Journal of APPLIED
BIOMEDICINEISSN 1214-0287 (on-line)
ISSN 1214-021X
(printed)
Volume 5 (2007)
Urtica dioica extract reduces platelet hyperaggregability in type 2
diabetes mellitus by inhibition of oxidant production, Ca2+ mobilization and
protein tyrosine phosphorylation Mohammed El Haouari, Isaac
Jardin, Hasane Mekhfi, Juan A. Rosado, Ginés M. Salido Address: J.
A. Rosado, Department of Physiology, University of Extremadura, Av. Universidad
s/n. Cáceres 10071, Spain
jarosado@unex.esFull text article
(pdf)Full
text article (html)Received 21th March 2007.
Revised 23rd
April 2007.
Published online 7th May 2007.
Summary
Platelet
hyperaggregability is involved in the pathogenesis of type 2 diabetes mellitus.
Thrombin-evoked platelet aggregation includes the activation of several
intracellular pathways, including endogenous generation of reactive oxygen
species (ROS), Ca2+ mobilization and protein tyrosine phosphorylation. Here we
show that crude aqueous extract from Urtica dioica reduces thrombin-evoked
aggregation in platelets from healthy donors and diabetics, in a
concentration-dependent manner. U. dioica extract showed a potent antioxidant
activity and prevented thrombin-evoked ROS generation in platelets from healthy
and diabetic donors. Treatment with U. dioica extract reduced Ca2+ entry induced
by thrombin or selective depletion of the two Ca2+ stores in platelets (without
altering Ca2+ release), reduced protein tyrosine phosphorylation and reversed
the abnormal Ca2+ mobilization and tyrosine phosphorylation in platelets from
diabetics. We conclude that extract from U. dioica shows antiaggregant actions
and might be used for the treatment and/or prevention of cardiovascular
complications associated with type 2 diabetes mellitus.
Keywords:
platelets - type 2 diabetes mellitus - Urtica dioica - calcium mobilization -
antioxidant - tyrosine phosphorylation
INTRODUCTION
Platelets are essential for normal haemostasis. However, excessive activation of platelets, which leads to their aggregation play a major role in the pathogenesis of cardiovascular disorders such as atherosclerosis, cardiac dysfunction and peripheral artery disease (Dogne et al. 2002, Willoughby et al. 2002, Belton et al. 2003). Moreover, it is well known that patients with type 2 diabetes mellitus and hypertension have hyperactive blood platelets (Mandal et al. 1993, Blankenship et al. 2000, Blann et al. 2003, Ruf 2004). Given the importance of platelet activation in the pathogenesis of cardiovascular diseases, research is oriented toward the discovery of new anti-platelet medicinal agents with fewer or no adverse effects.
Due to the hypotensive effect of Urtica dioica L. (Urticaceae) (Garnier et al. 1961), extracts of this plant are frequently used in the traditional medicine system as a natural remedy for hypertension and diabetes (Ziyyat et al. 1997). We have previously shown that U. dioica exerts antihyperglycaemic, bradycardial, hypotensive, diuretic and antiaggregant effects in rats (Tahri et al. 2000, Legssyer et al. 2002, Bnouham et al. 2003, El Haouari et al. 2006). It is known that physiological agonists, such as thrombin, induce platelet aggregation by increasing the cytosolic free calcium concentration ([Ca2+]c), which consists of two components: the release of Ca2+ from intracellular stores and Ca2+ entry through plasma membrane channels (Rosado and Sage 2000). Removal of Ca2+ from the cytosol is mediated by Ca2+ extrusion mainly by the plasma membrane Ca2+-ATPase (PMCA) and sequestration into the intracellular stores (LĂłpez et al. 2006). Furthermore, human platelets generate and release reactive oxygen species (ROS) under physiological stimulation (Pignatelli et al. 1998, Seno et al. 2001). Recently, we have reported that H2O2 and hydroxyl radicals are involved in the reduced platelet PMCA activity and Ca2+ extrusion in type 2 diabetes mellitus patients (JardĂn et al. 2006), an event that is likely to be responsible for the altered ionic homeostasis and hyperaggregability associated with type 2 diabetes mellitus (Rosado et al. 2004c, Saavedra et al. 2004).
In the present study, we have investigated the effect of crude extract obtained from U. dioica leaves on the production of oxidants, Ca2+ mobilization and aggregation in platelets from type 2 diabetes mellitus patients and healthy human donors. Pretreatment of platelets with crude aqueous extract reduced in a concentration-dependent manner the aggregation, the production of oxidants and Ca2+ mobilization, including Ca2+ entry, evoked by thrombin; however, this effect was greater in diabetic platelets. We conclude that the antiaggregant properties described for U. dioica are due to its reduction of intracellular calcium and its antioxidant action in human platelets.
MATERIALS AND METHODS
Materials
Fura-2 acetoxymethyl ester (fura-2/AM), calcein/AM and 5-(and-6)-chloromethyl-2´, 7´-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) were from Molecular Probes (Leiden, The Netherlands). Apyrase (grade VII), EGTA, aspirin, bovine serum albumin, thrombin, thapsigargin (TG) were from Sigma (Madrid, Spain). 2,5-di-(tert-butyl)-1,4-hydroquinone (TBHQ) was from Alexis (Nottingham, UK). Anti-phosphotyrosine monoclonal antibody (4G10) was from Upstate Biotechnology (Madrid, Spain). Horseradish peroxidase-conjugated ovine anti-mouse IgG antibody (NA931) was from Amersham (Buckinghamshire, U.K.). Enhanced chemiluminescence detection reagents were from Pierce (Cheshire, U. K.). Hyperfilm ECL was from Amersham (Arlington Heights, IL, U.S.A.). All other reagents were purchased from Panreac (Barcelona, Spain).
Subjects
Patients with type 2 diabetes mellitus (DM) and not showing other pathologies, and healthy drug-free volunteers were randomly obtained from normotensive patients of the Clinical Analysis Laboratory, Cáceres, Spain. Informed consent was obtained from every subject. Blood was obtained at 9:00 AM, in accordance with the principles of the Declaration of Helsinki, from 12 healthy (control) and 15 diabetic donors. Blood glucose concentration in DM patients was in the range of 180 to 240 mg/dl. The glycosylated Hb levels (HbA1c) were used as an index of metabolic control. Only DM patients with a level of HbA1c > 6 % were selected. The control subjects were normal age- and gender-matched healthy people whose HbA1c levels were within the normal range (3.5–5%).
Platelet preparation
Fura-2-loaded platelets were prepared as described previously (Rosado et al. 2004a). For measurements of [Ca2+]c platelet-rich plasma was incubated at 37 centigrade with 2 µM fura-2/AM for 45 min. Cells were collected by centrifugation and resuspended in HEPES-buffered saline (HBS) containing (in mM): 145 NaCl, 10 HEPES, 10 D-glucose, 5 KCl, 1 MgSO4, pH 7.45, supplemented with 0.1% w/v bovine serum albumin and 40 µg/ml apyrase.
Cell viability
Cell viability was assessed using calcein and trypan blue. For calcein loading, cells were incubated for 30 min with 5 µM calcein-AM at 37 centigrade, centrifuged, and the pellet was resuspended in fresh HBS. Then, resting cells or treated with the tested extract (1.5 mg/ml) were incubated at 37 oC for 1 min. Fluorescence was recorded from 2 ml aliquots using a Cary Eclipse fluorescence spectrophotometer (Varian Ltd, Madrid, Spain). Samples were excited at 494 nm and the resulting fluorescence was measured at 535 nm. The calcein fluorescence remaining in the cells after treatment with the tested extract was the same as in the controls, suggesting that under our conditions there was no cellular damage. The results obtained with calcein were confirmed using the trypan blue exclusion technique. 95% of cells were viable in our platelet preparations, at least during the performance of the experiments.
Measurement of intracellular free calcium concentration ([Ca2+]c)
Fluorescence was recorded from 2 ml aliquots of magnetically stirred cell suspensions (2 × 108 cells/ml) at 37°C with excitation wavelengths of 340 and 380 nm and emission at 510 nm. Changes in [Ca2+]c were monitored using the fura-2 340/380 fluorescence ratio and calibrated according to Grynkiewicz et al. (1985). Ca2+ entry was estimated using the integral of the rise in [Ca2+]c for 3 min after the addition of CaCl2 (Ben Amor et al. 2006). Ca2+ release was estimated using the integral of the rise in [Ca2+]c for 3 min after agonist addition.
Platelet aggregation
Platelets prepared as described above were suspended in HBS supplemented with 0.1 % w/v bovine serum albumin. Platelet aggregation was monitored in a Chronolog (Havertown, Pa, U.S.A.) aggregometer at 37 centigrade C under stirring at 1200 rpm (Redondo et al. 2005).
Intracellular oxidants production
CM-H2DCFDA is a fluorescent probe that can be used to monitor oxidant production in living cells (Rosado et al. 2004b). Platelets were incubated at 37 centigrade with 10 µM CM-H2DCFDA acetyl ester for 30 min, then centrifuged and the pellet was resuspended in fresh HBS. Samples were excited at 488 nm and the resulting fluorescence was measured at 530 nm. The production of oxidants after treatment of cells with thrombin in the absence or presence of antioxidants was quantified as the integral of the rise in DCF fluorescence for 5 min after the addition of the agent.
Protein tyrosine phosphorylation
Protein tyrosine phosphorylation was detected by gel electrophoresis and Western blotting (Rosado et al. 2001). Platelet stimulation (5 × 108 cells/ml) was terminated by mixing with an equal volume of 2 × Laemmli’s buffer with 10% dithiothreitol followed by heating for 5 min at 95 centigrade. One-dimensional SDS-electrophoresis was performed with 10% polyacrylamide minigels (50 µg total protein loaded/sample) and the separated proteins were electrophoretically transferred onto nitrocellulose for subsequent probing. Blots were incubated overnight with 10% (w/v) BSA in tris-buffered saline with 0.1% Tween 20 (TBST) to block residual protein binding sites. Detection of tyrosine phosphorylation was achieved using the anti-phosphotyrosine antibody 4G10 diluted 1:1500 in TBST for 1 h. The primary antibody was removed and blots washed six times for 5 min each with TBST. The blots were then incubated with horseradish peroxidase-conjugated ovine anti-mouse IgG antibody diluted 1:10000 in TBST, washed six times in TBST, and exposed to enhanced chemiluminescence reagents for 5 min. The blots were then exposed to photographic films and their integrated optical density was estimated using scanning densitometry.
Plant material
Urtica dioica leaves (Urticaceae) were collected in spring 2006 in the proximity of Oujda (Morocco). The collected plant was identified by Prof. A. Khalil, from the Biology Department, Faculty of Sciences (Oujda, Morocco), where a voucher specimen (n° 10 ZL) is deposited.
Preparation of crude aqueous extract
Dried and powdered leaves from U. dioica (20 g) were infused into 250 ml of boiled distilled water for 30 min. After decantation and filtration, the extract was evaporated at 50 oC to give a crude residue (yield: 22.2 %).
Statistical analysis
All data provided were expressed as mean ± standard error of the mean (SEM). Analysis of statistical significance was performed using the Student’s t-test and only values with p<0.05 were accepted as significant.
RESULTS AND DISCUSSION
Effect of U. dioica extract on thrombin-induced platelet aggregation
Treatment of platelets from healthy donors for 1 min at 37 centigrades with increasing concentrations of crude aqueous extract from U. dioica (0.05–3 mg/ml) significantly reduced thrombin-induced aggregation in a concentration-dependent manner (Fig. 1A and Table 1; n=6). A clear inhibitory effect of the extract was observed at concentrations as low as 0.05 mg/ml, which significantly increased the lag-time and reduced the percentage and rate of aggregation, and a maximal effect was observed after treatment with 3 mg/ml (Fig. 1A and Table 1; statistically significant).
Fig. 1B shows that treatment of platelets from DM donors with 0.5 U/ml thrombin induced rapid aggregation with an amplitude and rate of aggregation that were significantly greater than that observed in platelets from healthy donors. Similarly, the treatment of platelets from diabetics for 1 min at 37 centigrades with increasing concentrations of crude aqueous extract from U. dioica (0.05–3 mg/ml) significantly reduced thrombin-evoked aggregation in a concentration-dependent manner (Fig. 1B and Table 1; n=6). It is important to note that a significant inhibitory effect was observed at 0.05 mg/ml (Fig. 1B and Table 1; statistically significant).
Effect of U. dioica extract on ROS generation
The treatment of platelets from healthy donors in a medium containing 1 mM Ca2+ with 0.5 U/ml thrombin resulted in a rapid increase in DCF fluorescence, reaching a maximum after 1.5 min of treatment (Fig. 2A). As previously reported (Redondo et al. 2005), in platelets from diabetic patients, thrombin evoked an increase in DCF fluorescence that was significantly greater than in platelets from healthy donors.
Thrombin-evoked ROS production in platelets from DM donors reached a maximum approximately 1 min after treatment (Fig. 2B). Treatment of platelets for 1 min at 37 centigrades with 0.05 mg/ml U. dioica extract abolished the increase in the DCF fluorescence induced by the addition of thrombin in platelets from both healthy and DM donors (Fig. 2A,B statistically significant), which demonstrate that the U. dioica extract exerts antioxidant activity.
Table 1. Effect of crude aqueous extract of U. dioica on thrombin-induced aggregation in platelets from healthy donors and diabetic patients
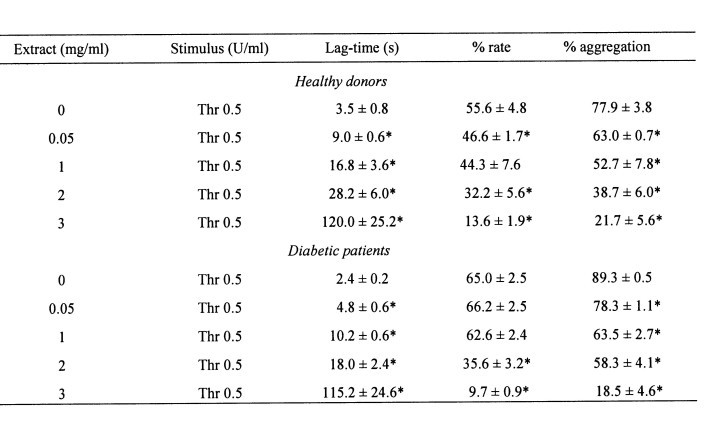
Platelets suspended in HBS (1 mM Ca2+) were treated for 1 min in the absence or presence of increasing concentrations of the crude aqueous extract of U. dioica (0.05–3 mg/ml). Cells were then stimulated at 37 oC with 0.5 U/ml thrombin (Thr) and platelet aggregation was determined as described under Material and methods. Values given are presented as mean ? S.E.M of six separate determinations. * statistically significant versus thrombin-induced response.
Effect of U. dioica extract on thrombin-induced Ca2+ mobilization
In a Ca2+-free medium (100 µM EGTA was added), treatment of platelets with 0.5 U/ml thrombin induced a transient increase in [Ca2+]c. The subsequent addition of Ca2+ (300 µM) to the external medium induced a sustained elevation in [Ca2+]c indicative of Ca2+ entry. Since it has been previously reported that endogenous ROS generation is required for Ca2+ mobilization in platelets (Rosado et al. 2004b), and in view of the antioxidant activity of U. dioica extract, we examined the effect of the U. dioica extract on Ca2+ mobilization by thrombin.
In platelets from healthy donors, treatment for 1 min with 0.05 U/ml extract, in a Ca2+-free medium, did not significantly alter thrombin-evoked Ca2+ release from the intracellular stores (Ca2+ release was 95.8 ± 7.9% of control; Fig. 3A, statistically significant). However, thrombin-induced Ca2+ entry was significantly reduced in the presence of the extract by 21.4 ± 4.1% (Fig. 3A). Higher concentrations of the extract showed a significant effect on fura 2 fluorescence and have not been used.
Treatment for 1 min with 0.05 U/ml extract, in a Ca2+-free medium, significantly reduced thrombin-evoked Ca2+ release from the intracellular stores and Ca2+ entry (Ca2+ release and entry were 53.9 ± 3.3 and 35.2 ± 3.1% of the controls, respectively; Fig. 3B, statistically significant). In the presence of the extract, thrombin-evoked Ca2+
mobilization was similar to that found in platelets from healthy donors, which further suggests the role of ROS in the pathogenesis of DM (Dixon et al. 2005, Redondo et al. 2005). In addition, these findings show that U. dioica extract might reduce the cardiovascular complications of type 2 DM.
Thrombin releases Ca2+ from the dense tubular system (DTS) and the acidic stores in human platelets (López et al. 2006), which activates store-operated Ca2+ entry by two mechanisms (Rosado et al. 2004a). We have further investigated the effect of U. dioica extract on Ca2+ mobilization mediated by selective depletion of the DTS or acidic stores using 10 nM TG or 20 µM TBHQ, respectively (Rosado et al. 2004a, Ben Amor et al. 2006). In a Ca2+-free medium TG and TBHQ induce a slow and sustained increase in [Ca2+]c due to depletion of the respective sensitive stores. The subsequent addition of extracellular Ca2+ revealed SOCE (Fig. 3C-F). In the presence of 0.05 mg/ml U. dioica extract SOCE induced by TG or TBHQ in healthy and diabetics were significantly reduced by 28.7 ± 8.2 and 28.9 ± 6.4%, respectively, in healthy donors and 42.2 ± 6.0 and 34.0 ± 3.6%, respectively, in diabetics (statistically significant). The extract did not alter TG- or TBHQ-induced Ca2+ release, either in healthy or diabetic donors, which suggests that the extracts did not modify the accumulation of Ca2+ in the stores (Figs. 3C-F). The inhibitory effect of the extract on store depletion-mediated Ca2+ entry might be responsible for the effect induced in thrombin-evoked Ca2+ influx.
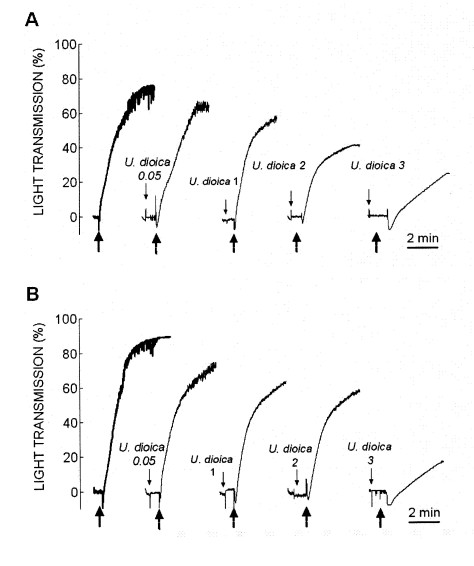
Fig. 1. Effect of crude aqueous extract of U. dioica on thrombin-evoked platelet aggregation. Human platelets from healthy (A) and diabetic donors (B) were suspended in HBS containing 1 mM Ca2+ and then were treated for 1 min in the absence or presence of increasing concentrations of the crude aqueous extract of U. dioica (0.05–3 mg/ml). Cells were then stimulated with 0.5 U/ml thrombin. The traces shown are representative of 6 separate experiments.
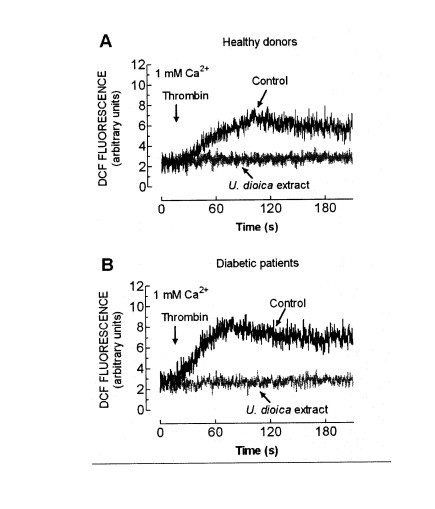
Fig. 2. Effect of U. dioica extract on ROS generation by thrombin. Human platelets from healthy donors (A) or diabetic patients (B) were loaded with CM-H2DCFDA and then pretreated for 1 min in the absence (Control) or the presence of 0.05 mg/ml extract (U. dioica), as indicated. Cells were then stimulated with 0.5 U/ml thrombin in a medium containing 1 mM Ca2+. The traces are representative of six independent experiments.
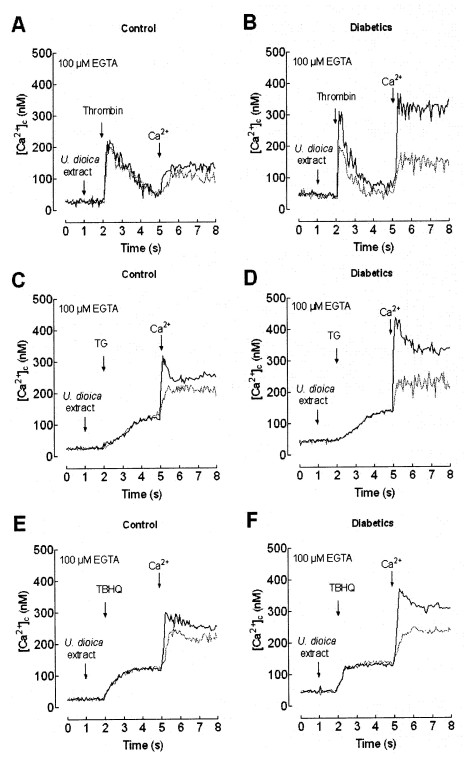
Fig. 3. Effect of U. dioica extract on Ca2+ mobilization. Fura 2-loaded human platelets from healthy (A, C and E) or diabetics (B, D and F) were suspended in Ca2+-free HBS (100 µM EGTA was added) and treated for 1 min in the absence (black traces) or presence (grey traces) of 0.05 mg/ml extract of U. dioica. Cells were then stimulated with 0.5 U/ml thrombin (A and B), 10 µM TG (C and D) or 20 µM TBHQ (E and F) and 3 min later 300 µM CaCl2 was added to the medium to initiate Ca2+ entry. The traces are representative of six independent experiments.
Effect of U. dioica extract on tyrosine phosphorylation induced by thrombin
Tyrosine phosphorylation has been shown to be required for aggregation and SOCE in human platelets (Rosado et al. 2000, Minuz et al. 2006). To test whether the observed effects of the U. dioica extract is mediated by the inhibition of tyrosine kinase activity we have examined their effect on thrombin-evoked tyrosine phosphorylation in normal and diabetic platelets. As shown in Fig. 4, thrombin increases the phosphotyrosine content in healthy and diabetic platelets, although, as reported (Rosado et al. 2004c), tyrosine phosphorylation was greater in diabetics. Platelet treatment for 1 min with 0.05 mg/ml extract significantly reduced resting and thrombin-stimulated tyrosine phosphorylation by 43 and 22% in platelets from healthy donors and 40 and 43% in platelets from diabetics, respectively (Fig. 4; statistically significant, n=4). These findings suggest that the inhibitory effect of U. dioica extract on platelet aggregation and Ca2+ entry might be mediated by inhibition of protein tyrosine phosphorylation. As for Ca2+ mobilization, in the presence of the extract the phosphotyrosine content was similar in platelets from healthy and diabetic donors.
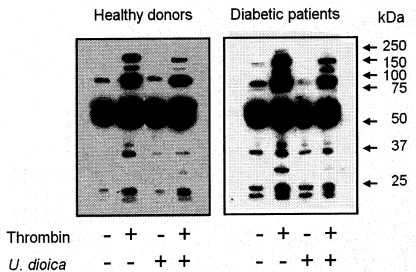
Fig. 4. Effect of U. dioica extract on thrombin-evoked tyrosine phosphorylation. Platelets from healthy and diabetic donors were suspended in HBS containing 1 mM Ca2+ and treated for 1 min at 37 °C with 0.05 mg/ml extract, as indicated, or the vehicle (Control). Cells were stimulated with 0.5 U/ml thrombin for 3 min or left untreated. Molecular size is indicated on the right. The panel shows the results from one experiment representative of three others.
In conclusion, our results indicate that U. dioica extracts reduce platelet aggregation in healthy and type 2 diabetic donors probably due to the inhibition of the production of endogenous ROS, Ca2+ mobilization and protein tyrosine phosphorylation. The crude aqueous extract of U. dioica reduces the hyperaggregability and hypersensitivity of platelets from diabetic donors, so that in the presence of the extract their responses are comparable to that observed in platelets from healthy donors. Antioxidants, which are ubiquitously included in vegetables, fruits and teas, might prevent or palliate cardiovascular complications associated with type 2 diabetes mellitus and might be used to design therapeutic strategies to prevent complications associated with platelet hyperactivity.
ACKNOWLEDGEMENTS
This study has been financially supported by Junta de Extremadura-ConsejerĂa de Sanidad y Consumo (SCSS0619) and AECI-PCI Spain-Morocco (A/7360/06). We thank Mercedes GĂłmez Blázquez for her technical assistance.
REFERENCES
Belton OA, Duffy A, Toomey S, Fitzgerald DJ: Cyclooxygenase isoforms and platelet vessel wall interactions in the apolipoprotein E knockout mouse model of atherosclerosis. Circulation 108:3017–3023, 2003.
Ben-Amor N, Redondo PC, Bartegi A, Pariente JA, Salido GM, Rosado JA: A role for 5,6-epoxyeicosatrienoic acid in calcium entry by de novo conformational coupling in human platelets. J. Physiol. 570:309–323, 2006.
Blankenship KA, Dawson CB, Aronoff GR, Dean WL: Tyrosine phosphorylation of human platelet plasma membrane Ca2+-ATPase in hypertension. Hypertension 35:103–107, 2000.
Blann AD, Nadar S, Lip GY: Pharmacological modulation of platelet function in hypertension. Hypertension 42:1–7, 2003.
Bnouham M, Merhfour FZ, Ziyyat A, Mekhfi H, Aziz M, Legssyer A: Antihyperglycemic activity of the aqueous extract of Urtica dioica. Fitoterapia 74:677–681, 2003.
Dixon LJ, Hughes SM, Rooney K, Madden A, Devine A, Leahey W, Henry W, Johnston GD, McVeigh GE: Increased superoxide production in hypertensive patients with diabetes mellitus: role of nitric oxide synthase. Am. J. Hypertens. 18:839–843, 2005.
Dogne JM, Leval XD, Benoit P, Delarge J, Masereel B, David JL: Recent advances in antiplatelet agents. Curr. Med. Chem. 9:577–589, 2002.
El Haouari M, Bnouham M, Bendahou M, Aziz M, Ziyyat A, Legssyer A, Mekhfi H: Inhibition of rat platelet aggregation by Urtica dioica leaves extracts. Phytother. Res. 20:568–572, 2006.
Garnier G, Bezanger-Beauquesne L, Debraux G: Ressources medicinales de la flore francaise, Vol 2, Vigot Freres, Paris 1961, pp. 962–964.
Grynkiewicz G, Poenie M, Tsien RY: A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440–3450, 1985.
JardĂn I, Redondo PC, Salido GM, Pariente JA, Rosado JA: Endogenously generated reactive oxygen species reduce PMCA activity in platelets from patients with non-insulin dependent diabetes mellitus. Platelets 17:283–288, 2006.
Legssyer A, Ziyyat A, Mekhfi H, Bnouham M, Tahri A, Serhrouchni M, Hoerter J, Fischmeister R: Cardiovascular effects of Urtica dioica L. in isolated rat heart and aorta. Phytother. Res. 16:503–507, 2002.
Lopez JJ, Redondo PC, Salido GM, Pariente JA, Rosado JA: Two distinct Ca2+ compartments show differential sensitivity to thrombin, ADP and vasopressin in human platelets. Cell. Signal. 18:373–381, 2006.
Mandal S, Sarode R, Dash S, Dash RJ: Hyperaggregation of platelets detected by whole blood platelet aggregometry in newly diagnosed noninsulin-dependent diabetes mellitus. Am. J. Clin. Pathol. 100:103–107, 1993.
Minuz P, Fumagalli L, Gaino S, Tommasoli RM, Degan M, Cavallini C, Lecchi A, Cattaneo M, Lechi Santonastaso C, Berton G: Rapid stimulation of tyrosine phosphorylation signals downstream of G-protein-coupled receptors for thromboxane A2 in human platelets. Biochem. J. 15:127–134, 2006.
Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, Violi F: Hydrogen peroxide is involved in collagen-induced platelet activation. Blood 91: 484–490, 1998.
Redondo PC, JardĂn I, Hernandez-Cruz JM, Pariente JA, Salido GM, Rosado JA: Hydrogen peroxide and peroxynitrite enhance Ca2+ mobilization and aggregation in platelets from type 2 diabetic patients. Biochem. Biophys. Res. Commun. 334:779–786, 2005.
Rosado JA, Graves D, Sage SO: Tyrosine kinases activate store-mediated Ca2+ entry in human p latelets through the reorganization of the actin cytoskeleton. Biochem. J. 351:429–437, 2000.
Rosado JA, Lopez JJ, Harper AG, Harper MT, Redondo PC, Pariente JA, Sage, SO, Salido GM: Two pathways for store-mediated calcium entry differentially dependent on the actin cytoskeleton in human platelets. J. Biol. Chem. 279:29231–29235, 2004a.
Rosado JA, Porras T, Conde M, Sage SO: Cyclic nucleotides modulate store-mediated calcium entry through the activation of protein-tyrosine phosphatases and altered actin polymerization in human platelets. J. Biol. Chem. 276:15666–15675, 2001.
Rosado JA, Redondo PC, Salido GM, Gomez-Arteta E, Sage SO, Pariente JA: Hydrogen peroxide generation induces pp60src activation in human platelets: evidence for the involvement of this pathway in store-mediated calcium entry. J. Biol. Chem. 279:1665–1675, 2004b.
Rosado JA, Saavedra FR, Redondo PC, Hernández-Cruz JM, Salido GM, Pariente JA: Reduced plasma membrane Ca2+-ATPase function in platelets from patients with non-insulin-dependent diabetes mellitus. Haematologica 89:1142–1144, 2004c.
Rosado JA, Sage SO: The actin cytoskeleton in store-mediated calcium entry. J. Physiol. 526: 221–229, 2000.
Ruf JC: Alcohol, wine and platelet function. Biol. Res. 37:209–215, 2004.
Saavedra FR, Redondo PC, Hernandez-Cruz JM, Salido GM, Pariente JA, Rosado JA: Store-operated Ca2+ entry and tyrosine kinase pp60src hyperactivity are modulated by hyperglycemia in platelets from patients with non insulin-dependent diabetes mellitus. Arch. Biochem. Biophys. 432:261–268, 2004.
Seno T, Inoue N, Gao D, Okuda M, Sumi Y, Matsui K, Yamada S, Hirata K, Kawashima S, Tawa R, Imajoh-Ohmi S, Sakurai H, Yokoyama M: Involvement of NADH/NADPH oxidase in human platelet ROS production. Thromb. Res. 103:399–409, 2001.
Tahri A, Yamani S, Legssyer A, Aziz M, Mekhfi H, Bnouham M, Ziyyat A: Acute diuretic, natriuretic and hypotensive effects of a continuous perfusion of aqueous extract of Urtica dioica. J. Ethnopharmacol. 73:95–100, 2000.
Willoughby S, Holmes A, Loscalzo J: Platelets and cardiovascular disease. Eur. J. Cardiovasc. Nurs. 1:273–288 , 2002.
Ziyyat A, Legssyer A, Mekhfi H, Dassouli A, Serhrouchni M, Benjelloun W: Phytotherapy of hypertension and diabetes in oriental Morocco. J. Ethnopharmacol. 58:45–54, 1997.
BACK





