-296, 2009.
[CrossRef]
[PubMed]
Waddell BJ, Benediktsson R, Brown RW, Seckl JR. Tissue-specific messenger ribonucleic acid expression of 11beta-hydroxysteroid dehydrogenase types 1
and 2 and the glucocorticoid receptor within rat placenta suggests exquisite local control of glucocorticoid action. Endocrinology. 139: 1517-1523,
1998.
[CrossRef]
[PubMed]
Yamamoto J, Ihara K, Nakayama H, Hikino S, Satoh K, Kubo N, Iida T, Fujii Y, Hara T. Characteristic expression of aryl hydrocarbon receptor repressor
gene in human tissues: organ-specific distribution and variable induction patterns in mononuclear cells. Life Sci. 74: 1039-1049, 2004.
[CrossRef]
[PubMed]
Zhu BT, Lee AJ. NADPH-dependent metabolism of 17beta-estradiol and estrone to polar and nonpolar metabolites by human tissues and cytochrome P450
isoforms. Steroids. 70: 225-244, 2005.
[CrossRef]
[PubMed]
|
BACK
|




