Journal of APPLIED
BIOMEDICINEISSN 1214-0287 (on-line)
ISSN 1214-021X
(printed)
Volume 5 (2007)
Antihyperglycaemic and antiperoxidative effect of Helicteres isora L.
bark extracts in streptozotocin-induced diabetic rats Ganesan
Kumar, Gani Sharmila Banu, Arunachalam Ganesan Murugesan, Moses Rajasekara
Pandian
Address: Ganesan Kumar, Faculty in Biochemistry, Selvamm
Arts and Science College, Namakkal (DT)-637 003, Tamilnadu, India
pgsvedhakumar@yahoo.com.Full text article
(pdf)Full text
article (html)
Received 21st December 2006.
Revised 22nd January 2007.
Published online 9th March 2007.
Summary
The present investigation shows the antihyperglycaemic activity of aqueous extract
of bark of Helicteres isora L. (100, 200mg/kg b.w/p.o) in streptozotocin (STZ)
induced diabetic rats. Blood glucose levels, body weight, food and liquid intake
were measured on every 5th day over a period of 14 days. A single injection of
STZ at a dose of 60mg/kg b.w/i.p elevated the glucose levels >240mg/dl after
5 days. Administration of H.isora at a dose of 100, 200mg/kg/p.o resulted in a
significant (p<0.05) reduction in blood glucose levels. Body weights were
significantly (p<0.05) reduced in STZ-induced diabetic rats when compared to
normal rats while the extract significantly (p<0.05) prevented a decrease in
body weight in the H.isora treated animals. The study also evaluated the
antioxidant potential of H.isora in STZ-induced diabetic rats. Decreased levels
of thiobarbituric acid reactive substances (TBARS), increased levels of reduced
glutathione (GSH) and the activities of superoxide dismutase (SOD) and catalase
(CAT) resulted in the reduction of free radical formation in various tissues
such as liver, kidney, and brain of the diabetic rats. Tolbutamide was used as a
standard reference drug. The results clearly indicate that the aqueous extract
of bark of Helicteres isora exhibits significant antihyperglycaemic and in vivo
antioxidant activity in STZ-induced diabetic rats and the results were found to
be dose dependent.
Keywords: Helicteres isora – streptozotocin – antihyperglycemic activity – antioxidant potential
INTRODUCTION
Diabetes mellitus is a non-communicable disease, which is considered one of the five leading causes
of death in the world. About 100 million people around the world have been diagnosed with diabetes and by the year 2010, it is projected that 215 million people will have the disease (Zimmet 1999). Recently, the search for appropriate hypoglycaemic agents has been focused on plants used in traditional medicine, partly because of leads provided by traditional medicine to natural products that may be better treatments than currently used drugs (Rates 2001).
The bark of Helicteres isora Linn. (Sterculiaceae) has been used in the indigenous systems of medicine in India for the treatment of diabetes mellitus since time immemorial. The plant is a shrub or small tree available in forests throughout the Central and Western India. The roots and the bark are expectorant, demulcent and useful in the treatment of colic, scabies, gastropathy, diabetes, diarrhoea and dysentery (Kirtikar and Basu 1995). The fruits are astringent, refrigerant, stomachic, vermifugal, vulnerary and useful in bowel gripes, flatulence in children (Chopra et al. 1956) and has an antispasmodic effect (Pohocha and Grampurohit 2001). The aqueous extract of the bark showed a significant hypoglycaemic effect (Kumar et al. 2006a) and a lowering effect on hepatic enzymes (Kumar et al. 2006b). There are no available reports on the antihyperglycaemic potential of H. isora against diabetes. Therefore, we undertook the present study to determine the antihyperglycaemic efficacy of H. isora in STZ-induced diabetic rats.
MATERIALS AND METHODS
Chemicals
All the drugs and biochemicals used in this experiment were purchased from Sigma Chemical Company Inc., St. Louis, USA. The chemicals were of analytical grade.
Collection and processing of plant material
The bark of Helicteres isora L. was collected from Solakkadu, Kollimalai, Namakkal District, Tamil Nadu, India and authenticated by Fr. K.M. Matthew, Director, Rapinat Herbarium, St. Joseph’s College, Tiruchirapalli. Voucher Herbarium specimens have been deposited in the Herbarium (collection number 23644, 27406) for future reference.
The dried bark of H. isora L. was ground to fine powder with an auto-mix blender. Then the fine powder was suspended in an equal amount of water, stirred intermittently and left overnight. The macerated pulp was then filtered through a coarse sieve and the filtrate was dried at a reduced temperature. This dry mass (yield 185g/kg of powdered bark) served as the aqueous extract of H. isora L. for experimentation.
Animals
Male Wistar albino rats (weighing 160–200 g) were procured from the Animal house, Bharathidasan University, Tiruchirapalli under standard environmental conditions (12 h light/dark cycles at 25–28 centigrades, relative humidity 60–80 %). They were fed with a standard diet (Hindustan Lever, India) and water ad libitum and allowed to acclimatize for 14 days before the procedure. All studies were conducted in accordance with the National Institute of Health Guide (1985).
Induction of experimental diabetes
Rats were made diabetic by a single intraperitoneal administration of streptozotocin (60 mg/kg) dissolved in 0.1M citrate buffer, pH 4.5 (Siddique et al. 1987). Forty-eight hours later, blood samples were collected and glucose levels were determined to confirm the development of diabetes. Only those animals which showed hyperglycaemia (blood glucose levels > 240mg/dl) were used in the experiment (Ewart et al.1975, Cetto et al. 2000).
Experimental design
In the experiment, a total of 30 rats (24 diabetic surviving rats, 6 normal rats) were used. The rats were divided into 5 groups of 6 rats each.
Group 1: Normal rats.
Group 2: Diabetic control rats
Group 3: Diabetic rats given H. isora bark extract (100 mg/kg b.w.) in an aqueous solution daily for 14 days using an intragastric tube.
Group 4: Diabetic rats given H. isora bark extract (200 mg/kg b.w.) in an aqueous solution daily for 14 days using an intragastric tube.
Group 5: Diabetic rats given tolbutamide (250mg/kg b.w.) in an aqueous solution daily for 14 days using an intragastric tube (Kumar et al. 2006).
The effect of H. isora on STZ induced diabetic rats were determined by measuring blood glucose levels, food and fluid intake amount and changes in body weights (Ewart et al. 1975, Kamtchouing et al. 1998). After 14 days of treatment, all the rats were decapitated after fasting for 16 h. The animals were dissected and a drop of blood from the heart was used for the estimation of blood glucose. Tissues (brain, liver, and kidney) were removed and cleared of blood. They were immediately transferred to ice-cold containers containing 0.9% NaCl and homogenized in 0.1M Tris-HCl buffer (pH 7.4). After centrifugation at 2000 g for 10 min, the clear supernatant was used to measure the assay of enzyme activities.
Biochemical analysis
Estimation of blood glucose
Body weight and blood glucose levels were measured at the beginning of the experiment and at 5-day intervals. Blood samples were obtained by tail-vein puncture of the normal and STZ-induced diabetic rats on day zero (0), day 5, day 10 and on day 15. Blood glucose levels were determined by the O-toluidine method (Sasaki et al. 1972)
Acute toxicity study
Albino rats of 6 animals per group and weighing 200-220 g were administered a graded dose (100–5000 mg/kg b.w./p.o.) of the aqueous extract of H. isora. After administration of the H. isora the rats were observed for toxic effects for 48 h. The toxicological effects were observed in terms of mortality expressed as LD50. The number of animals dying during a period was noted (Ghosh 1984).
Determination of in vivo antioxidants
TBARS in tissues was estimated by the method of Nichans and Samuelson (1968). GSH was determined by the method of Ellman (1959). SOD and CAT were assayed utilizing the technique of Kakkar et al. (1978) and Sinha (1972) respectively.
Statistical analysis
All data were expressed as Mean ± SD of number of experiments (n=6). The statistical significance was evaluated by one-way analysis of variance (ANOVA) using SPSS version 7.5 (SPSS, Cary, NC, USA) and the individual com¬parisons were obtained by Duncan's Multiple Range Test (DMRT). A value of p<0.05 was considered to indicate a significant difference between groups (Duncan 1957).
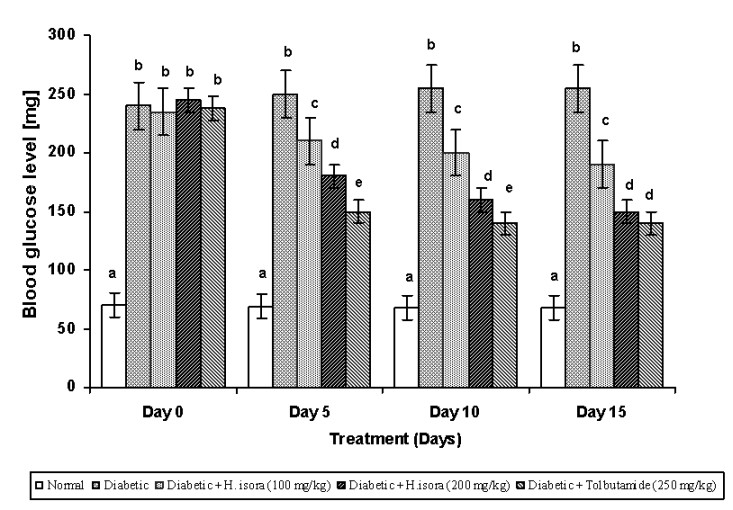
Fig. 1. Effect of aqueous extract of bark of Helicteres isora L. on blood glucose levels in STZ induced diabetic rats
Values are given as means ± SD of six animals in each group. Values not sharing a common superscript (a, b, c and d) differ significantly (Duncan’s Multiple Range Test).
RESULTS
Effect of H. isora on blood glucose levels
Blood glucose levels were measured in normal and STZ-induced diabetic rats. The results are shown in Fig. 1. There was a significant increase in blood glucose levels in STZ-induced diabetic rats (Group 2). Administration of H. isora at a dose of 100 mg and 200 mg/kg b.w. and tolbutamide (250mg/kg b.w.) significantly decreased blood glucose levels in STZ-induced rats (Group 3, 4 and 5). The results were found to be dose dependent .
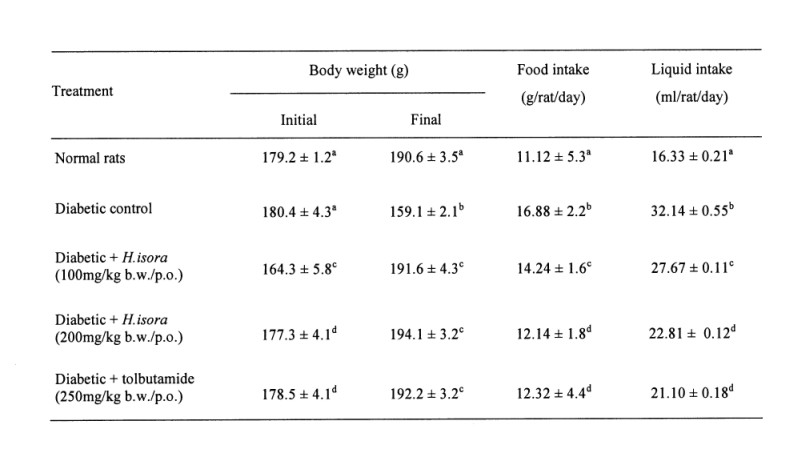
Effect of H. isora on body weight, food and liquid intake
Body weight, food and liquid intake were measured and are summarized in Table 1. The initial body weights were similar in normal and diabetic groups, whereas the final body weights were significantly decreased in the diabetic control (Group 2) when compared with the normal control (Group 1). At the same time, there was no significant difference in the body weights in H. isora and Tolbutamide treated diabetic rats (Group 3, 4 and 5). Food and fluid intake amount were significantly higher in the diabetic group than the normal (Table 1).
Effect of H. isora on the levels of TBARS and GSH
The concentration of TBARS and the GSH in tissues in the experimental diabetic rats are shown in Figs. 2 and 3. There was a significant elevation of TBARS in STZ-diabetic control rats when compared to normal rats. There was found to be a significant decrease in the levels of TBARS in liver, kidney and brain during diabetes in H. isora and Tolbutamide treated animals.
Table 1. Effect of Helicteres isora L. on body weight, food and liquid intake in rats
Symbols as in Fig. 1
There was a significant decrease in the concentration of GSH in the STZ-diabetic control group when compared with the normal. Administration of H. isora at the dose of 100 mg, 200 mg/kg b.w. reduced the levels of GSH in the liver and brain during diabetes while the kidneys did not show any significant change when compared with the normal group.
Effect of H. isora on the activity of SOD and CAT
The activities of SOD and CAT in experimental animal tissues are summarized in Figs. 4 and 5. There was a significant reduction in the activity of SOD in liver kidney and brain during diabetes. Administration of H. isora at 100 mg, 200 mg/kg b.w. and Tolbutamide 250mg/kg b.w. increased the activity of SOD in liver kidney and brain to near normal. There was a significant reduction in the activity of CAT in liver kidney and brain during diabetes. Administration of H. isora (100 mg, 200 mg/kg b.w.) and Tolbutamide (250 mg/kg b.w.) increased the activity of CAT in liver kidney and brain to near normal.
DISCUSSION
Hyperglycaemia, the primary clinical manifestation in diabetes, is associated with the development of certain complications of diabetes (Brownlee et al. 1981). Streptozotocin causes a significant elevation in the level of blood glucose in rats. But the administration of H. isora at doses of 100 mg, 200 mg/kg b.w. to diabetic rats caused a significant decrease in the blood glucose. Thus, H. isora has an antihyperglycaemic activity. Changes in body weight show that H. isora has a significant effect in controlling the loss of body weight, which is caused during diabetes. The activities were found to be dose dependent.
STZ is toxic to pancreatic Ăź-cells and is thus widely used for induction of experimental diabetes mellitus in animals, resulting in the production of reactive oxygen species (ROS) (Rakieten et al. 1963). ROS induced oxidative tissue damage plays an important role in many clinical disorders such as heart disease, diabetes, gout and cancer (Slater 1984, Vuillaume 1987, Meneghini 1988). Oxidative stress can be associated with the
peroxidation of cellular lipids, which is determined by measurement of TBARS (Nistico et al. 1992). The concentration of lipidperoxidation products may reflect the degree of oxidative stress in diabetes. It has been reported previously (Baynes 1991, Kakkar et al. 1995) that, in the tissues and blood of rats with STZ-induced diabetes, malondialdehyde, the production of lipidperoxidation, is increased. Further, the increased level of TBARS results in increased levels of oxygen free radicals, which attack the polyunsaturated fatty acids in cell membranes. STZ can also give rise to oxygen free radicals because of the increased blood glucose level in diabetes (Rakieten et al. 1963). We evaluated TBARS to determine the activity of H. isora in protection from oxidative damage in diabetes. The level of TBARS in the liver, kidney and brain of diabetic rats treated with H. isora was significantly decreased when compared to the STZ-induced diabetic rats. The decreased level of TBARS indicates that H. isora can improve the defective state of diabetes by means of inhibition of lipid peroxidation.
GSH is a major endogenous antioxidant which counterbalances free radical mediated damage (Mazumder et al. 2005). It is well known that GSH is involved in the protection of normal cell structure and function by maintaining the redox homeostasis, quenching free radicals and by participating in detoxification reactions (Matcovis et al. 1982). A significant decrease in the levels of GSH has been observed in H. isora-treated rats when compared to STZ-induced diabetic rats. The decrease in liver GSH levels represents an increased utilization due to oxidative stress (Anuradha et al. 1993). SOD scavenges the superoxide ions produced as cellular byproducts. SOD is a major defense for aerobic cells in combating the toxic effects of superoxide radicals (McCrod et al. 1976, Mazumder et al. 2005). CAT reduces the hydrogen peroxide produced by a dismutation reaction and prevents the generation of hydroxyl radicals thereby protecting the cellular constituents from oxidative damage in peroxisomes (Mazumder et al. 2005). The reduced activities of SOD and CAT in STZ-treated rats result in the accumulation of superoxide radicals and H2O2 respectively, which produce deleterious effects. H. isora supplementation showed normalization of SOD and CAT in liver, kidney and brain. Hence the present investigation exhibits strong antihyperglycaemic activity. Experiments suggest that H. isora also shows antioxidant activity.
Thus, H. isora seems to be effective in reducing oxidative stress and free radical-related diseases including diabetes.
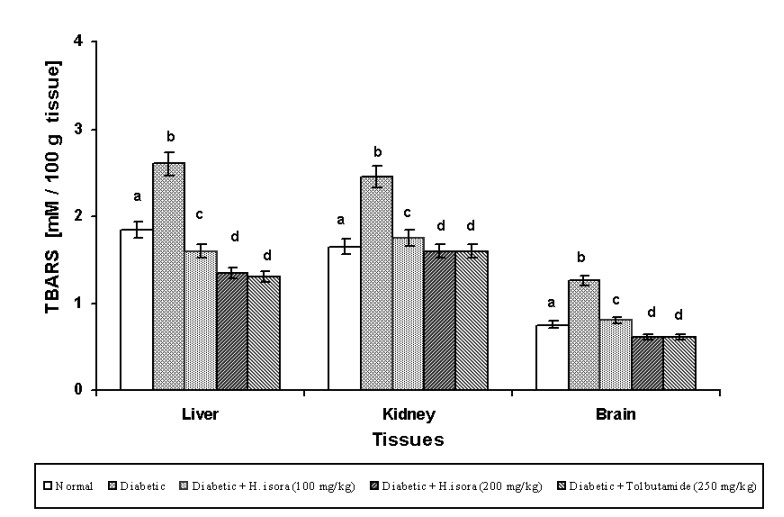
Fig. 2. Effect of aqueous extract of bark of Helicteres isora L. on TBARS levels in rat tissues. Symbols as in Fig. 1.
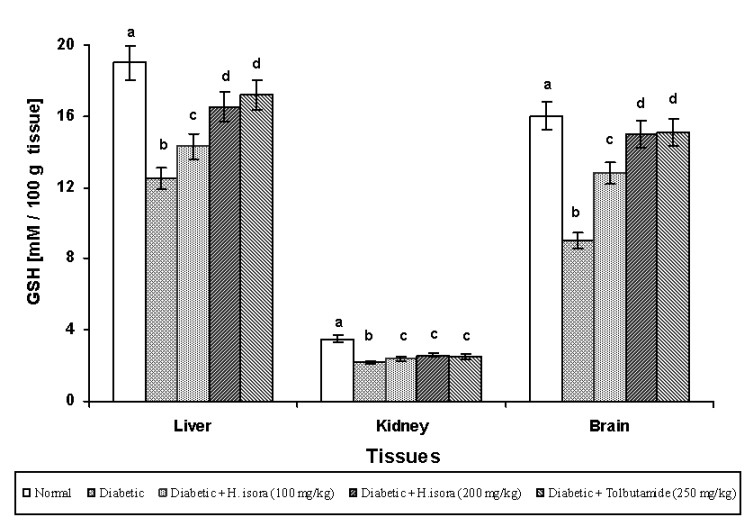
Fig. 3. Effect of aqueous extract of bark of Helicteres isora L. on GSH levels in rat tissues. Symbols as in Fig. 1.
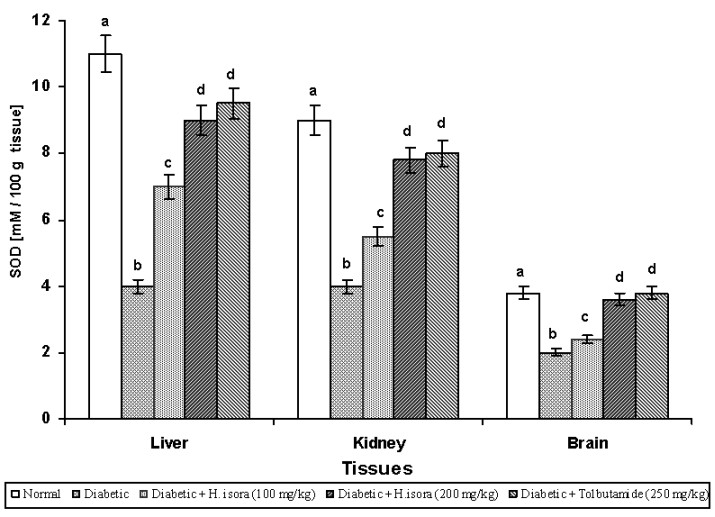
Fig. 4. Effect of aqueous extract of bark of Helicteres isora L. on SOD levels in rat tissues. Symbols as in Fig. 1.
REFERENCES
Anuradha CV, Selvam R: Effect of oral methionine on tissue lipid peroxidation and antioxidants in alloxan induced diabetic rats. J. Nutr. Biochem. 4:212–217, 1993.
Baynes JW: Role of oxidative stress in development of complications in diabetes. Diabetes 40:405–412, 1991.
Brownlee M, Cerami A: The biochemistry of the complications of diabetes mellitus. Annu. Rev. Biochem. 50:385–432, 1981.
Cetto AA, Weidenfeld H, Revilla MC, Sergio IA: Hypoglycemic effect of Equisetum mriochaetum aerial parts on STZ diabetic rats. J. Ethnopharmacol. 72:129–133, 2000.
Chopra RN, Nayar SL, Chopra IC: Glossary of Indian Medicinal Plants (1st ed.), CSIR, New Delhi, India 1956, p. 131.
Duncan BD: Multiple range tests for correlated and heteroscedastic means. Biometrics 13:359–364, 1957.
Ellman GL: Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82:70–77, 1959.
Ewart RBL, Kornfeld S, Kipnis DM: Effect of lectins on hormone release from isolated rat islets of langerhans. Diabetes 24:705–714, 1975.
Ghosh MN: Fundamentals of Experimental Pharmacology (2nd ed.), Scientific Book Agency, Calcutta 1984.
Kakkar P, Das B, Viswanathan PN: A modified spectrophotometric assay of SOD. Indian. J. Biochem.Biophys. 21:130–132, 1978.
Kakkar R, Kalra J, Mantha SV, Prasad K: Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol. Cell. Biochem. 151:113–119, 1995.
Kamtchouing P, Sokeng SD, Moundipa PF, Watcho P, Jatsa HB, Lontsi D: Protective role of Anacardium occidentale extract against streptozotocin-induced diabetes in rats. J. Ethnopharmacol. 62:95–99, 1998.
Kirtikar KR, Basu BD: Indian Medicinal Plants. Vol.1, International book distributors, Dehradun, India 1995, pp. 371–372.
Kumar G, Murugesan AG, Rajasekara Pandian M: Effect of Helicteres isora bark extract on blood glucose and hepatic enzymes in experimental diabetes. Pharmazie 61:353–355, 2006a.
Kumar G, Sharmila Banu G, Murugesan AG, Rajasekara Pandian M: Hypoglycaemic effect of Helicteres isora bark extract in rats. J. Ethnopharmacol. 107:304–307, 2006b.
Matcovis B, Varga SI, Szaluo L, Witsas H: The effect of diabetes on the activities of the peroxide metabolic enzymes. Horm. Metab. Res. 14:77–79, 1982.
Mazunder UK, Gupta M, Rajeshwar Y: Antihyperglycemic effect and antioxidant potential of Phyllanthus niruri (Euphorbiaceae) in streptozotocin induced diabetic rats. Eur. Bull. Drug Res. 13:1–9, 2005.
McCrod JM, Keele BB, Fridovich I Jr.: An enzyme based theory of obligate anaerobiosis; the physiological functions of superoxide dismutase. Proc. Natl. Acad. Sci. U.S.A. 68:1024–1027, 1976.
Meneghini R: Genotoxicity of active oxygen species in mammalian cells. Mutat. Res. 195:215–230, 1988.
National Institute of Health Guide for the Care and Use of Laboratory Animals. DHEW Publication (NIH), revised, Office of Science and Health Reports, DRR/NIH, Bethesda, USA, 1985.
Nichans WG, Samuelson B: Formation of MDA from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 6:126–130, 1968.
Nistico G, Cirilol HR, Fiskin K, Iannone M, Martino A, Rotilio G: NGF restores decrease in catalase activity and increases superoxide dismutase and glutathione peroxidase activity in the brain of aged rats. Free Radic. Biol. Med. 12:177–181, 1992.
Pohocha N, Grampurohit ND: Antispasmodic activity of the fruits of Helicteres isora Linn. Phytother. Res. 15:49–52, 2001.
Rakieten N, Rakieten ML, Nadkarni MV: Studies on the diabetogenic action of streptozotocin. Cancer Chemother. Rep. 29:91–98, 1963.
Rates SM: Plants as source of drugs. Toxicon 39:603–613, 2001.
Sasaki T, Matsui S, Sonae A: Effect of acetic acid concentration of a colour reaction in the O-toludine-boric acid method for blood glucose estimation. Rinshokagaku 1:346–353, 1972.
Siddique O, Sun Y, Lin JC, Chien YW: Facilitated transdermal transport of insulin. J. Pharm. Sci. 76:341–345, 1987.
Sinha KA: Colorimetric assay of catalase. Anal. Biochem. 47:389–394, 1972.
Slater TF: Free-radical mechanisms in tissue injury. J. Biochem. 222:1–15, 1984.
Vuillaume M: Reduced oxygen species, mutation, induction and cancer initiation. Mutat. Res. 186:43–72, 1987.
Zimmet PZ: Diabetes epidemiology as a tool to trigger diabetes research and care. Diabetologia 42:499–518, 1999.
BACK





