Journal of APPLIED
BIOMEDICINEISSN 1214-0287 (on-line)
ISSN 1214-021X
(printed)
Volume 4 (2006)
Effects of micro-ketoglutarate on antioxidants and lipid peroxidation
products in rats treated with sodium valproate Murugesan Vidya,
Perumal Subramanian Address:Perumal Subramanian, Reader, Department
of Biochemistry, Faculty of Science, Annamalai University, Annamalainagar – 608
002, Tamil Nadu, India
psub@rediffmail.comFull text article
(htm)Full text
article (pdf)Received 2nd September 2005.
Revised 9th January
2006.
Published online 15th March 2006.
Summary
Oxidative
stress may cause free radical reactions to produce deleterious modifications in
membranes, proteins, enzymes and DNA. Valproic acid is a major anti-epileptic
drug with a broad spectrum of anti-epileptic activity. Chronic treatment with
valproic acid can lead to elevated serum ammonia levels and specific oxidative
metabolites of valproic acid have been associated with the drug’s toxicity. The
influence of sodium valproate treatment on lipid peroxidation and lipid profiles
and the detoxifying effects of alpha-ketoglutarate on sodium valproate induced
toxicity were studied in rats. The levels of thiobarbituric acid reactive
substances, hydroperoxides and lipid profile variables (cholesterol,
phospholipids, triglycerides and free fatty acids) were significantly increased
in sodium valproate treated rats. Further, non-enzymic antioxidants (reduced
glutathione) and the activities of he enzymic (superoxide dismutase, catalase,
glutathione peroxidase) antioxidants were significantly decreased in sodium
valproate treated rats. The levels were observed to be normal in alpha-KG +
sodium valproate treated rats. These biochemical alterations during alpha-KG
treatment could be due to (i) its ubiquitous collection of amino groups in body
tissues, (ii) the participation of alpha-KG in non-enzymatic oxidative
decarboxylation of the hydrogen peroxide decomposition process and (iii) its
role in the metabolism of fats which could suppress oxygen radical generation
and thus prevent the lipid peroxidative damage.
Keywords: alpha-ketoglutarate – sodium valproate – antioxidants – lipid peroxidation
INTRODUCTION
Valproate is often prescribed as a long-term therapeutic mood-stabilizing agent for individuals
with bipolar disorder (Wang et al. 2003). Valproate (Valproic acid – VPA) is a major anti-epileptic drug with a broad spectrum of anti-epileptic activity. It has been the drug of choice in the treatment of most forms of primary generalized epilepsies and is also efficient against partial seizures (Rowan 1997). Valproic acid affects hepatocellular defence mechanisms and suggests that a predisposition of hepatocytes to oxidative stress may play a role in the fatal hepatotoxicity of valproic acid in epileptic patients (Klee et al. 2000). Specific oxidative metabolites of valproic acid have been associated with the clinically defined toxicity of the drug (Graf et al. 1998). Hyperammonemia is a documented side effect of valproate (VPA) treatment (Stephens and Levy 1994). Lipid peroxidation may be involved as an additional mechanism of valproic acid induced liver damage in rats. Alterations in mental status may develop in patients receiving sodium valproate through a direct hepatotoxic effect of the agent or its metabolites (Zaret et al. 1982).
Alpha-ketoglutarate (alpha-KG) is an intermediate of the citric acid cycle and is the natural ubiquitous collector of amino groups in body tissues (Lehninger et al. 2000). Because of its chemical structure, ?-KG is a potent natural detoxifying agent (Velvizhi et al. 2002a, Dakshayani et al. 2002) and is used as an antidote to cyanide poisoning where it reacts with the cyanide molecule to form cyanohydrin as a reaction product (Dunaley et al. 1999). Alpha-KG also improves myocardial protection in patients undergoing coronary operations (Kjellman et al. 1997). Further, alpha-KG also decreases muscle protein catabolism (Wernerman et al. 1990) and improves recovery after trauma. Another important function of alpha-KG consists in the formation of carnitine (Copper and Kristal 1997). Carnitine is a molecule that acts as a carrier of fatty acids into cell mitochondria so that proper metabolism of fats can proceed (Roe et al. 2000).
The detoxifying characteristics of alpha-KG were analysed in the present study by estimating the levels of thiobarbituric acid reactive substances (TBARS), hydroperoxides (the products of lipid peroxidation), non-enzymic antioxidant (reduced glutathione), activities of enzymic antioxidants (superoxide dismutase, catalase, glutathione peroxidase) and the lipid profile variables (cholesterol, triglycerides, phospholipids and free fatty acids) in blood and tissues (liver and brain) so as to assess its role in suppressing the side effects induced by sodium valproate.
MATERIALS AND METHODS
Animals
Adult Wistar rats (180-220g) obtained from Central Animal House, Faculty of Medicine, Annamalai University, were kept at room temperature (32±2°C). All animal experiments were approved by the ethical committee, Annamalai University and were in accordance with the National Institutes of Health: Guide for the Care and Use of laboratory animals (NIH 1985). Animals were fed with the standard pellet diet (Agro Corporation Private Limited, Bangalore, India) which, as with water was available to the animals ad libitum.
Alpha-ketoglutarate (disodium salt) was purchased from SRL Private Limited, Mumbai, India. Sodium valproate and all other chemicals used in this study were of analytical grade. The animals were randomized and divided into four groups (n=6 in each group). Group I animals served as controls. Group II animals were treated with sodium valproate (300 mg/kg body weight) every day orally for 8 weeks. Group III animals received sodium valproate at the same dose as in Group II along with alpha-KG (2 g/kg body weight) and Group IV rats received alpha-KG at the same dose as Group III animals for 8 weeks.
At the end of the experimental period, animals were sacrificed by cervical dislocation. Liver and brain tissues were excised for the determinations of biochemical parameters. The levels of TBARS (Nichans and Samuelson 1968), hydroperoxides (Jiang et al. 1992), GSH (Ellman 1959), cholesterol (Zlatkis et al. 1953), phospholipids (Zilversmit and Davis 1950), triglycerides (Foster and Dunn 1973), free fatty acids (Falholt et al. 1973) and the activities of superoxide dismutase (Kakkar et al. 1984), catalase (Sinha 1972), glutathione peroxidase (Rotruk et al. 1973) were analyzed in the liver and brain tissues.
Statistical analysis
The Mean±SD of the measured variables in each group was calculated. The Analysis of variance (ANOVA) followed by Least Significant Difference (LSD) test was carried out to detect significant differences between the control and the experimental groups.
RESULTS
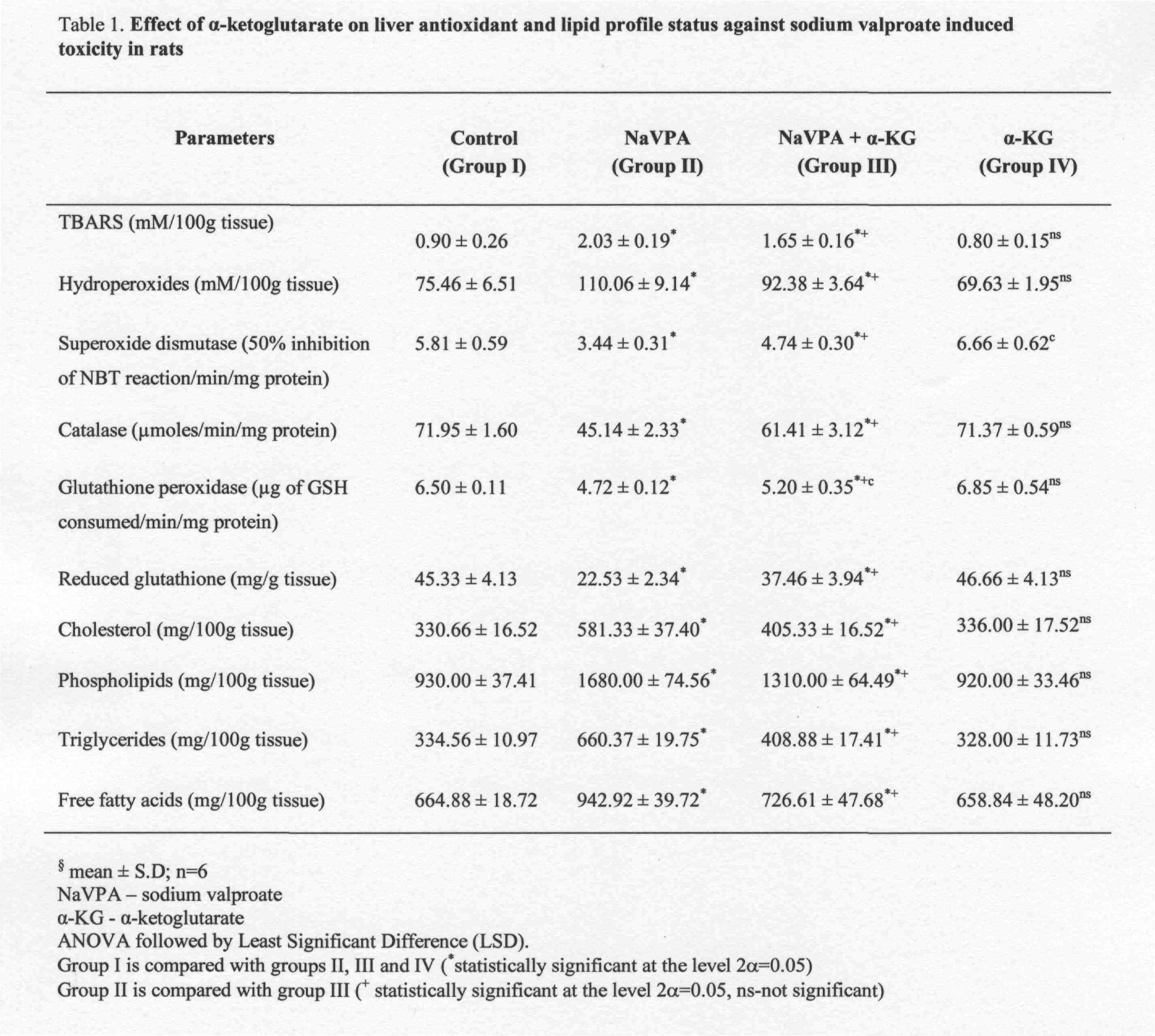
The levels of TBARS, hydroperoxides, lipid profiles such as cholesterol, phospholipids, triglycerides, free fatty acids and the antioxidant status of SOD, catalase, GPx, GSH in the liver and kidney tissues are shown in Table 1 and 2. A decrease in the status of antioxidants such as catalase, SOD, GPx and GSH with a concomitant increase in the rate of TBARS and hydroperoxides were observed in the sodium valproate (Group II) treated rats when compared with the control (Group I) rats. On treatment with alpha-Ketoglutarate KG (Group III) there was a significant increase in the activities of antioxidant enzymes and glutathione with a corres-ponding decrease in TBARS and hydroperoxides when compared to the sodium valproate (Group II) treated rats. The alpha-ketoglutarate KG treated group (Group IV) alone did not show any significant change.
The levels of cholesterol, phospholipids, triglycerides and free fatty acids were increased significantly in the sodium valproate treated rats (Group II). The sodium valproate and alpha-KG treated (Group III) rats showed significantly lower levels when compared with the sodium valproate treated rats. The levels of these parameters were found to be normal in the alpha-KG (Group IV) treated rats.
DISCUSSION
The cytotoxic activity of valproate is the result of the generation of hydrogen peroxide and the production of highly reactive hydroxyl radicals (Tabatabaei and Abbott 1999). This could lead to the increased levels of TBARS and hydroperoxides and decreased levels of enzymatic (SOD, CAT and GPx) and non-enzymatic (GSH) antioxidants in the Group II rats. Further sodium valproate treatment through a process of free radical damage causes functional damage in the liver (Siddique et al. 1999). The SOD catalyzes the dismutation of superoxide anion to H2O2. The latter can be converted to the more harmful hydroxyl radicals (OH). Subsequently, H2O2 is reduced to H2O and O2 by peroxidases (e.g., GPx) or CAT. GPx scavenges H2O2 in the presence of reduced glutathione (GSH) to form H2O and oxidized glutathione (Michiels et al. 1994). Earlier reports showed that alpha-KG offers protection against oxidative damage by participating in the non-enzymatic oxidative decarboxylation in the hydrogen peroxide decomposition process (Sokolowska et al. 2000). Reports have also shown that alpha-KG is a glutamine precursor from which GSH is formed (Cynober 1999) which is essential for the activity of GPx. It is known that the ammonia-induced inhibition of antioxidant enzymes is mediated by the activation of NMDA receptors of nitric oxide synthase and the formation of nitric oxide, which inhibits the activities of antioxidant enzymes (Kosenko et al. 2000).
 In the present study, the administration of sodium valproate caused a significant increase in the levels of lipid profile variables (cholesterol, triglycerides, phospholipids and free fatty acids). It has been reported that sodium valproate could deplete the levels of alpha-KG (Kifune et al. 2000) and this could elevate the levels of acetyl CoA. This acetyl CoA may be used for the synthesis of fatty acids and cholesterol, since fatty acids of different
sources are used as substrates for synthesizing triglycerides and phospholipids. Further treatment with valproate causes a kind of drug induced mitochondrial cytopathy with microvesicular lipid deposition. The lipid deposits are likely to be a result of the inhibited mitochondrial fatty acid oxidation (Melegh and Trombitas 1997).
In the present study, the administration of sodium valproate caused a significant increase in the levels of lipid profile variables (cholesterol, triglycerides, phospholipids and free fatty acids). It has been reported that sodium valproate could deplete the levels of alpha-KG (Kifune et al. 2000) and this could elevate the levels of acetyl CoA. This acetyl CoA may be used for the synthesis of fatty acids and cholesterol, since fatty acids of different
sources are used as substrates for synthesizing triglycerides and phospholipids. Further treatment with valproate causes a kind of drug induced mitochondrial cytopathy with microvesicular lipid deposition. The lipid deposits are likely to be a result of the inhibited mitochondrial fatty acid oxidation (Melegh and Trombitas 1997).
The enhanced level of lipid profile variables could be due to the carnitine transport defect associated with the treatment of sodium valproate. Our results are in accordance with the report of Heldenberg et al (1983) who found an increase in cholesterol levels in epileptic children treated with VPA. Increased levels of free fatty acids in the sodium valproate treated rats may be due to the decrease in the alpha-KG levels (Velvizhi et al. 2002b) which lead to the accumulation of free fatty acids which might be reversed during the treatment by alpha-KG as reflected in group III and group IV. Exogenous administration of alpha-KG could lead to the normalization of fat metabolism and could increase the oxidation of fats (Bellei et al. 1989) offering protection against lipid peroxidation and oxidative stress (Dakshayani et al. 2002, Velvizhi et al. 2002b).
In conclusion, exogenous administration of alpha-KG could cause the biochemical alterations by (i) participating in the non-enzymatic oxidative decarboxylation in the hydrogen peroxide decomposition process and (ii) enhancing the proper metabolism of fats, which could suppress oxygen radical generation and prevent lipid peroxidative damage in rats.
REFERENCES
Bellei M., Batelli P., Guarrier D.M.: Changes in mitochondrial activity caused by ammonium salts and the protective effect of carnitine. Biochem. Biophys. Res. Commun. 158:181–188, 1989.
Copper A.J.L., Kristal B.S.: Multiple roles of glutathione in the central nervous system. Biol. Chem. 378:798–802, 1997.
Cynober L.A.: The use of ?-ketoglutarate salts in clinical nutrition and metabolic care. Curr. Opin. Nutr. Metab. Care 2:33–37, 1999.
Dakshayani K.B., Velvizhi S., Subramanian P.: Effects of ornithine ?-ketoglutarate on circulatory antioxidants and lipid peroxidation products in ammonium acetate treated rats. Ann. Nutr. Metab. 46:93–96, 2002.
Dunaley M.D., Brumeley M., Willis J.T., Hume A.S.: Production against cyanide toxicity by oral ?-ketoglutaric acid. Vet. Hum. Toxicol. 33:537–540, 1999.
Ellman G.L.: Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82:70–77, 1959.
Falholt K., Lund B., Falholt W.: An easy colorimetric micromethod for routine determination of free fatty acids in plasma. Clin. Chim. Acta 46: 105–111, 1973.
Foster L.B., Dunn R.T.: Stable reagents for determination of serum triglycerides by a colorimetric Hantzsch condensation method. Clin. Chem. 19:338–340, 1973.
Graf W.D., Oleinik O.E., Glauser T.A. et al.: Altered antioxidant enzyme activities in children with a serious adverse experience related to valproic acid therapy. Neuropediatrics 29: 195–201, 1998.
Heldenberg O., Harel S., Holtzman M. et al.: The effect of chronic anticonvulsant therapy on serum lipids and lipoproteins in epileptic children. Neurology 33:510–513, 1983.
Jiang Z.Y., Hunt J.V., Wolff S.P.: Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 202:384–387, 1992.
Kakkar P., Das B., Viswanathan P.N.: A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 21:130–132, 1984.
Kifune A., Kuboto F., Shibata N., Kikuchi S.: Valproic acid-induced hyperammonemia encephalopathy with triphasic waves. Epilepsia 41:9909–9912, 2000.
Kjellman V., Bjork K., Ekroth R. et al.: Addition of alpha-ketoglutarate to blood cardioplegia improves cardioprotection. Ann. Thorac. Surg. 63:1625–1634, 1997.
Klee S., Johanssen S., Ungemach F.R.: Evidence for a trigger function of valproic acid in xenobiotic-induced hepatotoxicity. Pharmacol. Toxicol. 87:89–95, 2000.
Kosenko E., Kaminsky Y., Stavroskaya I.G., Felipo V.: Alteration of mitochondrial calcium homeostasis by ammonia-induced activation of NMDA receptors in rat brain in vivo. Brain Res. 880, 139–146, 2000.
Lehninger A.L., Nelson D.L., Cox M.M.: Principles of Biochemistry. 3rd edn: CBS publishers, New Delhi, 2000.
Melegh B., Trombitas K.: Valproate treatment induces lipid globule accumulation with ultrastructural abnormalities of mitochondria in skeletal muscle. Neuropediatrics 28: 256–261, 1997.
Michiels C., Raes M., Toussaint O., Remacle J.: Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic. Biol. Med. 17:235–248, 1994.
National Institutes of Health: Guide for the Care and Use of Laboratory Animals, DHEW Publication (NIH), revised, Office of Science and Health Reports, DRR/NIH, Bethesda, USA, 1985.
Nichans W.G., Samuelson B.J.: Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 6:126–130, 1968.
Roe D.S., Roe C.R., Brivet M., Sweetman L.: Evidence for a short-chain carnitine-acylcarnitine translocase in mitochondria specifically related to the metabolism of branched-chain amino acids. Mol. Genet. Metab. 69:69–75, 2000.
Rotruk J.T., Pope A.L., Gauther H.E. et al.: Selenium: Biochemical roles as a component of glutathione peroxidase. Science 179: 588–590, 1973.
Rowan A.J.: Valproate. In Engel J., Pedley T.A (eds.): Epilepsy: A comprehensive textbook, Lippincott-Raven Publ., Philadelphia 1997, pp. 1599–1607.
Siddique M.A.A., Nazmi A.S., Razia K. et al.: Oxidative damage in mice liver induced by sodium valproate: protection by melatonin. Indian J. Pharmacol. 31: 427–431, 1999.
Sinha A.K.: Colorimetric assay of catalase. Anal. Biochem. 47:389–394, 1972.
Sokolowska M., Oleszek A., Wlodek L.: Protective effect of alpha-ketoacids on the oxidative hemolysis. Pol. J. Pharmacol. 51: 429–434, 2000.
BACK


 In the present study, the administration of sodium valproate caused a significant increase in the levels of lipid profile variables (cholesterol, triglycerides, phospholipids and free fatty acids). It has been reported that sodium valproate could deplete the levels of alpha-KG (Kifune et al. 2000) and this could elevate the levels of acetyl CoA. This acetyl CoA may be used for the synthesis of fatty acids and cholesterol, since fatty acids of different
sources are used as substrates for synthesizing triglycerides and phospholipids. Further treatment with valproate causes a kind of drug induced mitochondrial cytopathy with microvesicular lipid deposition. The lipid deposits are likely to be a result of the inhibited mitochondrial fatty acid oxidation (Melegh and Trombitas 1997).
In the present study, the administration of sodium valproate caused a significant increase in the levels of lipid profile variables (cholesterol, triglycerides, phospholipids and free fatty acids). It has been reported that sodium valproate could deplete the levels of alpha-KG (Kifune et al. 2000) and this could elevate the levels of acetyl CoA. This acetyl CoA may be used for the synthesis of fatty acids and cholesterol, since fatty acids of different
sources are used as substrates for synthesizing triglycerides and phospholipids. Further treatment with valproate causes a kind of drug induced mitochondrial cytopathy with microvesicular lipid deposition. The lipid deposits are likely to be a result of the inhibited mitochondrial fatty acid oxidation (Melegh and Trombitas 1997).