Journal of APPLIED
BIOMEDICINEISSN 1214-0287 (on-line)
ISSN 1214-021X
(printed)
Volume 6 (2008), No 2
Electrochemical biosensors principles and applications
Miroslav Pohanka, Petr Skladal
Address: Miroslav Pohanka, Centre of Advanced Studies,Faculty of Military Health Sciences, University
of Defense, Hradec Kralove,Czech Republic
rau@atlas.cz
Received 2nd January 2008.
Revised 7th January 2008.
Published online 24th April 2008.
Full text
article (pdf)
Abstract in xml formatSUMMARY
The first scientifically proposed as well as successfully commercialized biosensors were those based on
electrochemical sensors for multiple analytes. Electrochemical biosensors have been studied for a long
time. Currently, transducers based on semiconductors and screen printed electrodes represent a typical
platform for the construction of biosensors. Enzymes or enzyme labeled antibodies are the most common
biorecognition components of biosensors. The principles of, and the most typical applications for
electrochemical biosensors are described in this review. The relevant systems are divided into three types
according to the operating principle governing their method of measurement: potentiometric,
amperometric and impedimetric transducers, and the representative devices are described for each group.
Some of the most typical assays are also mentioned in the text.
KEYWORDS
enzyme electrode; immunosensor; potentiometric; amperometric; impedimetric transducer
INTRODUCTION
Electrochemical biosensors have been the subject
of basic as well as applied research for nearly fifty
years. Leland C. Clark introduced the principle of
the first enzyme electrode with immobilized
glucose oxidase at the New York Academy of
Sciences Symposium in 1962 (Clark and Lyons was
the YSI 23A Blood Glucose Analyzer; Yellow
1962). The first commercially produced biosensor
Springs Instruments (Yellow Springs, OH, USA)
placed on the market in 1975. This device was
applied to the fast glucose assay in blood samples
from diabetics. At present, there are many proposed
and already commercialized devices based on the
biosensor principle including those for pathogens
and toxins, some even based on a multi-channel
configuration (Pohanka et al. 2007a, b).
The most typical part of electrochemical
biosensors is the presence of a suitable enzyme in
the biorecognition layer providing electroactive
substances for detection by the physico-chemical
transducer providing the measurable signal. A
native enzyme can be used as the biorecognition
component; in this case the analyte is equal to the
enzyme substrate; alternatively it may function as
its inhibitor. In addition, enzymes can be used as
labels bound to antibodies, antigens and
oligonucleotides with a specific sequence, thus
providing affinity-based sensors (Bakker 2004). A
rather limited number of enzymes processed in
biotechnology were chosen for the monitoring of
clinical metabolites and, especially from the group
of oxidoreductases: glucose oxidase (Kafi et al.
2006) and glucose dehydrogenase (Antiochia et al.
2007) for glucose assays, alcohol oxidase for
ethanol (Yildiz and Toppare 2006), NADH
dependent lactate dehydrogenase (DÄŹAuria et al.
2000) and lactate:cytochrome c oxidoreductase for
lactate (Stein and McShane 2003; Garjonyte et al.
2006; Pohanka and Zboýil 2007), urease for urea
(Barhoumi et al. 2005) and cholesterol oxidase
co-immobilized with cholesterol esterase for the
cholesterol assay (Singh et al. 2007). Peroxidase
and alkaline phosphatase are the most common
enzyme labels for electrochemical affinity
biosensors (Skl dal 1997).
Based on their operating principle, the
electrochemical biosensors can employ
potentiometric, amperometric and impedimetric
transducers converting the chemical information
into a measurable amperometric signal.
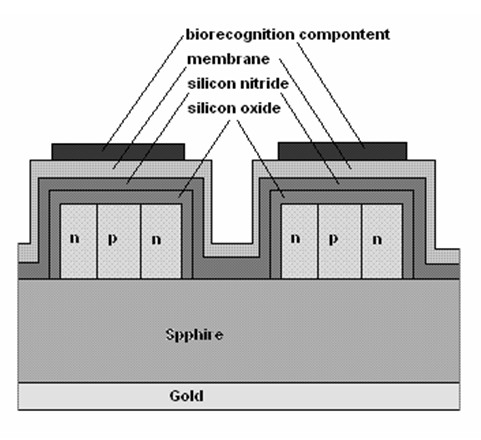
Fig. 1. Schematic drawing of a field effect transistor (n-p-n type) based biosensor.
POTENTIOMETRIC BIOSENSORS
Potentiometric biosensors are based on
ion-selective electrodes (ISE) and ion-sensitive
field effect transistors (ISFET). The primary
outputting signal is possibly due to ions
accumulated at the ion-selective membrane
interface. Current flowing through the electrode is
equal to or near zero. The electrode follows the
presence of the monitored ion resulting from the
enzyme reaction (Kauffmann and Guilbault 1991).
For example, glucose oxidase can be immobilized
on a surface of the pH electrode. Glucose has only
minimal influence on pH in the working medium;
however, the enzymatically formed gluconate
causes acidification. A biorecognition element is
immobilized on the outer surface or captured inside
the membrane. In the past the pH glass electrode
was used as a physicochemical transducer
(Newman and Setford 2006). The Nernst potential
of the pH glass electrode is described by the
Nicolsky-Eisenman equation, of which the
generalized form for ISE is as follows (Buerk
1993):

(E potential, R the universal gas constant, T
temperature, F Faraday constant, za followed and zi
interfering ion valence, aa activity of measured and
ai activity of interfering ion and Ka,i represents the
selectivity coefficient).
Nowadays, semiconductor based
physico-chemical transducers are more common.
ISFETs and LAPS (light addressable potentiometric
sensor) based systems especially are convenient for
biosensor construction. The ISFET principle
(Yuqing et al. 2003 and 2005) is based on a local
potential generated by surface ions from a solution.
This potential modulates the current flow across a
silicon semiconductor. The transistor gate surface in
ISFET is covered by a selective membrane; for pH
detection this could be made from compounds such
as silicon nitride (Si3N4), alumina (Al2O3),
zirconium oxide (ZrO2) and tantalum oxide (Ta2O5).
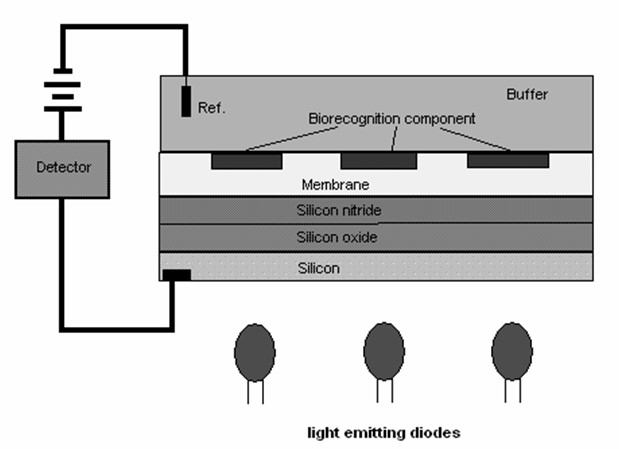
Fig. 2. Block diagram of the light addressable potentiometric sensor with biorecognition component bound into
membrane and with buffered reaction cell.
The LAPS principle (Yoshinobu et al. 2005) is
based on semiconductor activation by a
light-emitting diode (LED). The sensor is made
from an n-type silicon typically coated with 30 nm
of silicon oxide, 100 nm of silicon nitride, and
indium-tin oxide. The LAPS measures a voltage
change as a function of medium pH in the LED
activated zone. This opens the way for
multiposition sensing and construction of an array
of biorecognition zones.
A potentiometric biosensor with a molecularly
imprinted polymer constructed for the herbicide
atrazine assay allows detecting from 3Ĺľ10-5 to
1Ĺľ10-3 M (DÄŹAgostino et al. 2006); molecularly
imprinted polymer was also used for tracking the
level of neurotransmitter serotonin (Kitade et al.
2004). Another potentiometric biosensor with
co-immobilized urease and creatinase on the
poly(vinylchloride) ammonium membrane was used
for creatine analysis (Karakus et al. 2006).
ISFET with immobilized butyrylcholinesterase
was employed for the glycoalkaloids assay (Korpan
et al. 2006). A simple pH electrode modified with
acetylcholinesterase (AChE) was used for the
detection of organophosphate pesticides (Timur and
Telefoncu 2004). The LAPS biosensor was used for
the Escherichia coli assay allowing detection as low
as 10 cells/ml when the specific primary capture
antibody was immobilized on the LAPS
flow-through cell, and the secondary antibody
labeled by urease for sandwich complex formation
was used (Ercole et al. 2002). A commercial device
Bio-Detector (Smiths Detection, Warrington, UK)
based on the LAPS type biosensor is found in
mobile laboratories for automated 8-channel
analysis of biological agents.
AMPEROMETRIC BIOSENSORS
Amperometric biosensors are quite sensitive and
more suited for mass production than the
potentiometric ones (Ghindilis et al. 1998). The
working electrode of the amperometric biosensor is
usually either a noble metal or a screen-printed
layer covered by the biorecognition component
(Wang 1999). Carbon paste with an embedded
enzyme is another economic option (Cui et al.
2005). At the applied potential, conversion of
electroactive species generated in the enzyme layer
occurs at the electrode and the resulting current
(typically nA to mA range) is measured (Mehrvar
and Abdi 2004). The principle of the previously
mentioned YSI 23A (Magner 1998) can serve as an
example:
glucose + GOD(FAD) gluconolactone + GOD(FADH2) (1)
GOD(FADH2) + O2 GOD(FAD) + H2O2 (2)
H2O2 O2 + 2H+ + 2e- (3)
The reactions (1) and (2) are catalyzed by glucose
oxidase (GOD) containing FAD as a cofactor. The
last reaction is the electrochemical oxidation of
hydrogen peroxide at the potential of around +600
mV.
Amperometric biosensors can work in two- or
three-electrode configurations. The former case
consists of reference and working (containing
immobilized biorecognition component) electrodes.
The main disadvantage of the two-electrode
configuration is limited control of the potential on
the working electrode surface with higher currents,
and because of this, the linear range could be
shortened. To solve this problem, a third auxiliary
electrode is employed. Now voltage is applied
between the reference and the working electrodes,
and current flows between the working and the
auxiliary electrodes. A common screen-printed
three electrode sensor is shown in Fig. 3.
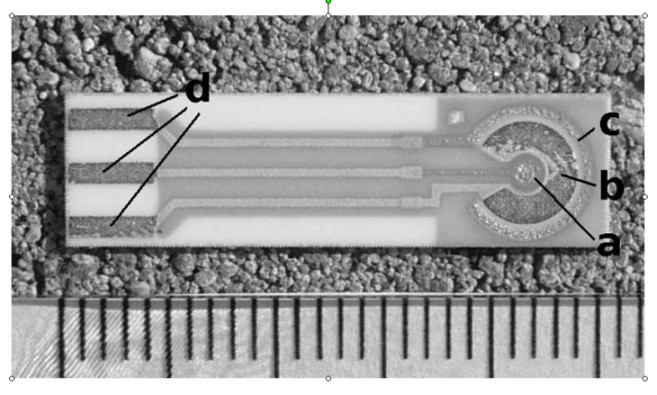
Fig. 3. Example of the three-electrode screen-printed sensor produced by BVT (Brno, Czech Rep.). The sensor body is
made from ceramics. A gold working electrode (a) is surrounded by an Ag/AgCl reference electrode (b) and gold auxiliary
electrode (c). Letter d means silver output contacts. The ruler in the bottom is in millimeter scale.
The amperometric biosensors are often used on
a large scale for analytes such as glucose, lactate
(Ohnuki et al. 2007), and sialic acid (Marzouk et al.
2007). Biological agents such as model Bacillus
cereus and Mycobacterium smegmatis (Yemini et
al. 2007), the serological diagnosis of Francisella
tularensis (Pohanka and Skl dal 2007), a
pharmacology study (Pohanka et al. 2007c) and the
detection of pesticides and nerve agents (Liu et al.
2006) have also been described. A metabolism
apparatus of whole cells can be used for certain
analytes such as the measurement of phenol with
immobilized Pseudomonas sp. cells (Skl dal et al.
2002). Biosensors based on AChE and
butyrylcholinesterase (BChE) can be employed for
rapid detection of organophosphates and
carbamates (Skl dal 1996) due to strong enzyme
inhibition (Krejźov et al. 2005). The AChE
amperometric biosensor based on a nanoporous
carbon matrix was used for the dichlorvos assay
(Sotiropoulou and Chaniotakis 2005) and a similar
device based on the screen-printed carbon electrode
modified with Prussian blue was tested for aldicarb,
paraoxon and parathion-methyl (Suprun et al.
2005). Amperometric biosensors were evaluated
also for assays with nucleic acid acting as a marker
and/or biorecognition component; uropathogens
were assayed using their 16S rRNA (Liao et al.
2006).
Several commercial amperometric biosensors
exist. The glucose biosensors are most well known
and commonly available; examples include SIRE
P201 (Chemel AB, Lund, Sweden), FreeStyle
Freedom Blood Glucose Monitoring System,
Precision Xtra (Abbot Diabetes Care, Alameda,
CA, USA), and GlucoWatch Biographer (Cygnus,
Redwood City, CA, USA). The device Midas Pro
(Biosensori SpA, Milan, Italy) is widely employed
for the analysis of surface waters (Rosseti et al.
2001).

Fig. 4. Simplified scheme of analytical device based on impedimetric biosensor. Scheme picture screen printed
transducer with typical labyrinth electrodes.
IMPEDIMETRIC BIOSENSORS
Such devices follow either impedance (Z) or its
components resistance (R) and capacitance (C);
inductance typically has only a minimal influence
in a typical electrochemical setup. Thus, the
expression of impedance is as follows:

The inverse value of resistance is called
conductance and for this reason some investigators
name such systems as conductometric. Impedance
biosensors include two electrodes with applied
alternating voltage, amplitudes from a few to 100
mV are used. The impedance biosensor is
commonly a functional part of the Wheatstone
bridge. These systems are considered for the assay
of urea when urease is used as a biorecognition
component. The following reaction takes place in
the medium:
(NH2)2CO + 3 H2O 2NH4+ HCO3- + OH- (4)
The principle is obvious; urea and water
molecules on the left side of the equation exhibit
only minimal influence on the measured impedance.
The enzymatically produced ions are able to
provide a significant increase of impedance.
Alternatively, impedance biosensors have been
successfully used for microorganism growth
monitoring due to the production of conductive
metabolites (Silley and Forsythe 1996). False
positive results due to electrolytes from the samples
are the main disadvantage of impedance biosensors.
Impedimetric biosensors are less frequent
compared to potentiometric and amperometric
biosensors; nevertheless, there have been some
promising approaches. Hybridization of DNA
fragments previously amplified by a polymerase
chain reaction has been monitored by an impedance
assay (Davis et al. 2007). A model impedance
immunosensor containing electrodeposited
polypyrrole film with captured avidin connected
through biotin to anti-human IgG was able to detect
antibodies as low as 10 pg/ml present in a sample
(Ouerghi et al. 2002). The ethanol level in some
alcoholic beverages was evaluated by an impedance
biosensor with immobilized yeast (Saccharomyces
cerevisiae; Korpan et al. 1994). The
impedance-based commercial device Malthus 2000
(Malthus Instruments, Crawley, UK) was used for
an assay of the pathogenic fungus Ichthyophonus
hofery (Spanggaard et al. 1994) and the Erwinia
carotovora rot (Fraaje et al. 1997).
CONCLUSION
Electrochemical biosensors have existed for nearly
fifty years and seem to possess great potential for
the future. This technology gains practical
usefulness from a combination of selective
biochemical recognition with the high sensitivity of
electrochemical detection. Thanks to current
technological progress, such biosensors profit from
miniaturized electrochemical instrumentation and
are thus very advantageous for some sophisticated
applications requiring portability, rapid
measurement and use with a small volume of
samples. Numerous commercial applications
confirm the attractive advantages of
electrochemical biosensors.
ACKNOWLEDGEMENT
Authors would like to thank to the Ministry of
Industry and Trade of the Czech Republic for the
Project No. 2A-1TP1/007. This work was
supported by the Ministry of Defence of the Czech
Republic Grant No. FVZ 000604.
REFERENCES
Antiochia R, Gorton L: Development of a carbon
nanotube paste electrode osmium
polymer-mediated biosensor for determination
of glucose in alcoholic beverages. Biosens.
Bioelectron. 22:2611-2617, 2007.
Bakker E: Electrochemical sensors. Anal. Chem.
76:3285-3298, 2004.
Barhoumi H, Maaref A, Rammah M, Martelet C,
Jaffrezic-Renault N, Mousty C, Cosnier S,
Perez E, Rico-Lattes E: Insulator semiconductor
structures coated with biodegradable latexes as
encapsulation matrix for urease. Biosens.
Bioelectron. 20:2318-2323, 2005.
Buerk DG: Biosensors. Theory and Applications.
Technomic Publ. Co., Lancaster, Pennsylvania
1993. p. 54.
Clark L, Lyons C: Electrode system for continuous
monitoring in cardiovascular surgery. Ann. N.
Y. Acad. Sci. 148:133-153, 1962.
Cui X, Liu G, Lin Y: Amperometric biosensors
based on carbon paste electrodes modified with
nanostructured mixed-valence manganese
oxides and glucose oxidase. Nanomedicine 1:
130-135, 2005.
DÄŹAgostino G, Alberti G, Biesuz R, Pesavento M:
Potentiometric sensor for atrazine based on a
molecular imprinted membrane. Biosens.
Bioelectron 22:154-152, 2006.
DÄŹAuria S, Gryczynski Z, Gryczynski I, Rossi M,
Lakowicz JR: A protein biosensor for lactate.
Anal. Biochem. 283:83-88, 2000.
Davis F, Hughes MA, Cossins AR, Higson SP:
Single gene differentiation by DNA-modified
carbon electrodes using an AC impedimetric
approach. Anal. Chem. 79:1153-1157, 2007.
Ercole C, Gallo MD, Pantalone M, Santucci S,
Mosiello L, Laconi C, Lepidi AA: A biosensor
for Escherichia coli based on a potentiometric
alternating biosensing (PAB) transducer. Sens.
Actuators B Chem. 83:48-52, 2002.
Fraaje BA, Appels M, de Boer SH, van Vuurde
JWL, van den Bulk RW: Detection of soft rot
Erwinia spp. on seed potatoes: conductimetry in
comparison with dilution plating, PCR and
serological assays. Eur. J. Plant Pathol. 103:
183-193, 1997.
Ghindilis AL, Atanasov P, Wilkins M, Wilkins E:
Immunosensors: electrochemical sensing and
other engineering approaches. Biosens.
Bioelectron. 13:113-131, 1998.
Karakus E, Erden PE, Pekyardimci S, Kilic E:
Determination of creatine in commercial
creatine powder with new potentiometric and
amperometric biosensors. Artif. Cells Blood
Substit. Immobil. Biotechnol. 34:337-347,
2006.
Kafi AK, Lee DY, Park SH, Kwon YS: DNA as a
support for glucose oxidase immobilization at
Prussian blue-modified glassy carbon electrode
in biosensor preparation. J. Nanosci.
Nanotechnol. 6:3539-3542, 2006.
Kauffmann JM, Guilbault GG: Potentiometric
enzyme electrodes. Bioprocess Technol. 15:
63-82, 1991.
Kitade T, Kitamura K, Konishi T, Takegami S,
Okuno T, Ishikawa M, Wakabavashi M,
Nishikawa K, Muramatsu Y: Potentiometric
immunosenor using artificial antibody based on
molecularly imprinted polymers. Anal. Chem.
76:6802-6807, 2004.
Korpan YI, Dzyadevich SV, Zharova VP, ElÄŹskaya
AV: Conductometric biosensor for ethanol
detection based on whole yeast cells. Ukr.
Biokhim. Zh. 66:78-82, 1994.
Korpan YI, Raushel FM, Nazarenko EA, Soldatkin
AP, Jaffrezic-Renault N, Martelet C: Sensitivity
and specificity improvement of an ion sensitive
field effect transistors-based biosensor for
potato glycoalkaloids detection. J. Agric. Food
Chem. 54:707-712, 2006.
Krejźova G, Kuźa K, ćevelova L: Cyclosarin-an
organophosphate nerve agent. Def. Sci. J. 55:
105-115, 2005.
Liao JC, Mastali M, Gau V, Suchard MA, Moller
AK, Bruckner DA, Babbitt JT, Li Y, Gornbein
J, Landaw EM, McCabe ERB, Churchill BM et
al.: Use of electrochemical DNA biosensors for
rapid molecular identification of uropathogens
in clinical urine specimens. J. Clin. Microbiol.
44:561-570, 2006.
Liu G, Lin Y: Biosensor based on self-assembling
acetylcholinesterase on carbon nanotubes for
flow injection/amperometric detection of
organophosphate pesticides and nerve agents.
Anal. Chem. 78:835-843, 2006.
Magner E: Trends in electrochemical biosensors.
Analyst 123:1967 1970, 1998.
Marzouk SA, Ashraf SS, Tayyari KA: Prototype
amperometric biosensor for sialic acid
determination. Anal. Chem. 79:1668-1674,
2007.
Mehrvar M, Abdi M: Recent developments,
characteristics, and potential applications of
electrochemical biosensors. Anal. Sci. 20:
1113-1126, 2004.
Newman JD, Setford SJ: Enzymatic biosensors.
Mol. Biotechnol. 32:249-268, 2006.
Ohnuki H, Saiki T, Kusakari A, Endo H, Ichihara
M, Izumi M: Incorporation of glucose oxidase
into languir-blodgett films based on prussian
blue applied to amperometric glucose
biosensor. Langmuir 23:4675-4681, 2007.
Ouerghi O, Touhami A, Jaffrezic-Renault N,
Martelet C, Ouada HB, Cosnier S:
Impedimetric immunosensor using
avidin-biotin for antibody immobilization.
Bioelectrochemistry 56:131-133, 2002.
Pohanka M, Jun D, KuĹşa K: Mycotoxin assays
using biosensor technology a review. Drug
Chem. Toxicol. 30:253-261, 2007a.
Pohanka M, Skl dal P, Kroźa M: Biosensors for
biological warfare agent detection. Def. Sci. J.
57:185-193, 2007b.
Pohanka M, Jun D, KuĹşa K: Amperometric
biosensor for evaluation of competitive
cholinesterase inhibition by the reactivator HI-6.
Anal. Lett. 40:2351-2359, 2007c.
Pohanka M, Skl dal P: Serological diagnosis of
tularemia in mice using the amperometric
immunosensor. Electroanalysis 24:2507-2512,
2007d.
Pohanka M, Zboýil P: Amperometric biosensor for
D-lactate assay. Food Technol. Biotechnol.
46:107-110, 2008.
Rosseti C, Pomati F, Calamari D: MicroorganismsÄŹ
activity and energy fluxes in Lake Varese
(Italy): a field method. Water Res.
35:1318-1324, 2001.
Silley P, Forsythe S: Impedance microbiology: a
rapid change for microbiologists. J. Appl.
Bacteriol. 80:233-243, 1996.
Singh S, Singhal R, Malhotra BD: Immobilization
of cholesterol esterase and cholesterol oxidase
onto sol-gel films for application to cholesterol
biosensor. Anal. Chim. Acta 582:335-343,
2007.
Skl dal P: Biosensors based on cholinesterase for
detection of pesticides. Food Technol.
Biotechnol. 34:43-49, 1996.
Skl dal P: Advances in electrochemical
immunosensors. Electroanalysis 9:737-745,
1997.
Skl dal P, Morozova NO, Reshetilov AN:
Amperometric biosensors for detection of
phenol using chemically modified electrodes
containing immobilized bacteria. Biosens.
Bioelectron. 17:867-873, 2002.
Sotiropoulou S, Chaniotakis NA: Lowering the
detection limit of the acetylcholinesterase
biosensor using a nanoporous carbon matrix.
Anal. Chim. Acta. 530:199-204, 2005.
Spanggaard B, Gram L, Okamoto N, Huss HH:
Growth of the fish-pathogenic fungus,
Ichthyophonus hoferi, measured by
conductimetry and microscopy. J. Fish Dis.
17:145-153, 1994.
Stein EW, McShane MJ: Multilayer lactate oxidase
shells on colloidal carriers as engines for
nanosensors. IEEE Trans. Nanobioscience
2:133-137, 2003.
Suprun E, Evtugyn G, Budnikov H, Ricci F,
Moscone D, Palleschi G: Acetylcholinesterase
sensor based on screen-printed carbon electrode
modified with Prussian blue. Anal. Bioanal.
Chem. 383:597-604, 2005.
Timur S, Telefoncu A: Acetylcholinesterase
(AChE) electrodes based on gelatin and
chitosan matrices for the pesticide detection.
Artif. Cells Blood Substit. Immobil. Biotechnol.
32:427-442, 2004.
Wang J.: Amperometric biosensors for clinical and
therapeutic drug monitoring: a review.
J. Pharm. Biomed. Anal. 19:47 53, 1999.
Yildiz HB, Toppare L: Biosensing approach for
alcohol determination using immobilized
alcohol oxidase. Biosens. Bioelectron. 21:
2306-2310, 2006.
Garjonyte R, Melvydas V, Malinauskas A:
Mediated amperometric biosensors for lactic
acid based on carbon paste electrodes modified
with bakerÄŹs yeast Saccharomyces cerevisiae.
Bioelectrochemistry 68:191-196, 2006.
Yemini M, Levi Y, Yagil E, Rishpon J: Specific
electrochemical phage sensing for Bacillus
cereus and Mycobacterium smegmatis.
Bioelectrochemistry 70:180-184, 2007.
Yoshinobu T, Iwasaki H, Ui Y, Furuichi K,
Ermolenko Y, Mourzina Y, Wagner T, Nather
N, Schoning MJ: The light-addressable
potentiometric sensor for multi-ion sensing and
imaging. Methods 37:94-102, 2005.
Yuqing M, Jianquo G, Jianrong C: Ion sensitive
field effect transducer-based biosensors.
Biotechnol. Adv. 21:527-534, 2003.
Yuqing M, Jianrong C, Keming F: New technology
for the detection of pH. J. Biochem. Biophys.
Methods 63:1-9, 2005.
BACK






