Journal of APPLIED
BIOMEDICINEISSN 1214-0287 (on-line)
ISSN 1214-021X
(printed)
Volume 4 (2006)
Reactivation of acetycholinesterase inhibited by the pesticide
chlorpyrifos Veronika Racakova, Daniel Jun, Veronika Opletalova,
Kamil Kuca Address: Kamil Kuca, Department of Toxicology, Faculty of
Military Health Sciences, University of Defence, Trebesska 1575, 500 01, Hradec
Kralove, Czech Republic
kucakam@pmfhk.czFull text article
(pdf)Received 2nd March 2006
Revised 4th April 2006
Published online 1st June 2006
Summary
Organophosphorus
pesticides such as parathion or chlorpyrifos are substances used worldwide for
agricultural purposes. These compounds are able to inhibit an enzyme
acetylcholinesterase (AChE; EC 3.1.1.7) by phosphorylation in its active site.
AChE reactivators and anticholinergics are generally used as antidotes in the
case of intoxication by these agents. In this work, the reactivation potency of
nine structurally different AChE reactivators was tested in vitro. Chlorpyrifos
was chosen as an appropriate member of the pesticide family. The result is that
bisquaternary reactivators with two oxime groups in position four at the
pyridinium rings (trimedoxime and K074) seem to be the most potent reactivators
of chlorpyrifos-inhibited AChE.
Keywords: acetylcholinesterase – reactivator – pesticide – organophosphate
INTRODUCTION
Self-poisoning with pesticides is a major problem across the world. It is estimated that hundreds of thousands of people die each year due to the into-
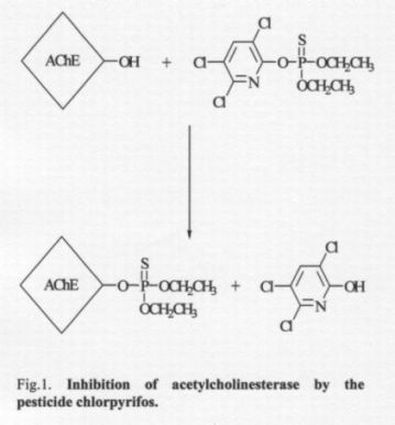
xication by organophosphorus pesticides (Gunnell and Eddleston, 2003; Meggs 2003). Although these compounds act in the same way as nerve agents [inhibition of the enzyme organophosphorus pesticides (Gunnell and Eddleston 2003, Meggs 2003). acetylcholinesterase (AChE; EC 3.1.1.7)], nerve agent antidotes are not generally as satisfactory in the treatment of pesticide poisoning as in the case of nerve agent poisoning (Worek et al. 1996, 1999). This is due to the fact that pesticides create a different enzyme-inhibitor complex than with nerve agents. Pesticides create the dimethoxy- (methylchlorpyrifos, fenitrothion, trichlorfon), diethoxy- (chlorpyrifos, parathion, paraoxon) or diisopropoxy- (DFP) phosphoryl complex with enzyme (Figure 1). As mentioned above, these complexes are poorly reactivated by the commonly used AChE reactivators. Of the commercially available oximes, only obidoxime seems to be satisfactorily effective in the case of pesticide poisonings (Thiermann et al. 1997, MusĂlek et al. 2005).
However, it depends on the pesticide used and on the time of therapy administration (Thiermann et al. 1997, Eddleston et al. 2005).
Currently, there is a lack of the information necessary for the successful development of antidotes against pesticide poisoning (Buckley et al. 2005). Because of this, we would like to focus on the relationship between the structure of cholinesterase reactivators and their biological activity. This study could be helpful in the future to scientists working on the development of new antidotes.
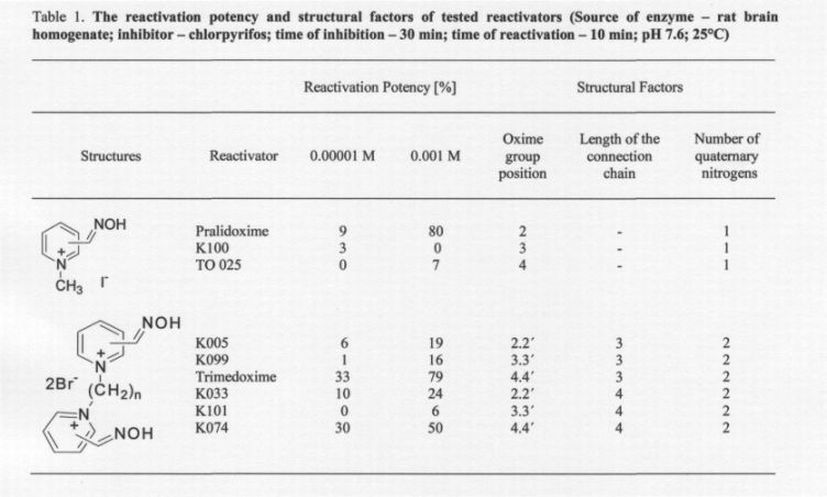
To obtain the basic structural requirements of AChE reactivators for the treatment of pesticide poisonings, we have selected nine known AChE reactivators differing in their chemical structure. Three compounds tested were monoquaternary AChE reactivators differing in the position of the oxime group (position 2, 3 or 4 on the pyridinium ring). Among them, pralidoxime, an oxime currently available for first aid in the US, is included. The remaining six AChE reactivators were bisquaternary, differing in the position of the oxime groups on the pyridinium rings (position 2, 3 or 4) and in the length of the connection chain (three or four membered linker) between both pyridinium rings. As described earlier, all these structural factors (the number of quaternary nitrogens, number and position of oximes groups, and length of the connection chain) play a very important role in the biological activity of AChE reactivators (KuÄŤa et al. 2006).
In this work, all the above-mentioned reactivators were tested in vitro as potential reactivators of AChE inhibited by pesticide chlorpyrifos.
MATERIAL AND METHODS
All tested AChE reactivators were synthesized in our department (MusĂlek et al. 2006). Their purity was tested by TLC using DC-Alufolien Cellulose F (Merck, Germany) and elution BuOH-CH3COOH-H2O 5: 1: 2, detection by solution of Dragendorf´s reagent (solution containing 10 ml CH3COOH, 50 ml H2O and 5 cm3 of basic solution prepared by mixing of two fractions – fraction I.: 0.85 mg Bi(NO3)3, 40 cm3 H2O, 10 cm3 CH3COOH; fraction II.: 8 g KI, 20 cm3 H2O). The pesticide chlorpyrifos (O,O-diethyl-O-(3,5,6-trichloropyrid-2-yl)-phosphorothioate) was obtained from Riedel-de Haen of analytical standard with 99.2 % purity. All other chemicals used in this experiment were purchased from Fluka and Aldrich (Czech Republic) and used without further purification.
The in vitro testing of the synthesized reactivators involved the standard collection of experimental procedures. The whole method is described in detail in the work of KuÄŤa and Cabal (2005). The reactivation efficacy of tested reactivators was evaluated in 10% rat brain homogenate that was incubated with chlorpyrifos for 30 minutes and then the tested oxime of appropriate concentration (10-5 and 10-3 M) was added. After 10 min of reactivation, the activity of brain AChE was measured using the potentiostatic method with an automatic titrator RTS 822 (Radiometer, Denmark). The data on the initial rate of enzyme reaction with the substrate made it possible to calculate the percentage of increase in the activity of reactivated enzyme in the reaction mixture.
RESULTS
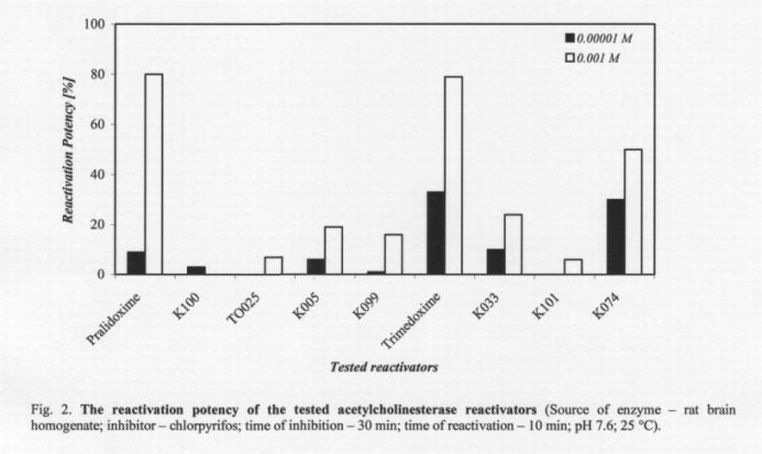
All results obtained are shown in Fig. 2 and Table 1. As can be seen clearly, all the tested oximes were able to reactivate chlorpyrifos-inhibited AChE. Six oximes reached a reactivation potency of over 10 %, which is believed to be sufficient for the survival of intoxicated rats. The highest reactivation potency was achieved for pralidoxime (80%) and obidoxime (79%). Unfortunately, this reactivation potency was not reached at the concentration 10-53 M sufficient for humans (Bajgar 2004). At relevant doses for humans, trimedoxime (33%) and K074 (30%) seem to be the most potent reactivators tested.
If we focus on structural factors, both the most potent AChE reactivators (trimedoxime and K074) have in their structure two quaternary nitrogens, connected with three or four membered linking chain. Moreover, both reactivators have two oxime groups in position four at two pyridinium rings.
DISCUSSION
Owing to the fact that all the earlier developed AChE reactivators were prepared with the aim of treating nerve agent intoxication, there is a lack of reactivators for treatment of pesticide poisonings, because of the low efficacy of nerve agent-reactivators in the case of pesticide intoxications. It is generally known that oxime HI-6, so promising in the case of sarin, cyclosarin and VX poisoning, is practically ineffective in the case of pesticide reactivation (Worek et al. 1996, 1999, MusĂlek et al. 2005). Currently, obidoxime seems to be the reactivator of the first choice for the treatment of pesticide-intoxication (Thiermann 1997). Its structural analogue, trimedoxime, has achieved according to our former results similar to reactivation potency (Musilek et al. 2005). However due to the potential toxicity of both reactivators, these substances should not be used over the long period of treatment necessary for achieving satisfactory cholinesterase activity in blood (Bismuth et al. 1992, Balali-Mood and Shariat 1998, Jun et al., 2006).
Therefore the development of new more potent and less toxic antidotes, especially AChE reactivators, for the treatment of pesticide poisonings should continue. To design a new, more potent, AChE reactivator, it would be very important to know the relationship between the structure of reactivators and their biological activity (Gray 1984, KuÄŤa et al. 2006). This was a goal in this work.
As is known from our previous results, there are several structural factors (the number of quaternary nitrogens, number and position of oxime groups, and the length of the connection chain) influencing the activity of currently used nerve agent-inhibited AChE reactivators (KuÄŤa et al. 2003, KuÄŤa et al. 2006). We wanted to find out if these factors influence the reactivation potency of the tested oximes in the same manner as they did in the case of nerve agents.
It transpired that bisquaternary are more potent than monoquaternary reactivators. The same results were obtained also for nerve agents-inhibited AChE. Secondly, the ideal position for the oxime group seems to be position four. This result is in good correlation with reactivators of tabun-inhibited AChE (Cabal et al. 2004). The position of the oxime group is different in cyclosarin-inhibited AChE reactivators, which have the oxime group in position two at the pyridinium ring (except methoxime – due to its size) (Kassa and Cabal 1999, Kuča and Patočka 2004). In this manuscript, we could not discuss the dependence of the reactivatioin potency of AChE reactivators on the length of linker, because in the study we concentrated only three and four membered connection chains, which are believed to be the best for the reactivation of nerve agent inhibited AChE (Kuča et al. 2003).
The aim of our work was to focus the attention of scientists working in this field on the targeted development of new pesticide-inhibited AChE reactivators and, moreover, to point out that there are several structural factors, which should be followed in producing new AChE reactivators with a promising reactivation potency
ACKNOWLEDGEMENT
The authors express their appreciation to Mrs. Hrabiinováa and Mr. Stodůlka Stodůlka for their technical assistance. This work was supported by a grant from Charles University (Czech Republic) No. 126/2005/B-BIO/FaF.
REFERENCES
Bajgar J.: Organophosphates/nerve agents poisoning: mechanism of action, diagnosis, prophylaxis and treatment. Adv. Clin. Chem. 38:151–216, 2004.
Balali-Mood M., Shariat M.: Treatment of organophosphate poisoning. Experience of nerve agents and acute pesticide poisoning on the effects of oximes. J. Physiol.ogy (Paris) 92:375–378, 1998.
Bismuth C., Ins R.H., Marrs T.C.: Efficacy, toxicity and clinical use of oxime in anticholinesterase poisoning., inIn: Ballantyne B., Marrs T.C., Aldrige W.N. (eds.): Clinical and experimental toxicology of organophosphates and carbamates, Butterworth-Heinmann Ltd., Oxford, 1992, pp. 555–577.
Buckley N.A., Eddleston M., Dawson A.H.: The need for translational research on antidotes for pesticide poisoning. Clin. Exp. Pharmacol. Physiol. 32:999–1005, 2005.
Cabal J., Kuča K., Kassa J.: Specification of the structure of oximes able to reactivate tabun inhibited acetylcholinesterase. Basic. Clin. Pharmacol. Toxicol. 95:81–86, 2004.
Eddleston M., Eyer P., Worek F. et al.: Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet 366: 1452–1459, 2005.
Gray A.P.: Design and structure-activity relationships of antidotes to organophosphorus anticholinesterase agents. Drug Metab. Rev. 15:557–589, 1984.
Gunnell D., Eddleston M.: Suicide by intentional ingestion of pesticides: A continuing tragedy in developing countries. Int. J. Epidemiol. 32: 902–909, 2003.
Jun D., Kuca K., Hronek M., Opletal L.: Effect of some acetylcholinesterase reactivators on human platelet aggregation in vitro. J. Appl. Toxicol. 26:262–268, 2006.
Kassa J., Cabal J.: A comparison of the efficacy of acetylcholine reactivators against cyclohexyl methylphosphonofluoridate (GF agent) by in vitro and in vivo methods. Pharmacol. Toxicol. 84:41–45, 1999.
Kuča K., Cabal J.: Evaluation of newly synthesized reactivators of the brain cholinesterase inhibited by sarin-nerve agent. Toxicol. Mech. Methods 15:247–252, 2005.
Kuča K., Patočka J.: Reactivation of cyclosarin-inhibited rat brain acetylcholinesterase by pyridinium–oximes. J. Enzyme Inhib. Med. Chem. 19:39–43, 2004.
Kuča K., Patočka J., Cabal J.: Reactivation of organophosphate inhibited acetylcholinesterase activity by ??,?-bis-(4-hydroxyiminomethylpyridinium)alkanes in vitro. J. Appl. Biomed. 1:207–211, 2003.
KuÄŤa K., Jun D., MusĂlek K.: Structural requirements of acetylcholinesterase reactivators. Mini Rev. Med. Chem. in press, 2006.
Meggs W.J.: Permanent paralysis at sites of dermal exposure to chlorpyrifos. J. Toxicol. Clin. Toxicol. 41:883–886, 2003.
MusĂlek K., KuÄŤa K., Jun D. et al.: Synthesis of the novel series of bispyridinium compounds bearing xylene linker and evaluation of their reactivation activity against chlorpyrifos-inhibited acetylcholinesterase. J. Enzyme Inhib. Med. Chem. 20:409–415, 2005.
Musilek K., Lipka L., Racakova V. et al.: New methods in synthesis of acetylcholinesterase reactivators and evaluation of their potency to reactivate cyclosarin-inhibited AChE. Chem. Pap. in press, 2006.
Thiermann H., Mast U., Klimmek R. et al.: Cholinesterase status, pharmacokinetics and laboratory findings during obidoxime therapy in organophasphate poisoned patients. Hum. Exp. Toxicol. 16:473–480, 1997.
Worek F., Kirchner T., Bäcker M., Szinicz L.: Reactivation by various oximes of human erythrocyte acetylcholinesterase inhibited by different organophosphorus compounds. Arch. Toxicol. 70:497–503, 1996.
Worek F., Diepold C., Eyer P.: Dimethylphosphoryl-inhibited human cholinesterases: inhibition, reactivation, and aging kinetics. Arch. Toxicol. 73:7–14, 1999.
BACK



