Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 11 (2013), No 3, p 131-141
DOI 10.2478/v10136-012-0019-6
Regulatory effect of beta-catenin on proliferation of hair follicle stem cells involves PI3K/Akt pathway
Yi Zhang, Jin Yu, Chunying Shi, Yun Wang, Jin Yang, Tian Yang
Address: Yi Zhang, Department of Cell Biology, Third Military Medical University, Chongqing, China
honeyzhang2@gmail.com
Received 29th May 2012.
Revised 28th August 2012.
Published online 30th August 2012.
Full text article (pdf)
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
Acknowledgements
References
SUMMARY
Beta-catenin signaling is required for hair follicle development and regeneration which are involved in the resuscitation of hair follicle stem cells (HFSCs). To further characterize the role of beta-catenin in the regulation of proliferation of HFSCs, the beta-catenin expression was measured in the defined stages of hair follicle cycle and the proliferative potency was determined by using an in vitro cell growth assay. Our results showed that activation of beta-catenin correlated with HFSCs proliferation, which appeared to be mediated by the nuclear translocation of stabilized beta-catenin and the activation of responsible cell cycle genes (cyclin D1 and p21). In addition, PI3K/Akt pathway was also involved in the HFSCs proliferation, partly regulated by beta-catenin signaling pathway. These results demonstrate that beta-catenin is an essential factor in the regulation of HFSCs proliferation via PI3K/Akt pathway and might be a potential therapeutic target for the regulation of the yield of keratinocytes from HFSCs.
KEY WORDS
beta-catenin; hair follicle stem cell; proliferation; PI3K/Akt; cyclin D1; p21
INTRODUCTION
Hair follicle is one of the important appendages in the skin with a complex structure, including outer root sheath (ORS), inner root sheath (IRS) and hair shaft (HS). Studies have confirmed that a repository of progenitor cells exist in the bulge region, specialized portion in the ORS of upper hair follicle (Oshima et al. 2001). In previous studies, epithelial stem cells in the bulge region, termed as hair follicle stem cells (HFSCs), were found to be unable to proliferate in vivo, while behavior with superior clonogenicity and proliferative capacity in the cultural medium (Kobayashi et al. 1993). Subsequent studies displayed that epidermal appendages were related to the epidermal repair and regeneration (Taylor et al. 2000, Oshima et al. 2001), which was explained by the fact that HFSCs were able to migrate not only downwards to participate in the formation of hair follicles, but also upwards to maintain the renewal of epidermis and sebaceous gland. Thus, HFSCs were regarded as the stem cells with the most potent proliferative potential in the skin and as an essential cell resource of epidermis, hair follicle and sebaceous gland.
Generally, the hair follicle undergoes dynamic changes from an actively growing phase (anagen), to a remodeling phase (catagen), and finally to a quiescent phase (telogen). Investigators have shown that the hair follicle can enter a new anagen from the last telogen when the quiescent stem cells are stimulated to proliferate in response to signals from the dermal papilla, resulting in the initiation of new hair follicle cycle. Under this condition, HFSCs may give rise to transient amplifying cells (TACs) by undergoing a transient period of cell proliferation. Then, the rapidly proliferative TACs differentiate and regenerate daughter cells of hair follicle. Wnt signal has been identified as a key regulator responsible for hair follicle development and cycle maintenance (Suzuki et al. 2009). beta-catenin is an essential molecule in the Wnt signaling pathway, and can bind to the E-cadherin tightly at the plasma membrane when the cells receive no Wnt signal. In the cytoplasm, free beta-catenin is phosphorylated by glycogen synthase kinase-3beta (GSK-3beta) and undergoes degradation via the ubiquitin-proteasome mediated pathway. However, inhibition of GSK-3beta results in beta-catenin stabilization and accumulation (Reya and Clevers 2005). Excessive beta-catenin will translocate into the nucleus where it binds to the TCF/LEF transcription factors and then induces the transcription of target genes. Investigators have reported that the activation of beta-catenin signaling is essential during the first stage of hair follicle morphogenesis, as evidenced by the absence of hair formation on conditional ablation of beta-catenin or over-expression of the Wnt inhibitor Dkk during the embryogenesis (Sick et al. 2006, Ito et al. 2007).
Increasing evidence indicates that the phosphoinositide-3 kinase (PI3K/Akt) signaling can regulate the cell proliferation, growth, migration and apoptosis, and plays essential roles in the cancer development and progression (Ciraolo et al. 2011). Recent studies prove that PI3K/Akt signaling also regulates the self-renewal and differentiation of stem cells (Kim et al. 2010). Perry et al. found that the self-renewal and expansion of hematopoietic stem cells depended on the simultaneous activation of both Wnt/beta-catenin and PI3K/Akt signaling (Perry et al. 2011). Furthermore, there is evidence showing that the PI3K/Akt signaling may impact the activation of Wnt pathway by phosphorylation the GSK-3beta, leading to the beta-catenin ubiquitination and degradation (Zhou et al. 2004, Lee et al. 2010). Although PI3K/Akt pathway has been reported to regulate the activation of Wnt/beta-catenin signaling, the role of crosstalk between Wnt and PI3K in HFSCs proliferation remains poorly understood.
In this study, to reveal the mechanism underlying the regulation of HFSCs proliferation, we focus on the Wnt signaling pathway. Results showed that beta-catenin mediated the proliferation of HFSCs via up-regulating cyclin D1 expression or down-regulating p21 expression, which is partly dependent on the activity of PI3K/Akt signaling. Our findings provide evidence on the direct link between beta-catenin and HFSCs proliferation.
MATERIALS AND METHODS
Tissue preparation and cell culture
Back skin specimens were obtained from 15, 20, 28 days-old rats by plastic surgery. Pieces were washed with PBS, fixed in 4% paraformaldehyde overnight and embedded in paraffin. Serial sections (5 micro) were obtained with a cryostat (Leica).
Primary HFSCs were obtained from the bulge region in the upper hair follicle as previously described (Zhang et al. 2006). These cells were initially maintained in the dulbecco's modified Eagle's medium (DMEM) and Ham's F12 medium (3:1) (Hyclone), supplemented with 10% fetal bovine serum (FBS; Hyclone), 10 ng/ml epidermal growth factor (EGF; Sigma), 0.5 microg/ml hydrocortisone (Sigma), and antibiotics (100 U/ml penicillin and 100 microg/ml streptomycin). Then, the medium was refreshed with K-SFM (Invitrogen) followed by culture and passaging. HFSCs were treated with Wnt agonist (LiCl, Sigma), PI3K inhibitor (ly294002, Sigma) or agonist (insulin, Sigma) for further experiments. Cell morphology was observed under a microscope once daily. To evaluate the effects of LiCl at different concentrations on cell growth, mouse myeloma cells (SP2/0, ATCC), as control, were grown in RPMI 1640 medium containing 10% donor calf serum. All these cells were maintained at 37 °C in a humidified environment with 5% CO2.
Immunohistochemistry and immunocytochemistry
Tissue sections were deparaffinized in xylene, rehydrated in graded ethanol and washed in PBS. After endogenous peroxidase and non specific binding were blocked with 1% H2O2 and 10% goat serum (Sigma), respectively, the specimens were incubated with anti-beta-catenin antibody overnight at 4 °C. Visualization was done by using a streptavidin-horseradish peroidase labeling kit (SP kit) and diaminobenzidine (DAB, Beijing Zhongshan Biotechnology Co.) staining according to the manufacturer's instructions. Counterstaining with hematoxylin was performed if necessary.
For cell detection, cells seeded on glass coverslips were washed with PBS after treatment and fixed in ice cold acetone for 10 min. The endogenous peroxidase and non-specific binding were blocked by incubation with H2O2 and serum, respectively, at room temperature followed by incubation with antibody overnight at 4 °C. Immunolabeling was done according to the manufacturer's instructions.
Cell proliferation assay
MTT (3-[4, 5-dimethylthiazol-2-yl]-2,5diphenyltetrazolium bromide) solution (Sigma) was used to evaluate the cell growth. Cells were plated in 96-well plates. At 24 h after culture at 37 °C, LiCl at different concentrations (0, 5, 10, 20, 40 and 100 microM) was added into these cells 2 d before the examination. In other experiments, cells were grown in medium containing 10 mM LiCl, and the proliferation was determined at 0, 1, 2, 3 and 4 d after treatment. For inhibition or activation of PI3K/Akt signaling pathway, 10 microM LY294002 (Sigma) and 1 microg/ml insulin (Sigma) was added independently. Then, the optical density (OD) was measured using a spectrophotometer (Bio-Rad) in the presence or absence of insulin or LiCl. The cell growth was expressed as a percentage compared to that in the control group. The experiments were repeated at least three in triplicate.
BrdU incorporation
Before immunostaining, HFSCs were incubated with 10 microM 5-bromo-2-deoxyuridine (BrdU, Sigma) for 2 h. Cells were incubated with mouse anti-BrdU antibody (1:100, Roche, UK) overnight at 4 °C and then with goat anti-mouse secondary antibody for 60 min at room temperature. After addition of DAB, the proportion of BrdU-positive cells was calculated and t test was used for statistical analysis.
Western blot assay
The proteins of equal amount were subjected to SDS-PAGE, and then transferred onto a PVDF membrane (Amersham) by electroblotting. After incubation overnight at 4 °C with primary antibody (Santa Cruz), the proteins were detected by using a protein detection kit (Pierce, USA) according to the manufacturer's instructions, and densitometry was performed by using the QuanlityOne 4.3 (Bio-Rad). Membranes were stripped and reprobed with primary antibody against beta-actin as a control.
RNA interference
An unscrambling control shRNA (NC) and a lentiviral particle expressing beta-catenin specific shRNA were used to infect HFSCs in the presence of 5 microg/ml polybrene (Sigma-Aldrich). The cell growth was measured by MTT assay as described above, and cellular protein was obtained for western blot assay.
Luciferase assay
TOPflash and FOPflash are widely used to evaluate the activation of beta-catenin-dependent signaling. After transient transfection of the TOP-Flash and FOP-Flash plasmids, the cells were incubated for 24 h, and the luciferase activity in the cell lysates was determined by using a luminometer. The Luciferase Reporter Assay System was purchased from Promega.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). Statistical analysis was performed in the Microsoft EXCEL. The statistically significant difference was evaluated using the Student's t-test, and the difference was compared at the significance level at 2alpha=0.05.
RESULTS
beta-catenin expression in hair follicle cycles in vivo and cultured HFSCs in vitro
The expression of beta-catenin was measured at different stages of the hair follicle cycle using immunohistochemistry (Fig. 1A). In the anagen follicles, beta-catenin positive cells were confined to the ORS and IRS. Especially, the matrix strong positive for beta-catenin contained the epithelial cells that could migrate upwards to form the cortex and hair shaft. In addition, beta-catenin located in the nucleus of dermal papilla (DP) cells, but not at the cell membrane (Fig. 1B), suggesting a regulatory mechanism related to the Wnt/beta-catenin signaling pathway. In the catagen, a process typified by apoptosis of epithelial cells in the bulb and ORS, the beta-catenin was expressed weakly in the ORS, IRS and DP. In the telogen follicles, the dermal papilla migrated upwards to the base of the permanent epithelial portion of the follicle. beta-catenin protein was less detected in the cells of bulge or ORS region.
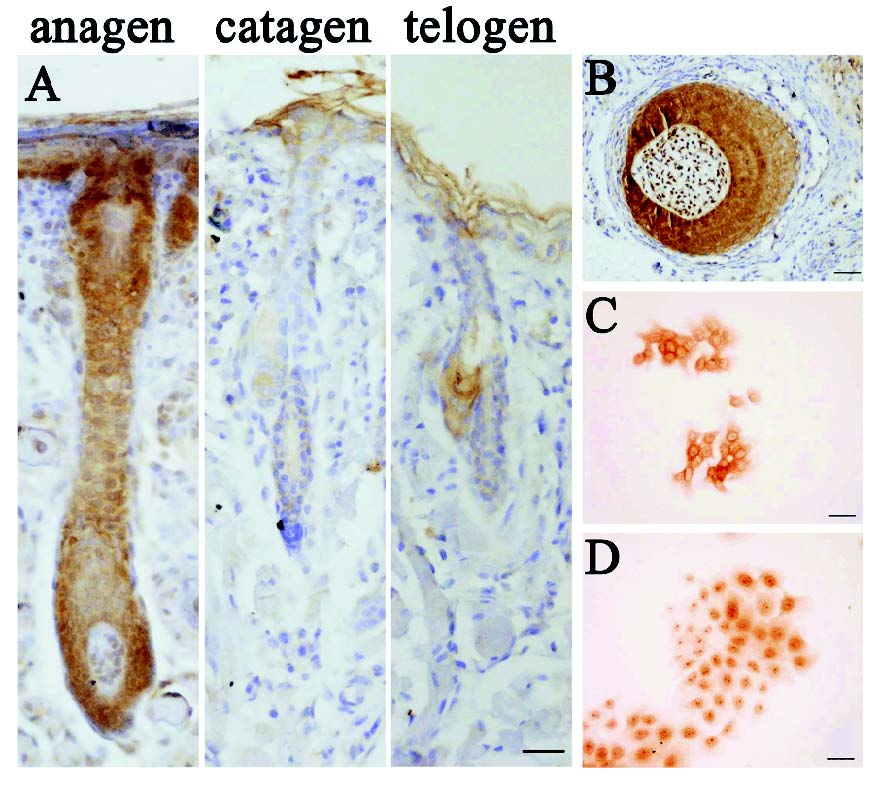
Fig. 1. Expression of beta-catenin in the normal hair follicle and the cultured HFSCs.
(A)Immunohistochemistry showed a cyclic expression of beta-catenin in different phases of hair cycle. (B) The expression of beta-catenin in the bulb of hair follicle at the anagen. (C-D) The location of beta-catenin in the HFSCs at 2 d (C) and 6 d (D) after culture. (Bars: A, B: 50 microm; C, D: 20 microm).
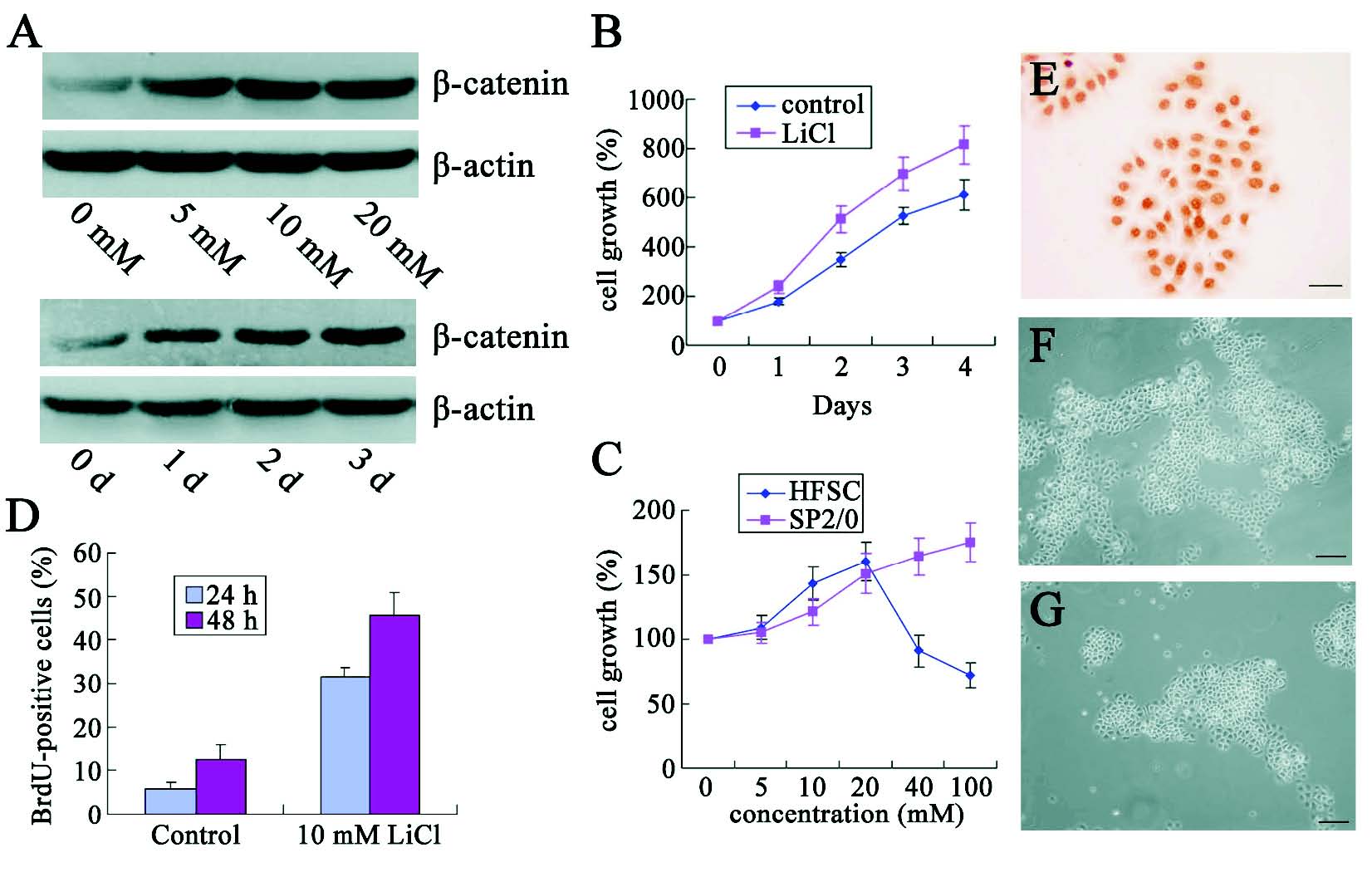
Fig. 2. beta-catenin promotes the proliferation of HFSCs in response to LiCl treatment. (A) Western blot assay was performed to demonstrate dose- (up) and time-dependent (down) increase in beta-catenin expression in the cells harvested after LiCl treatment. (B-C) MTT assay showed the cell growth after treatment at different days after culture (B) or with LiCl at different concentrations (C). (D) BrdU-incorporated assay was used to determinate the cell proliferation after LiCl treatment. (E) E-cadherin expression was detected in the nucleus. (-G) Morphology of cell clones with (F) or without (G) treatment. (Bars: E:20 microm; F, G: 50 microm). * Statistically significant as compared with controls.
Of interest, the expression of beta-catenin was also detected in the nucleus of cultured HFSCs in vitro. Our results showed that beta-catenin predominantly located at the cell membrane at 2 days before inoculation (Fig. 1C). At 6 days after culture, these cells showed an obvious tendency in intra-nuclear accumulation of beta-catenin (Fig. 1D).
Up-regulation of beta-catenin promotes HFSCs proliferation
LiCl can activate Wnt/beta-catenin signaling pathway by inhibiting the GSK-3beta, which induces the beta-catenin phosphorylation and degradation. In our experiments, immunoblotting assay were employed to detect the effects of LiCl on the beta-catenin expression. Results showed that total beta-catenin level in the HFSCs increased not only in dose, and time dependent manners (Fig. 2A).
Cell growth was determined by MTT assay at 0, 1, 2, 3, and 4 days after LiCl treatment. beta-catenin-positive cells exhibited a rapid expansion in a time-dependent manner (Fig. 2B). In addition, cell growth was also related to the concentration of LiCl. It seemed that LiCl at low concentration (<20 mM) could promote the HFSCs proliferation, but the LiCl at high concentration (above 40 mM) inhibited the cell growth (Fig. 2C). This might be explained by the excessive activation of beta-catenin which resulted in the differentiation of HFSCs. These phenomena suggested that beta-catenin could regulate the cell growth in a dose-dependent manner and the beta-catenin at optional concentration was essential for HFSCs survival and proliferation in vitro.
Furthermore, incorporation assay was used to determine the effect of LiCl on the HFSCs proliferation. As shown in Fig. 2D, when compared with the control group, LiCl significantly increased the number of BrdU-positive cells by approximately 3-fold after treatment for 24 h and 48 h, indicating the significant proliferation of HFSCs. To our surprise, ICC analysis of E-cadherin in LiCl -treated HFSCs revealed a relative increase in the nuclear E-cadherin (Fig. 2E). Morphologically, LiCl treated cells produced larger clones than those in the control group (Figs 2F and G).
Influence of beta-catenin on HFSCs proliferation via PI3k/Akt pathway
An efficient beta-catenin specific shRNA lentiviral particle was prepared to evaluate the influence of beta-catenin on HFSCs proliferation. Immunoblotting assay displayed that beta-catenin was weakly expressed in the shRNA-infected HFSCs, but highly expressed in the control group (Fig. 3A). MTT assay revealed that shRNA-infected cells exhibited a decreasing tendency of growth rates at 24 h, and the growth rates was reduced by 50% at 48 h when compared with the un-infected cells (Fig. 3B).
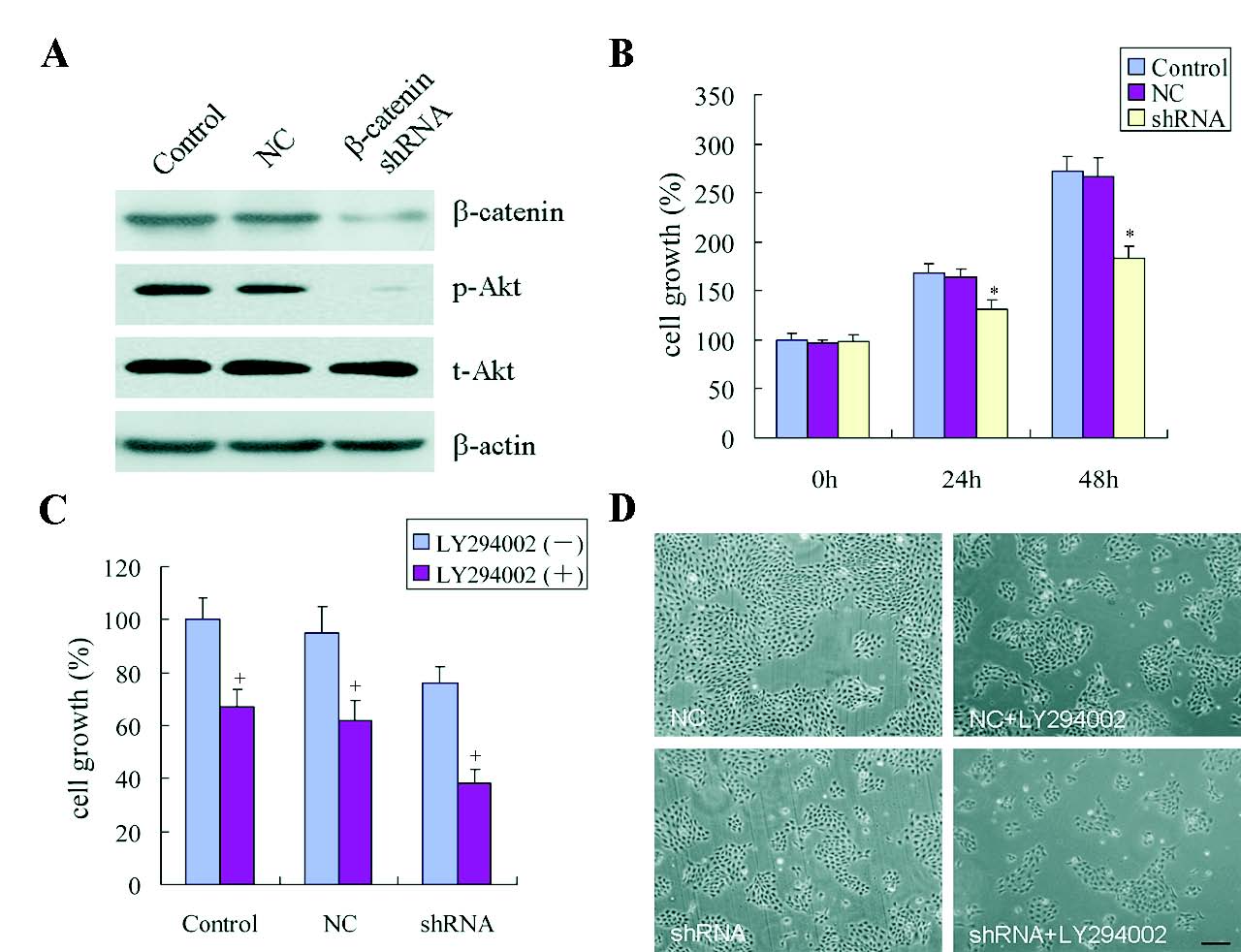
Fig. 3. beta-catenin deficiency causes disorder of HFSCs growth. (A) Western blot assay of beta-catenin, phosphorylated Akt (p-Akt), and total Akt (t-Akt) expression after beta-catenin shRNA treatmet for 48 h. (B) MTT assay was performed in the absence of beta-catenin and the OD was measured at 24 and 48 h. (C-D) HFSCs transfected with control shRNA or shRNA against beta-catenin were treated with LY294002 (10 micromol/l). Cell growth was evaluated by MTT assay (C) and morphological examination (D). (Bars: D: 100 microm). + Statistically significant versus animal untreated with LY294002. Other symbols as in Fig. 2.
To determine the intracellular signaling pathways mediating the HFSCs proliferation, PI3K/Akt growth-promoting signaling pathway was investigated. Western blot assay demonstrated that shRNA against beta-catenin led to a decrease in phosphorylated Akt in comparison with the controls, whereas the total Akt was remained relatively unchanged (Fig. 3A). The specific PI3K inhibitor LY294002 was used to assess whether the PI3k/Akt accounted for beta-catenin-induced cell growth. As shown in Fig3C and D, the growth rate of cells following LY294002 treatment was significantly suppressed, and the inhibition was more obvious in the beta-catenin shRNA-treated HFSCs.
To further understand whether the PI3K/Akt was responsible for beta-catenin-induced proliferation, the effect of activated Akt on cell growth was investigated following treatment with insulin. Inhibition of beta-catenin by specific shRNA resulted in obvious decrease in cell growth, which did not recover after insulin treatment (Fig. 4A). Likewise, when the beta-catenin was knocked down, the phosphorylation of Akt remained unchanged after insulin treatment. (Fig. 4B). As described above, cell growth was significantly suppressed by LY294002 as compared to the untreated controls. However, LY294002 in combination with LiCl still increased the cell growth as compared to cells treated with LY294002 alone (Fig. 4C). Although LY294002 profoundly reduced the protein expression of phosphorylated Akt, pretreatment with LY294002 followed by LiCl treatment also induced a remarkable phosphorylation of Akt as compared to the cells treated with LY294002 alone. (Fig. 4D). Taken together, these findings suggested that beta-catenin could regulate the proliferation of HFSCs in a PI3K/Akt dependent way.

Fig. 4. Roles of PI3K/Akt in cell proliferation regulated by beta-catenin. (A) Cell growth of beta-catenin-deficient cells was evaluated by MTT assay in the presence or absence of 1 microg/ml insulin. (B) Protein level of total and phosphorylated Akt was detected by western blot assay. NC: unscrambling control. (C) HFSCs were pre-incubated with LY294002 for 1 h prior to exposure to LiCl. MTT assay was performed to measure the cell growth at 48 h. (D) Phosphorylated Akt was also evaluated by western blot assay. Results are expressed as OD of pAkt normalized to that of tAkt. Other symbols as in Fig. 2.
Activation of beta-catenin-dependent gene expression in proliferative HFSCs
To assess that LiCl induced the TCF/LEF-dependent transcriptional activity depending on beta-catenin, reporter-gene analysis was performed with the TOPFlash containing multimerized LEF/TCF binding sites and the negative control FOPFlash containing mutated LEF/TCF binding sites. In the cells transfected with the TOPFlash luciferase reporter plasmid, 10 mM LiCl resulted in a 5-fold increase in beta-catenin-mediated transcriptional activation of TCF/LEF-responsive genes (Fig. 5A), but the luciferase activity reduced by 3-4 folds in HFSCs after specific shRNA treatment (Fig. 5B). Alternatively, no change was observed in the FOPflash activity after LiCl treatment or shRNA infection. These results were consistent with the nuclear translocation of beta-catenin in immunocytochemistry (Fig. 5C), and further indicated that Wnt/beta-catenin signaling pathway could be regulated by specifically activating the TCF/LEF-dependent transcription.
To elucidate the effect of beta-catenin on cell proliferation, the expression of cyclin D1 and p21, potential target genes of Wnt/beta-catenin signaling pathway, was detected. Over-expression of beta-catenin significantly elevated the cyclin D1 expression but lowered the p21 expression after LiCl treatment (Fig. 5D). In contrast, specific shRNA infection decreased the cyclin D1 expression but increased the p21 expression in beta-catenin-silencing cells (Fig. 5E). Taken together, these findings implied that cyclin D1 and p21 were able to exert effect on HFSCs proliferation via the beta-catenin signaling pathway.

Fig. 5. beta-catenin regulates cyclin D1 and p21 expression in proliferating HFSCs in TCF/LEF-dependent signaling pathway. (A-B) The transcriptional activity of Wnt was determined by luciferase reporter assay after LiCl treatment (A) or transfection of shRNA against beta-catenin (B). (C) Indirect immunofluorescence analysis was done to detect the subcellular distribution of beta-catenin protein in HFSCs following treatment with 10 microM LY294002 for 48 h. (Bars: C: 20 microm) (D-E) Expression of cyclin D1 and p21 was detected in HFSCs after treatment with 10 mM LiCl (D) or transfection with shRNA against beta-catenin (E) at different time points (0 h, 24 h and 48 h). The expression of target gene was normalized to that of beta-actin. Other symbols as in Fig. 2.
DISCUSSION
The regulatory effect of Wnt signal pathway on cell proliferation has been reported in many tissues (Masckauchan et al. 2005, Hirsch et al. 2007, Rulifson et al. 2007). As a key protein in the canonical Wnt signaling pathway, stable beta-catenin affects some cellular properties by the way of up-regulating target genes through stimulating the TCF/LEF mediated transcription, which regulates the cell fates in the embryogenesis, postnatal development and tumorigenesis. In the skin, over-expression of beta-catenin is frequently found in the pilomatricomas and squamous cell carcinomas (Xia et al. 2006, Malanchi et al. 2008), while the hair follicle depletion was present in the beta-catenindeficient mice (Huelsken et al. 2001). Thus, beta-catenin functions as an indispensable factor for the hair follicle development and cycle maintenance (Lo Celso et al. 2004, Zhang et al. 2008).
In the present study, results showed that beta-catenin expression displayed a cyclic pattern in the hair follicle cycle. As shown in Fig. 1A, beta-catenin was highly expressed in the anagen, but weakly expressed in the catagen and telogen, representing the regressive and quiescent process of hair follicle, which demonstrates that the effect of beta-catenin on hair growth is attributed to its influence on cell proliferation in vivo. Furthermore, a great quantity of beta-catenin positive cells was observed in the matrix where epithelial cells, migrating from the bulge region, could generate the cortex, medulla and cuticle of hair shaft. These results suggested that beta-catenin not only affected the proliferation of matrix cells, but was a critical factor to activate cell differentiation. It has been thought that DP presents with inductive signals required for hair outgrowth (Kishimoto et al. 2000). Enshell-Seijffers et al. reported that the specific deletion of beta-catenin gene in the DP during the anagen phase caused a reduction in hair growth (Enshell-Seijffers et al. 2010). Consistent with these, our results showed that beta-catenin obviously translocated into the nucleus of DP cells, demonstrating that the inductive activation of the DP cells by beta-catenin may be another pathway to indirectly regulate the cell proliferation.
Although the change in beta-catenin expression has been implicated in the follicle cycle, the mechanism involving in the proliferation of quiescent HFSCs is still unclear. To delineate the direct effect of beta-catenin on cell proliferation, LiCl was used to stabilize the free beta-catenin in the cytoplasm and mimics the Wnt signal in vitro. As expected, LiCl dose- and time-dependently increased the total beta-catenin level in the HFSCs and enhanced the TOP luciferase activity, suggesting that the LiCl induced cell growth was associated with the rapid activation of Wnt signaling pathway. In addition, excessive beta-catenin expression resulted in a decrease in the cell proliferation. Several studies had demonstrated that over-expression of beta-catenin would lead to an imbalance of proliferation and differentiation, which attributed to the activation of different beta-catenin-dependent target gene expression (Blanpain 2007). Therefore, we speculate that the beta-catenin signaling pathway is required for the HFSCs proliferation and there is a critical threshold of beta-catenin concentration for the activation of target genes.
It is well known that beta-catenin is a dual-functional protein contributing to the cell adhesion and Wnt signal transduction (Gottardi and Gumbiner 2004). As a structural component of adhesion junction, beta-catenin forms the cadherin-catenin complex to mediate the intercellular adhesion, controlling some essential steps in the embryogenesis and tumorigenesis (Perez-Moreno et al. 2003). Herein, our results showed that the translocation of beta-catenin from cell membrane to the nucleus appeared when the HFSCs were actively proliferative in vitro (Figs 1C, D). It implies that beta-catenin plays a dominant role in the signal transduction for cell proliferation. In previous study, the impairment of the cadherin-catenin complex at the adhesion junction contributed to the increase in free beta-catenin in the cytoplasm. The sequent translocation to the nucleus might lead to the activation of target genes via TCF/LEF transcription. In our experiments, the expression of E-cadherin significantly decreased in the membrane of proliferating cells. Simultaneously, its nuclear accumulation was similar to the expression pattern of beta-catenin after LiCl treatment, suggesting that E-cadherin was another transcription factor in the Wnt signaling pathway, but the regulatory mechanism has not been elucidated in the bulge cells.
The PI3K/Akt signaling pathway has been found to induce the epidermal hyperplasia and promote the stem cell proliferation (Le Belle et al. 2011, Chimge et al. 2012). Our results revealed that the activation of PI3K/Akt signaling pathway may be mediated by the crosstalk with the beta-catenin pathway in the HFSCs proliferation. Prior studies have documented that the activation of PI3K/Akt signaling pathway is known to be involved in the phosphorylation and inhibition of GSK-3beta (Liu et al. 2002), which is a major kinase for the phosphorylation of beta-catenin, and results in its consequent degradation. Conversely, in the present study, the phosphorylation of Akt was down-regulated in the beta-catenin-inactivated cells, suggesting that additional activation of PI3K/Akt was partly dependent on the beta-catenin. Consistent with these findings, LY294002, an inhibitor of PI3K, significantly attenuated the cell proliferation after beta-catenin was knocked down. Furthermore, in the beta-catenin-inactivated cells, the cell growth was not recovered even in the present of insulin. The findings revealed that the activation of beta-catenin was critical to promote the cell growth in the PI3K/Akt signaling pathway. However, when the PI3K was inhibited by LY294002, LiCl still increased the phosphorylation of Akt and promoted the cell growth as compared to the cells treated with LY294002 alone, suggesting that LiCl-induced cell proliferation was associated with the beta-catenin-dependent activation of Akt. These results imply that there is a positive feedback loop regulating the crosstalk between beta-catenin and PI3K/Akt and that beta-catenin plays a dominant role in the cell proliferation through activating the PI3K/Akt pathway.
Previous studies demonstrated that beta-catenin promoted the cell proliferation, owing to its ability to increase the expression of specific genes (Vlad et al. 2008). In the canonical Wnt/beta-catenin signaling pathway, the activation of target genes usually depends on the translocation of beta-catenin from the cytoplasm to the nucleus and binding of beta-catenin with TCF/LEF complex (MacDonald et al. 2009). Luciferase assay, determined by Top/Fop flash, showed that the activation of TCF/LEF was enhanced after LiCl treatment but inhibited by shRNA infection. These were accompanied by a positive connection to cell proliferation, supporting a role of beta-catenin signaling via the TCF/LEF pathway. Furthermore, the expression of beta-catenin influenced the downstream effectors of Wnt signaling pathway including cyclin D1 and p21. Cyclin D1, which participates in the regulation of cell cycle progression, has a TCF/LEF-binding site in the promoter region and is identified as a direct target gene of beta-catenin during the tumorigenesis (Saito et al. 2006). In the experiment, the expression of cyclin D1 showed an increasing tendency depending on the expression of beta-catenin protein. This suggested that the beta-catenin-mediated signal transduction might be responsible for the HFSCs proliferation by promoting the expression of cyclin D1. p21 is another target gene of TCF/LEF complex, functioning as an inhibitor of the cyclin-dependent kinases (Cdks) (Choi 2007). Previous studies showed that the transcriptional activation of p21 gene was inhibited by the beta-catenin/TCF signaling pathway in HEK293 cells (Kamei et al. 2003). Consistent with these findings, our results showed that the p21 expression was also down-regulated following beta-catenin over-expression, while beta-catenin deletion resulted in p21 up-regulation. Recent studies have indicated that p21 is involved in the regulation of cell proliferation by inducing cell cycle arrest in the G1 phase (Hoi et al. 2010, Cheng et al. 2011) or G2/M phase (Dvory-Sobol et al. 2006, Yoshida et al. 2009). In the skin, it has been shown that p21 is only expressed in non-proliferating keratinocytes and attenuated p21 would induce the epidermis hyperplasia (Cho et al. 2008). Thus, p21 expression might be associated with the keratinocyte growth. In addition, investigators have reported that Akt activation can also up-regulate the cyclin D1 expression (Guo et al. 2011, Parrales et al. 2011) and reduce the p21 expression (Fang et al. 2012), further demonstrating a possible connection between the Wnt/beta-catenin and PI3K/Akt signaling pathways. Furthermore, since the blockage of beta-catenin inhibits the PI3K/Akt pathway, we postulate that beta-catenin may contribute to the HFSCs proliferation through activating the beta-cateninPI3K/Akt-Cyclin D1/p21 signaling pathway. Thus, more studies are required to identify the target genes of beta-catenin signaling pathways.
In summary, our results show that beta-catenin plays an essential role in controlling the proliferation of HFSCs, which may be also important in the hair follicle development, epidermal repair and sebaceous gland maintenance. This will be helpful to increase the yield of hair follicle keratinocytes from stem cells for cell therapy and tissue engineering. To understand the related mechanism is beneficial for resolving problems in the hair regeneration and carcinogenesis.
ACKNOWLEDGEMENTS
This study was supported by the National Nature Science Foundation of China (NSFC, No. 30900737).
REFERENCES
Blanpain C. Impact of beta-catenin signaling pathway on stem cell differentiation in the skin. Med Sci (Paris). 23: 34-36, 2007.
[CrossRef]
[PubMed]
Cheng WL, Lin TY, Tseng YH, Chu FH, Chueh PJ, Kuo YH, Wang SY. Inhibitory Effect of Human Breast Cancer Cell Proliferation via p21-Mediated G1 Cell Cycle Arrest by Araliadiol Isolated from Aralia cordata Thunb. Planta Med. 77: 164-168, 2011.
[CrossRef]
[PubMed]
Chimge NO, Makeyev AV, Waigel SJ, Enkhmandakh B, Bayarsaihan D. PI3K/Akt-dependent functions of TFII-I transcription factors in mouse embryonic stem cells. J Cell Biochem. 113: 1122-1131, 2012.
[CrossRef]
[PubMed]
Cho YS, Bae JM, Chun YS, Chung JH, Jeon YK, Kim IS, Kim MS, Park JW. HIF-1alpha controls keratinocyte proliferation by up-regulating p21(WAF1/Cip1). Biochim Biophys Acta. 1783: 323-333, 2008.
[CrossRef]
[PubMed]
Choi EJ. Hesperetin induced G1-phase cell cycle arrest in human breast cancer MCF-7 cells: involvement of CDK4 and p21. Nutr Cancer. 59: 115-119, 2007.
[CrossRef]
[PubMed]
Ciraolo E, Morello F, Hirsch E. Present and future of PI3K pathway inhibition in cancer: perspectives and limitations. Curr Med Chem. 18: 2674-2685, 2011.
[CrossRef]
[PubMed]
Dvory-Sobol H, Cohen-Noyman E, Kazanov D, Figer A, Birkenfeld S, Madar-Shapiro L, Benamouzig R, Arber N. Celecoxib leads to G2/M arrest by induction of p21 and down-regulation of cyclin B1 expression in a p53-independent manner. Eur J Cancer. 42: 422-426, 2006.
[CrossRef]
[PubMed]
Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. Beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 18: 633-642, 2010.
[CrossRef]
[PubMed]
Fang Y, Yu S, Braley-Mullen H. TGF-beta promotes proliferation of thyroid epithelial cells in IFN-gamma(-/-) mice by down-regulation of p21 and p27 via AKT pathway. Am J Pathol. 180: 650-660, 2012.
[CrossRef]
[PubMed]
Gottardi CJ, Gumbiner BM. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol. 167: 339-349, 2004.
[CrossRef]
[PubMed]
Guo X, Li W, Wang Q, Yang HS. AKT Activation by Pdcd4 Knockdown Up-Regulates Cyclin D1 Expression and Promotes Cell Proliferation. Genes Cancer. 2: 818-828, 2011.
[CrossRef]
Hirsch C, Campano LM, Wohrle S, Hecht A. Canonical Wnt signaling transiently stimulates proliferation and enhances neurogenesis in neonatal neural progenitor cultures. Exp Cell Res. 313: 572-587, 2007.
[CrossRef]
[PubMed]
Hoi CS, Lee SE, Lu SY, McDermitt DJ, Osorio KM, Piskun CM, Peters RM, Paus R, Tumbar T. Runx1 directly promotes proliferation of hair follicle stem cells and epithelial tumor formation in mouse skin. Mol Cell Biol. 30: 2518-2536, 2010.
[CrossRef]
[PubMed]
Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 105: 533-545, 2001.
[CrossRef]
Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 447: 316-320, 2007.
[CrossRef]
[PubMed]
Kamei J, Toyofuku T, Hori M. Negative regulation of p21 by beta-catenin/TCF signaling: a novel mechanism by which cell adhesion molecules regulate cell proliferation. Biochem Biophys Res Comm. 312: 380-387, 2003.
[CrossRef]
[PubMed]
Kim JS, Kim BS, Kim J, Park CS, Chung IY. The phosphoinositide-3-kinase/Akt pathway mediates the transient increase in Nanog expression during differentiation of F9 cells. Arch Pharm Res. 33: 1117-1125, 2010.
[CrossRef]
[PubMed]
Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 14: 1181-1185, 2000.
[PubMed]
Kobayashi K, Rochat A, Barrandon Y. Segregation of keratinocyte colony-forming cells in the bulge of the rat vibrissa. Proc Natl Acad Sci USA. 90: 7391-7395, 1993.
[CrossRef]
Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 8: 59-71, 2011.
[CrossRef]
[PubMed]
Lee G, Goretsky T, Managlia E, Dirisina R, Singh AP, Brown JB, May R, Yang GY, Ragheb JW, Evers BM, Weber CR, Turner JR et al. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 139: 869-881, 2010.
[CrossRef]
[PubMed]
Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 108: 837-847, 2002.
[CrossRef]
Lo Celso C, Prowse DM, Watt FM. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 131: 1787-1799, 2004.
[CrossRef]
[PubMed]
MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 17: 9-26, 2009.
[CrossRef]
[PubMed]
Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W, Huelsken J. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 452: 650-653, 2008.
[CrossRef]
[PubMed]
Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 8: 43-51, 2005.
[CrossRef]
[PubMed]
Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicle from adult multipotene stem cells. Cell. 104: 233-245, 2001.
[CrossRef]
Parrales A, Lopez E, Lopez-Colome AM. Thrombin activation of PI3K/PDK1/Akt signaling promotes cyclin D1 upregulation and RPE cell proliferation. Biochim Biophys Acta. 1813: 1758-1766, 2011.
[CrossRef]
[PubMed]
Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 112: 535-548, 2003.
[CrossRef]
Perry JM, He XC, Sugimura R, Grindley JC, Haug JS, Ding S, Li L. Cooperation between both Wnt/{beta}-catenin and PTEN/PI3K/Akt signaling promotes primitive hematopoietic stem cell self-renewal and expansion. Genes Dev. 25: 1928-1942, 2011.
[CrossRef]
Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 434: 843-850, 2005.
[CrossRef]
[PubMed]
Rulifson IC, Karnik SK, Heiser PW, ten Berge D, Chen H, Gu X, Taketo MM, Nusse R, Hebrok M, Kim SK. Wnt signaling regulates pancreatic beta cell proliferation. Proc Natl Acad Sci USA. 104: 6247-6252, 2007.
[CrossRef]
[PubMed]
Saito T, Oda Y, Yamamoto H, Kawaguchi K, Tanaka K, Matsuda S, Iwamoto Y, Tsuneyoshi M. Nuclear beta-catenin correlates with cyclin D1 expression in spindle and pleomorphic sarcomas but not in synovial sarcoma. Hum Pathol. 37: 689-697, 2006.
[CrossRef]
[PubMed]
Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 314: 1447-1450, 2006.
[CrossRef]
[PubMed]
Suzuki K, Yamaguchi Y, Villacorte M, Mihara K, Akiyama M, Shimizu H, Taketo MM, Nakagata N, Tsukiyama T, Yamaguchi TP, Birchmeier W, Kato S et al. Embryonic hair follicle fate change by augmented beta-catenin through Shh and Bmp signaling. Development. 136: 367-372, 2009.
[CrossRef]
[PubMed]
Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 102: 451-461, 2000.
[CrossRef]
Vlad A, Rohrs S, Klein-Hitpass L, Muller O. The first five years of the Wnt targetome. Cell Signal. 20: 795-802, 2008.
[CrossRef]
[PubMed]
Xia J, Urabe K, Moroi Y, Koga T, Duan H, Li Y, Furue M. beta-Catenin mutation and its nuclear localization are confirmed to be frequent causes of Wnt signaling pathway activation in pilomatricomas. J Dermatol Sci. 41: 67-75, 2006.
[CrossRef]
[PubMed]
Yoshida M, Matsui Y, Iizuka A, Ikarashi Y. G2-phase arrest through p21(WAF1/Cip1) induction and cdc2 repression by gnidimacrin in human hepatoma HLE cells. Anticancer Res. 29: 1349-1354, 2009.
[PubMed]
Zhang Y, Xiang M, Wang Y, Yan J, Zeng Y, Yu J, Yang T. Bulge cells of human hair follicles: segregation, cultivation and properties. Colloids Surf B Biointerfaces. 47: 50-56, 2006.
[CrossRef]
[PubMed]
Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, Tobias JW, Piccolo S, Schmidt-Ullrich R, Nagy A, Taketo MM, Dlugosz AA, Millar SE. Activation of beta-catenin signaling programs embryonic epidermis to hair follicle fate. Development. 135: 2161-2172, 2008.
[CrossRef]
[PubMed]
Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition, Nature Cell Biol. 6: 931-940, 2004.
[CrossRef]
[PubMed]
|
BACK
|






