Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 6 (2008), No 4
Antioxidant efficacy of flavonoid-rich fraction from Spermacoce hispida
in hyperlipidemic rats
Kuppusamy Kaviarasan, Panneerselvam Kalaiarasi, Vishwanathan Pugalendi
Address: Kuppusamy Kaviarasan, Department of Biochemistry and Biotechnology, Faculty of Science, Annamalai University, Annamalainagar – 608 002, Tamilnadu, India
drpugalendi@sancharnet.in
Received 21st April May 2008.
Revised 25th August 2008.
Published online 16th September 2008.
Full text article (pdf)Abstract in xml format
SUMMARY
Phytochemicals in fruits, vegetables, spices and traditional herbal medicinal plants have been found to play a protective role against many human chronic diseases including cancer and cardiovascular diseases. These diseases are associated with oxidative stress caused by excess free radicals and other reactive oxygen species. Fractions rich in flavonoids obtained from
S. hispida seeds were orally administered at three different doses of 20, 40 and 80 mg/kg BW to HFD fed rats. The antioxidant activity of a flavonoid-rich fraction was measured both
in vitro and in vivo. The flavonoid-rich fraction effectively scavenged DPPH and ABTS+ radicals in vitro. Further, the results showed elevated activities of free radical-scavenging enzymes (SOD, CAT and GPx) and increased levels of non-enzymic antioxidants (GSH, vitamins C and E). TBARS and lipid hydroperoxides decreased significantly in flavonoid-rich fraction treated rats compared to HFD control. Among the doses used, 40 mg/kg BW dose showed maximum effect. Thus, the results indicate that a S. hispida seed flavonoid-rich fraction possesses free radical scavenging and antioxidant activity both in vitro and in vivo.
KEYWORDS
hyperlipidemia; lipid hydroperoxides; antioxidants; flavonoid
Abbreviations
ABTS.+ 2,2'-Azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid) radical
ATV Atorvastatin
BW Body weight
CAT Catalase
DMSO Dimethyl sulphoxide
DPPH. 2, 2-Diphenyl-1-picrylhydrazyl radical
GAE Gallic acid equivalents
GPx Glutathione peroxidase
GSH Reduced glutathione
HFD High fat diet
LDL-C Low density lipoprotein cholesterol
LOOH Lipid hydroperoxide
OH. Hydroxyl radical
PUFA Polyunsaturated fatty acids
QE Quercetin equivalents
ROS Reactive oxygen species
O2.- Superoxide radical
SOD Superoxide dismutase
TBARS Thiobarbituric acid reactive substances
alpha-Toc-O. Tocopheroxyl radical
INTRODUCTION
Oxidative stress in hyperlipidemia is thought to be a factor in the development of atherosclerotic plaques (Volkovova et al. 2006). Reactive oxygen
species (OH., O2.- and singlet oxygen) are constantly generated in our body due to normal metabolism, through leakage of electrons from the electron transport chain and by the activities of oxidoreductase enzymes. Polyunsaturated fatty acids in cellular membranes are especially prone to damage by lipid peroxidation. Therefore, cells and tissues have evolved both enzymatic and non-enzymatic antioxidant systems to combat the oxidative stress caused by reactive oxygen species (ROS). However, such endogenous antioxidants are not sufficient under the conditions of extreme oxidative stress, thus making it necessary to rely on exogeneous antioxidants. In this context, flavonoids and polyphenols are proving to be highly effective and are therefore gradually emerging as viable alternatives to conventional drugs for various diseases (Yoshida et al. 1999). Flavonoids are phenolic compounds widely distributed in plants, with over 4000 individual substances known and the numbers constantly increasing (Harborne 1986). In the present study, an attempt is made to evaluate the antioxidant activity of flavonoid-rich fraction from S. hispida seeds, which is listed in indigenous medicine as having high therapeutic value and is even now being used in various diseases. S. hispida L. (Rubiaceae) was popularly known as “Nattaiccuri” in Tamil or “Shaggy button weed” in English (Narayan and Kumar 2003). It is widely distributed in the Western ghats of Kerala (Puspagandan and Atal 1984) and in Maruthamalai forest, which is an extension of Western ghats (Sekar and Francis 1999) in Tamil Nadu. The seed-extract of the plant has been used as a remedy for the treatment of internal injuries of nerves and kidney. It is suggested that it can remove signs of old age, purify blood and improve vitality, and has been used by the tribals living in the forest regions in the Western ghats of Kerala since ancient times (Puspagandhan and Atal 1984). It has been also reported that S. hispida is an effective natural drug for the treatment of hypertension (Arnold and Schmidt 2004).
S. hispida was one of the five plants which contained the maximum amount of flavonoids among 25 plants analyzed (Sekar and Francis 1999). Plants containing flavonoids have been reported to possess strong antioxidant and hypolipidemic properties (Aviram 2004, Sudheesh and Vijayalakshmi 2005, Sweedy et al. 2007). Previously, methanolic extract of this whole plant extract exhibited strong antioxidant activity (Surveswaran et al. 2007). Further, oxidative stress is the causative factor that links hyperlipidemia in the pathogenesis of atherosclerosis. Hence, in the present study, the flavonoid-rich extract of S. hispida seeds was investigated for antioxidant activity in hyperlipidemic rats.
MATERIALS AND METHODS
Chemicals
2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) were purchased from Sigma Chemical Company Inc., St. Louis, USA and all the other chemicals used were of analytical grade purchased from E Merck or Himedia, Mumbai, India.
Animals
Eight-week-old adult male albino rats of Wistar strain, weighing approximately 150 to 180 g, were acclimatized for 7 days at room temperature (30+-3 oC) and relative humidity (55%) in a 12-hour light/dark cycle in a room under hygienic condition. They were given access to water and a commercial diet ad libitum. The experiments were carried out as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India, and approved by the Institutional Animal Ethics Committee (IAEC), Annamalai University (Approval number: 361).
Experimental protocol
Rats were randomly divided into seven groups of six rats each. Group 1 served as normal and received standard pellet diet (Table 1) + 0.5% dimethyl sulphoxide (DMSO). Group 2 received flavonoid-rich fraction at a dose of 80 mg/kg body weight (BW) in 0.5% DMSO. Groups 3, 4, 5, 6 and 7 received a high fat diet (Table 1) + 0.5% DMSO. Groups 4, 5 and 6 received flavonoid-rich fraction at a dose of 20, 40 and 80 mg/kg BW and group 7 received atorvastatin, a reference drug, at 2 mg/kg BW in 0.5% DMSO. The total duration of the experiment was 9 weeks (66 days). The experimental groups received 10% of groundnut oil from 0-7th day, 15% from 8th to 14th day and 20% from 15th to 66th day, all mixed with the diet as shown in Table 1. Different doses of flavonoid-rich fractions or atorvastatin were given by intragastric
tube to different groups from 4th week (i.e. 22nd day) onwards, along with 20% high fet diet (HFD). The animals were fasted for 12 h, anaesthetized using ketamine (24 mg/kg BW) intramuscular injection and sacrificed by cervical dislocation on the morning of the 67th day and tissues dissected out, washed, weighed, homogenized and centrifuged. The tissue supernatant and plasma were used for the biochemical analysis.
Biochemical analysis
Lipid peroxidation was estimated as evidenced by the formation of thiobarbituric acid reactive substances (TBARS), and lipid hydroperoxides (LOOH). TBARS were assayed in plasma and tissues by the method described by Ohkawa et al. (1979). Malondialdehyde (MDA) and other TBARS were measured by their reactivity with thiobarbituric acid in an acidic condition to generate pink coloured chromophore which was read at 535 nm. LOOH was estimated by the method of Jiang et al. (1992) based on the oxidation of ferrous ion (Fe2+) under acidic conditions in the presence of xylenol orange that leads to the formation of a chromophore with an absorbance maximum at 560 nm. The activity of superoxide dismutase (SOD) was estimated by the method of Kakkar et al. (1984) based on 50% inhibition of the formation of NADH-phenazine methosulphate nitroblue tetrazolium (NBT) formazan at 520 nm. The activity of catalase (CAT) was assayed by the method of Sinha (1972). Dichromate in acetic acid was converted to perchromic acid and then to chromic acetate, when heated in the presence of H2O2. The reaction was stopped by the addition of dichromate – acetic acid mixture and the chromic acetate formed was measured colorimetrically at 620 nm. Glutathione peroxidase (GPx) activity was assayed by the method of Rotruck et al. (1973). A known amount of enzyme preparation was allowed to react with H2O2 in the presence of GSH for a specified time period. GPx utilize GSH for the decomposition of H2O2. After a specific time, the remaining GSH content was measured by Ellman’s method.
GSH was estimated by the method of Ellman (1959). This method was based on the formation of 2-nitro-5-thiobenzoic acid (a yellow color compound) when dithionitrobenzoic acid was added to compounds containing sulfhydryl groups. The yellow colour developed was read at 412 nm. The level of vitamin C was assessed by the method of Roe and Kuether (1943). The ascorbic acid was converted to dehydroascorbic acid by mixing with norit and then coupled with 2, 4- dinitrophenylydrazine (DNPH) in the presence of thiourea, a mild reducing agent. The coupled dinitrophenylhydrazine was converted into a red coloured compound when treated with sulfuric acid and read at 520 nm. Vitamin E was estimated by the method of Baker and Frank (1980). The method involves the reduction of ferric ions to ferrous ions by alpha-tocopherol and the formation of a red colored complex with 2, 2-dipyridyl. Absorbance of the chromophore was measured at 520 nm.
Plant material
S. hispida seeds were collected from the local market, Chidambaram, Cuddalore district, Tamil Nadu, India and dried under shade. The plant was identified and a voucher specimen (No: AU 1917) was deposited at the Herbarium of Botany Directorate, Annamalai University. The dried seeds were made into fine powder with an auto-mix blendor and were kept separately in an airtight container until use.
Extraction of total flavonoids
Seed powder was extracted using a soxhlet apparatus with methanol (18 h) (Petra et al. 1999). The extracted solution was filtered and concentrated in a rotary evaporator under reduced pressure (rotary vacuum flash evaporator). The concentrated extract was again exhaustively defatted by refluxing with n-hexane and benzene (15 h twice). The two fractions were negative for polyphenols. Then the defatted bulk residue was successively extracted by refluxing with ethyl acetate (15 h twice). Total polyphenols (Fukumto and Mazza 2000) and total flavonoids (Jia et al. 1999) were spectrophotometrically estimated in the ethyl acetate fraction (total polyphenols content 693.24 +- 18.78 mg GAE/g dry weight) which was found to contain the bulk of flavonoids (total flavonoids content 410.57 +- 16.23 mg QE/g dry weight) and this fraction was evaporated in a rotary evaporator under reduced pressure, freeze-dried and used for the study.
DPPH radical scavenging activity
The radical scavenging activity of S. hispida flavonoid-rich fraction against DPPH., was determined spectrophotometrically by the method of Blios (1958). DPPH., is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule. DPPH., reacts with an antioxidant compound that can donate hydrogen and gets reduced. The change in colour (from deep violet to light yellow) was measured. The intensity of the yellow colour depends on the amount and
nature of radical scavenger present in the sample or standard compounds. The reaction mixture containing 1ml of 0.1mM DPPH., and various concentrations of extract (20, 40, 60, 80, 100 and 120 µg) were made up to 3ml with water and were incubated for 10 minutes. The blue colour formed was measured at 517 nm. Besides atorvastatin, ascorbic acid was used as a standard for comparison.
Evaluation of total antioxidant activity - (ABTS radical cation decolourization assay)
2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) radical cation forms the basis of one of the spectrophotometric methods that has been applied for the measurement of the total antioxidant activity of solutions of pure substances (Wolfenden and Willson 1982). The improved technique for the generation of ABTS+ described here involves the direct production of the blue/green ABTS.+ chromophore through the reaction between ABTS.+ and potassium persulphate. The addition of S. hispida flavonoid-rich fraction competes with ABTS.+ and diminishes the colour formation. ABTS.+ was dissolved in water at a concentration of 7mM. The stock solution was mixed with 2.45 mM potassium persulphate. The mixture was allowed to stand in the dark at room temperature for 12-16 h before use for incomplete oxidation of ABTS.+. The radical was stable in this form for more than two days when stored in the dark at room temperature. The incubation mixture in a total volume of 5 ml contained 0.54 ml of ABTS.+, 0.5 ml of phosphate buffer and varying concentrations of S. hispida (20, 40, 60, 80, 100 and 120 µg). The blank contained water in place of S. hispida or standard. The absorbance was read in spectrophotometer at 734 nm and compared with ascorbic acid and atorvastatin.
RESULTS
In vitro assay
The flavonoid-rich fraction of S. hispida exhibited strong antioxidant activity in DPPH. and ABTS.+ radical scavenging assay as evidenced by the low IC50 (concentration at 50% inhibition) values (Figs 1, 2). These values were found to be less than those obtained for ascorbic acid or atorvastatin suggesting more antioxidant ability.
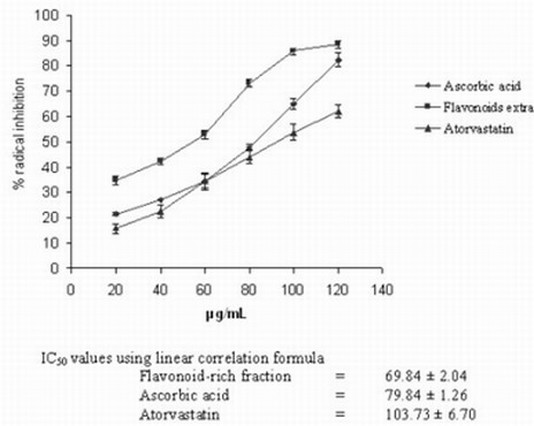
Fig. 1. DPPH radical scavenging activity of S. hispida and flavonoid-rich fraction
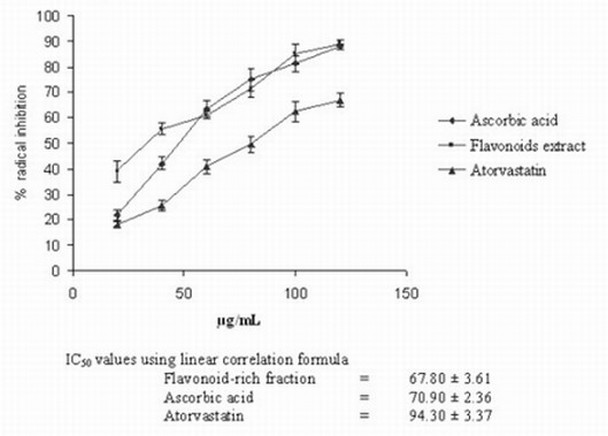
Fig. 2. Total antioxidant activity of S. hispida seed flavonoid-rich fraction by ABTS-radical cation decolourization assay
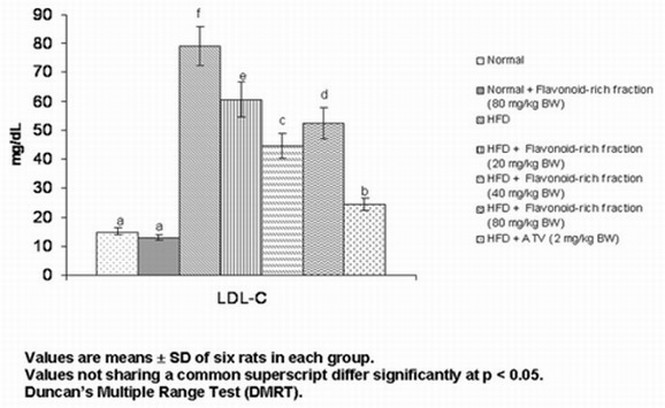
Fig. 3. Effect of S. hispida seed flavonoid-rich fraction on the plasma LDL-C of normal and hyperlipidemic rats
In vivo assay
Fig. 3 shows the effect of S. hispida seed flavonoid-rich fraction on low-density-lipoprotein cholesterol (LDL-C) levels in the plasma and tissues of normal and hyperlipidemic rats. The levels of LDL-C increased significantly in HFD rats and the administration of flavonoid-rich fraction decreased all these parameters significantly with a maximum at 40 mg/kg BW. However, the effect was still lower than that of atorvastatin.
Table 2 shows the effect of S. hispida seed flavonoid-rich fraction on the food intake, and body weight of normal and hyperlipidemic rats. HFD fed rats exhibited a significant increase in body weight and food intake. Administration of flavonoid-rich fraction or atorvastatin reduced the body weight significantly in HFD rats. There was no significant difference in body weight between flavonoid-rich fractions or atorvastatin treated rats. Food intake was the same in all the HFD-rats.
Table 1. Composition and varying percentages of experimental diet
Composition of
Standard pellet diet
|
Week I
(10%)
|
Week II
(15%)
|
Week III
(20%)
|
Composition of
High fat diet
|
|
Protein 17.7%
Fat 4.2%
Carbohydrate 50.5%
Fiber 3.4 %
Minerals 6.7 %
Vitamins 1.7%
|
Groundnut oil
(5.8 ml/100 g)
|
Groundnut oil
(10.8 ml/100 g)
|
Groundnut oil
(15.8 ml/100 g)
|
Protein 17.7%
Fat 20%
Carbohydrate 0.5%
Fiber 3.4 %
Minerals 6.7 %
Vitamins 1.7%
|
Table 2. Effect of S. hispida seed-flavonoid-rich fraction on the food intake, body weight and weight gain of normal and hyperlipidemic rats
Groups
|
Body weight (g)
|
Net gain (g)
|
Average
Food intake (g)
|
|
Initial day
|
Final day
|
|
Normal
|
161.67 +-
5.16
|
212.5 +- 8.80a
|
|
22.5 +- 0.93a
|
|
Normal +
flavonoid-rich fraction (80 mg/kg BW)
|
165.33 +-
6.05
|
206.67 +-
9.3a
|
|
|
|
HFD control
|
169.17 +-
7.35
|
286.67 +-
12.11c
|
117.5 +- 8.09d
|
25.83 +- 1.09b
|
|
HFD +
flavonoid-rich fraction (20 mg/kg BW)
|
170.83 +-
8.61
|
239.17 +-
7.35b
|
68.33 +- 6.06c
|
27.1 +- 1.41b
|
|
HFD +
flavonoid-rich fraction (40 mg/kg BW)
|
171.67 +-
9.30
|
234.17 +-
8.61b
|
62.5 +- 3.32b,c
|
26.72 +- 0.92b
|
|
HFD +
flavonoid-rich fraction (80 mg/kg BW)
|
170.00 +-
7.07
|
238.33 +-
8.16b
|
68.2 +- 8.01c
|
26.83 +- 0.91b
|
|
HFD +
atorvastatin (2 mg/kg BW)
|
171.65 +-
8.16
|
223.33 +-
6.83b
|
51.67 +- 4.29b
|
26.67 +- 1.07b
|
Values are means +- SD of six rats in each group.
Tables 3 and 4 show the effect of S. hispida seed flavonoid-rich fraction on the levels of TBARS and lipid hydroperoxides in the plasma and tissues of normal and hyperlipidemic rats. The high fat diet (HFD) fed rats exhibited a significant increase in TBARS and LOOH. The administration of flavonoid-rich fraction or atorvastatin reduced the levels of TBARS and LOOH significantly and the effect was more pronounced at 40 mg/kg BW dose.
Table 5 shows the effect of S. hispida seed flavonoid-rich fraction on the activities of SOD, CAT and GPx in the erythrocytes of normal and hyperlipidemic rats. The activities of SOD, CAT and GPx decreased significantly in HFD rats and the administration of flavonoid-rich fraction increased these parameters with a maximum at 40 mg/kg BW.
Tables 6 and 7 show the effect of S. hispida seed flavonoid-rich fraction on GSH, vitamin C and E levels in the plasma and tissues of normal and hyperlipidemic rats. The levels of GSH, vitamin C and E decreased significantly in HFD rats and administration of flavonoid-rich fraction increased significantly all these parameters with a maximum at 40 mg/kg BW.
Table 3. Effect of S. hispida seed-flavonoid-rich fraction on the plasma and tissue TBARS of normal and hyperlipidemic rats
|
Groups
|
Plasma TBARS
(mmol/dL)
|
Tissue TBARS (mmol/100g wet tissue)
|
|
Liver
|
Kidney
|
Heart
|
Aorta
|
|
Normal
|
0.17 +- 0.01b
|
0.91 +- 0.06b
|
1.51 +- 0.11b
|
0.53 +- 0.04b
|
0.52 +-0.04b
|
|
|
Normal +
flavonoid-rich fraction (80 mg/kg BW)
|
0.13 +- 0.01a
|
0.79 +-0.05a
|
1.21 +- 0.12a
|
0.42 +- 0.03a
|
0.40+- 0.03a
|
|
|
HFD control
|
0.49 +- 0.03f
|
1.93 +- 0.08f
|
4.04 +- 0.31d
|
1.87 +- 0.16f
|
1.92 +- 0.12f
|
|
|
HFD +
flavonoid-rich fraction (20 mg/kg BW)
|
0.28 +- 0.03e
|
1.55 +- 0.09d
|
3.28 +- 0.47c
|
1.28 +- 0.11e
|
1.37 +- 0.12e
|
|
|
HFD +
flavonoid-rich fraction (40 mg/kg BW)
|
0.23 +- 0.02c,d
|
1.38 +- 0.05c
|
2.41 +- 0.18b
|
0.78 +- 0.06c
|
0.77 +- 0.05c
|
|
|
HFD +
flavonoid-rich fraction (80 mg/kg BW)
|
0.27 +- 0.02d,e
|
1.52 +- 0.07d
|
2.5 +- 0.18b
|
1.18 +- 0.08d
|
1.12 +- 0.08d
|
|
|
HFD +
atorvastatin (2 mg/kg BW)
|
0.24 +- 0.02c
|
1.36 +- 0.06c
|
2.39 +- 0.18b
|
0.77 +- 0.06c
|
0.78 +- 0.06c
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 4. Effect of S. hispida seed-flavonoid-rich fraction on the plasma and tissue lipid hydroperoxide of normal and hyperlipidemic rats
|
Groups
|
Plasma LOOH
(X 10-5mmol/dl)
|
Tissue LOOH (mmol/100g wet tissue)
|
|
Liver
|
Kidney
|
Heart
|
Aorta
|
|
Normal
|
8.57 +- 0.63b
|
82.85 +- 6.38a
|
72.61 +- 5.37a
|
44.40 +- 3.07b
|
76.18 +-5.83a
|
|
Normal +
flavonoid-rich fraction (80 mg/kg BW)
|
6.90 +- 0.58a
|
72.62 +-7.02a
|
70.23 +- 5.37a
|
38.33 +- 3.40a
|
74.40 +- 6.56a
|
|
HFD control
|
16.60 +- 1.5e
|
140.47 +-
11.6d
|
128.57 +-
10.10d
|
89.28 +- 7.49f
|
124.99 +-
9.85d
|
|
HFD +
flavonoid-rich fraction (20 mg/kg BW)
|
14.87+- 1.38d
|
127.38 +-
8.35c
|
114.28 +-
6.38c
|
72.14 +- 5.62e
|
108.45 +-
10.40c
|
|
HFD +
flavonoid-rich fraction (40 mg/kg BW)
|
10.11 +- 0.86c
|
105.95 +-
9.49b
|
88.09 +- 7.38b
|
58.81 +- 4.81c
|
85.71 +- 7.82b
|
|
HFD +
flavonoid-rich fraction (80 mg/kg BW)
|
13.80 +- 1.25d
|
120.23 +-12.3b
|
96.42 +- 7.49b
|
65.47 +- 5.00d
|
98.81 +- 8.35c
|
|
HFD +
atorvastatin (2 mg/kg BW))
|
10.59 +- 1.03c
|
108.32 +-
8.35b
|
94.04 +- 8.35b
|
60.71 +- 5.36c,d
|
94.05 +- 8.35b
|
|
|
|
|
|
|
|
Table 5. Effect of S. hispida seed-flavonoid-rich fraction on the activities of erythrocyte SOD, CAT and GPx in normal and hyperlipidemic rats
|
Groups
|
SOD
(UX/mg Hb)
|
CAT
(UY/mg Hb)
|
GPx
(UZ/mg Hb)
|
|
|
Normal
|
6.63 +- 0.57a
|
182.57 +-
12.52a
|
15.29 +- 1.43a
|
|
Normal +
flavonoid-rich fraction (80 mg/kg BW)
|
7.15 +- 0.59a
|
183.32 +-
9.32a
|
16.40 +- 1.36a
|
|
HFD control
|
4.43 +- 0.29f
|
126.95 +-
11.22d
|
8.17 +- 0.60e
|
|
HFD + flavonoid-rich
fraction (20 mg/kg BW)
|
5.07 +- 0.34e
|
142.06 +-
7.95c
|
9.92 +- 0.76d
|
|
HFD +
flavonoid-rich fraction (40 mg/kg BW)
|
5.98 +- 0.54c
|
160.67 +-
12.57b
|
13.75 +- 0.93c
|
|
HFD +
flavonoid-rich fraction (80 mg/kg BW)
|
5.19 +- 0.40d,e
|
143.28 +-
10.29c
|
10.28 +- 0.46d
|
|
HFD +
atorvastatin (2 mg/kg BW)
|
5.71 +- 0.55c,d
|
157.83 +-
8.11b
|
11.65 +- 1.07b
|
UX- Enzyme concentration required for 50% inhibition of NBT reduction/min
UY-mmole of hydrogen peroxide consumed/min
UZ-mmole of reduced glutathione consumed/min
Table 6. Effect of S. hispida seed-flavonoid-rich fraction on the levels of reduced glutathione in the plasma and tissues of normal and hyperlipidemic rats
|
Groups
|
Plasma GSH
(mg/dL)
|
Tissue GSH (mg/mg protein)
|
|
Liver
|
Kidney
|
Heart
|
Aorta
|
|
Normal
|
24.09 +- 1.86a
|
14.33 +- 1.31a
|
11.40 +- 1.18a
|
7.68 +- 0.63a
|
6.53 +- 0.29a
|
|
Normal +
flavonoid-rich fraction (80 mg/kg BW)
|
25.07 +- 1.97a
|
15.01 +- 1.07a
|
12.20 +- 1.82a
|
8.21 +- 0.53a
|
6.72 +- 0.27a
|
|
HFD control
|
16.80 +- 1.34d
|
8.45 +- 0.70e
|
6.28 +- 0.41d
|
3.51 +- 0.3e
|
3.47 +- 0.3d
|
|
HFD + flavonoid-rich
fraction (20 mg/kg BW)
|
18.76 +- 1.49c
|
9.91 +- 0.67d
|
7.61 +- 0.52c
|
5.24 +- 0.39d
|
4.71 +- 0.40c
|
|
HFD + flavonoid-rich
fraction (40 mg/kg BW)
|
22.04 +- 1.15b
|
12.97 +- 0.97b,c
|
9.81 +- 0.96b
|
7.02 +- 0.0.39b,c
|
5.91 +- 0.41b
|
|
HFD + flavonoid-rich
fraction (80 mg/kg BW)
|
19.56 +- 1.38c
|
11.27 +- 0.91c
|
8.69 +- 0.64b,c
|
6.36 +- 0.0.43c
|
5.10 +- 0.45c
|
|
HFD +
atorvastatin (2 mg/kg BW)
|
21.16 +- 1.74b
|
12.56 +- 0.86b
|
9.60 +- 0.72b
|
6.82 +- 0.55b
|
5.71 +- 0.45b
|
Table 7. Effect of S. hispida seed-flavonoid-rich fraction on the levels of vitamin C and E in the plasma and tissues of normal and hyperlipidemic rats
|
Groups
|
Plasma
(mg/dL)
|
Tissues
(mg/mg
protein)
|
|
Vitamin
C
|
Vitamin
E
|
Vitamin
C
|
Vitamin
E
|
|
Liver
|
Kidney
|
Liver
|
Kidney
|
|
Normal
|
1.81
+-
0.10a
|
1.64
+-
0.11a
|
0.91
+-
0.09a
|
0.54
+-
0.06a
|
5.85
+-
0.52a
|
3.70
+-
0.35a
|
|
Normal + flavonoid-rich fraction
(80 mg/kg BW)
|
1.85
+-
0.12a
|
1.74
+-
0.15a
|
0.98
+-
0.07a
|
0.59
+-0.05a
|
6.13
+-
0.49a
|
3.69
+-
0.30a
|
|
HFD control
|
0.54
+-
0.12e
|
0.62
+-
0.06d
|
0.46
+-
0.03e
|
0.33
+-
0.03d
|
3.59
+-
0.34d
|
1.47
+-
0.08d
|
|
HFD + flavonoid-rich fraction
(20 mg/kg BW)
|
0.70
+-
0.03d
|
0.89
+-
0.06c
|
0.56
+-
0.03d
|
0.41
+-
0.04c
|
4.21
+-0.46c
|
2.37
+-
0.20c
|
|
HFD + flavonoid-rich fraction
(40 mg/kg BW)
|
1.04
+-
0.08b
|
1.14
+-
0.08b
|
0.79
+-
0.05b
|
0.47
+-
0.03b
|
4.98
+-
0.35b
|
2.91
+-
0.18b
|
|
HFD + flavonoid-rich fraction
(80 mg/kg BW)
|
0.88
+-
0.05c
|
0.97
+-
0.06c
|
0.66
+-0.04c
|
0.42
+-
0.04b,c
|
4.63
+-
0.22b,c
|
2.78
+-
0.16b
|
|
HFD + atorvastatin (2 mg/kg BW)
|
1.02
+-
0.08b
|
1.06
+-
0.10b
|
0.77
+-
0.07b
|
0.44
+-
0.04b,c
|
4.81
+-
0.35b
|
2.90
+-
0.27b
|
DISCUSSION
High fat diet brings about remarkable modifications in the antioxidant defense mechanism against the process of lipid peroxidation. Potential antioxidant therapy should, therefore, include either natural free radical scavenging enzymes or agents which are capable of augmenting the activity of the antioxidants. A number of studies have investigated the ability of flavonoid-rich fraction to act as antioxidants (Aviram 2004, Sudheesh and Vijayalakshmi 2004). Flavonoids can directly react with superoxide anions and lipid peroxyl radical (Torel et al. 1986) and consequently inhibit/break the chain of lipid peroxidation. This radical scavenging activity of extracts could be related to the antioxidant nature of polyphenols/flavonoids, thus contributing to their electron/hydrogen donating ability. Hagerman et al. (1998) have reported that the high molecular weight phenolics have more ability to quench free radicals and their effectiveness depends on the molecular weight, the number of aromatic rings and nature of hydroxyl group’s substitution than the specific functional groups. Chandel et al. (1996) have reported that S. hispida contains quercetin, hesperetin and isorhamnetin as major flavonoids, and beta-sitosterol, ursolic acid and borreline, as other components. The antioxidant activity of ursolic acid and beta-sitosterol has also been already reported (Somova et al. 2003, Vivancos et al. 2005). In our study also, the compounds such as quercetin, hesperetin and isorhamnetin may be the major contributor to antioxidant activity.
Membrane lipids are particularly susceptible to oxidation. This is true not only because of their high polyunsaturated fatty acid content, but also due to their association with enzymatic and non enzymatic systems that are able to generate free radical species. During the attack of free radicals on membrane lipoproteins and polyunsaturated fatty acids, a lot of by-products, particularly aldehydes such as malondialdehyde, LOOH and conjugated dienes are formed. In our study, elevation of TBARS and LOOH in HFD rats might be due to a depressed antioxidant defense system. Further, we also observed increased plasma concentration of LDL-C (Fig. 3) in HFD rats. This leads to an increased rate of entry of LDL particles into the artery wall. LDL particles are protected from oxidation in plasma by antioxidant compounds whereas LDL particles trapped within artery wall are prone to damage (Osterud 2003). Before being completely oxidized, LDL particles have to undergo some modifications. At first, LDL particles contain one intact polypeptide – apolipoprotein B-100 (apo B-100), no lipid peroxides or aldehydes, and are enriched in PUFA and antioxidants (Parthasarathy et al. 1999). Minimally modified oxidized low density lipoprotein particles are characterized by oxidation of phospholipids on the surface of particles (Parthasarathy et al. 1999).
At this stage apo-B moiety of LDL is intact. Tocopherol is the most active form of vitamin and the most abundant lipid-soluble antioxidant in LDL. alpha-Tocopherol is confined to the lipid-phase of LDL and, it is suggested, undergoes oxidation to alpha-Toc-O. at the surface of the particle by peroxyl radicals, which are confined largely to the aqueous phase. Because alpha-Toc-O. cannot escape from the LDL particle, Bowry and Stocker (1992) proposed that its life-time is sufficiently long to permit reaction with LOOH, the initiating and rate-limiting reaction of tocopherol-mediated peroxidation. Thus, alpha-tocopherol may act as a phase-transfer agent, transferring the oxidizing potential of peroxyl radicals from the aqueous phase into the hydrophobic core of LDL. Co-antioxidants such as ascorbate and ubiquinol-10, which can intercept alpha-Toc-O., are proposed as protection for LDL against tocopherol-mediated peroxidation by “exporting the radical” from the lipid phase back to the aqueous phase. Ascorbate can reduce alpha-Toc-O. directly from the aqueous phase (Osterud and Bjorklid 2003). Therefore, high plasma LDL concentration obviously has a chance to undergo LDL oxidation and tocopherol-mediated peroxidation since it enters into the artery wall. Animals who had received the flavonoid-rich fraction showed a reduction in LDL-C, TBARS and LOOH evidencing the antioxidant nature of the fraction. Free radical scavenging enzymes such as SOD, CAT and GPx are the first line of defense against injury, and are involved in the disposal of superoxide anions and hydrogen peroxide. We have observed decreased activities of SOD, CAT and GPx in the erythrocytes of HFD rats, which may be an important factor in limiting the antioxidant capacity because of increased TBARS and LOOH. Administration of the flavonoid-rich fraction along with a high fat diet caused a significant increase in the activities of these enzymes indicating that the compounds present in the fraction protect the tissues from lipid peroxidation by their antioxidant ability and consequent reduction in lipid peroxidation. We observed decreased levels of GSH, vitamins C and E in the HFD rats group. Depletion of GSH in HFD rats may be due to enhanced oxidation or its consumption by electrophilic compounds like lipoperoxidation aldehydes. Reduced vitamin C level may be due to increased utilization to trap the ROS or could be due to a decrease in GSH concentration because GSH is involved in the recycling of vitamin C. Reduced vitamin E levels may due to the increased utilization in scavenging the radicals or could be due to the decreased vitamin C because there is a well estabilised synergism between vitamin C and E. The treatment with flavonoid-rich fraction has elevated the levels of these parameters in the plasma and tissues of rats fed high fat diet. Flavonoids also suppress lipid peroxidation by recycling the antioxidant such as alpha-tocopherol (Kris-Etherton et al. 2002). The lower dose of flavonoid-rich fraction (20 mg/kg BW) was not effective, because its concentration might not have been enough to counteract the free radicals generated by high fat diet. The higher concentration of flavonoid-rich fraction might have resulted in the production of by-products that are interfering with the antioxidant activity, and consequently, decreasing its effect. Hence, 40 mg/kg BW flavonoid-rich fraction seems to be optimum for quenching free radicals. The antioxidant properties of the S. hispida seed-flavonoid-rich fraction, in this study, support its ethno medical use in India and several other parts of the world. It can be concluded that the flavonoid-rich fraction of S. hispida seed possesses strong antioxidant properties as evidenced by a significant increase in the levels of enzymic and non-enzymic antioxidants. The in vitro study also confirms this.
REFERENCES
Arnold N, Schmidt J: Plant and Fungal Metabolites/ Microanalytics In: Constituents of traditional medicinal plants. Scientific report, Leibniz Institute of Plant Biochemistry, Leibniz. 2004.
Aviram M: Flavonoids-rich nutrients with potent antioxidant activity prevent atherosclerosis development: the licorice example. International Congress Series 1262:320-327, 2004.
Baker H, Frank O, De Angelis B, Feingold, S: Plasma tocopherol in man at various time intervals after ingesting free or acetylated tocopherol. Nutr. Rep. Int. 21:531-536, 1980.
Blios MS: Antioxidant determinations by the use of a stable free radical. Nature 26: 1199-1200, 1958.
Bowry VW, Ingold KU, Stocker R: Vitamin E in human low-density lipoprotein. When and how this antioxidant becomes a pro-oxidant. Biochem. J. 288:341–344, 1992.
Chandel KPS, Shukla G, Sharma N: Biodiversity in Medicinal and Aromatic Plans in India: Conservation and Utilization. National Bureau of Plant Genetic Resources, Pusa Campus, New Delhi. pp. 235-239, 1996.
Ellman GL: Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82:70-77, 1959.
Fukumoto LR, Mazza G: Assessing antioxidant and pro-oxidant of phenolic compounds. J. Agric. Food. Chem. 48: 3597–3604, 2000.
Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, et al: High molecular weight plant polyphenolics (Tannins) as biological antioxidants. J. Agric. Food. Chem. 46, 1887-1892, 1998.
Harborne JB: Nature, distribution, and function of plant flavonoids, in plant flavonoids in Biology and Medicine: Biochemical, pharmacological, and structure-activity relationships (Cody V, Middleton E Jr and Harborne eds). Alan R, Liss, Inc., New York. 1986, pp. 15-24.
Jia Z, Tang M, Wu J: The determination of flavonoids content in mulbery and scavenging effect on superoxide radicals. Food. Chem. 64:555–599, 1999.
Jiang ZY, Hunt JV, Wolf SP: Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroxide in low-density lipoprotein. Anal Biochem 202:384–389,1992.
Kakkar PS, Das BB, Viswanathan PN: A modified spectrophotometric assay of superoxide dismutase. Ind. J. Biochem. Biophys. 21: 130 – 132, 1984.
Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF et al: Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 113(9B):71S-88S, 2002.
La Casa C, Villegas I, Alarcon-de-la-Lastra C, Motilva V, Martin Calero MJ: Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J. Ethnopharmacol. 71:45–53, 2000.
Narayan DP, Kumari U: Agro’s dictionary of medicinal plants. Agrobios publishers, Jodhpur, India 2005, pp. 323.
Ohkawa H, Ohishi N, Yagi K: Assay of lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95: 35 – 58, 1979.
Osterud B, Bjorklid E: Role of monocytes in atherogenesis. Physiol. Rev. 83:1069-112, 2003.
Parthasarathy S, Santanam N, Ramachandran S, Meilhac O: Oxidants and antioxidants in atherogenesis. An appraisal. J. Lipid. Res. 40:2143-57, 1999.
Petra M, Britta T, Macki K, Eckart E: Flavonoids sulfates from the convolvulaceae. Phytochemistry. 50: 267-271, 1999.
Pushpangandan P, Atal CK: Ethno-Medico-Botanical investigations in Kerala I. Some primitive tribals of Western Ghats and their herbal medicine. J. Ethnopharmacol. 11:59 – 77, 1984.
Roe HJ, Kuether CA: Detection of ascorbic acid in whole blood and urine through the 2, 4-dinitrophenyl-hydrazine derivative of dehydroascorbic acid. J. Biol. Chem. 147:399-407, 1943.
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Haseman DG, Hoekstra WG: Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588-590, 1973.
Sekar T, Francis K: A preliminary investigation of some Maruthamalai forest plants for phytochemical compounds. Biores. Tech. 70: 303 – 304, 1999.
Sinha KA: Colorimetric assay of catalase. Anal. Biochem. 47:89-94, 1972.
Somova LO, Nadar A, Rammanan P, Shode FO: Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension Phytomedicine 10:115–121, 2003.
Sudheesh S, Vijayalakshmi NR: Flavonoids from Punica granatum – potential antiperoxidative agents. Fitoterapia 76, 181-186, 2005.
Surveswaran S, Cai Y, Corke H, Sun, M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food. Chem. 102:938–953, 2007.
Sweedy ME, Hamid NA, Moselhy ME. The role of a mixture of green tea, turmeric and chitosan in the treatment of obesity-related testicular disorders. J Appl Biomed 5:131–138, 2007.
Torel J, Cilliard J, Cilliard P: Antioxidant activity of flavonoids and reactivity with peroxy radicals. Phytochemistry 25, 383-385, 1985.
Vivancos M, Moreno JJ: Beta-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radic. Biol. Med. 39: 91–97, 2005.
Volkovova K, Dusinska M, Collins AR. From oxidative DNA damage to molecular epidemiology. J. Appl. Biomed. 4: 39–43, 2006.
Wolfenden BS, Willson RL: Radical cations as reference chromogens in kinetic studies
of one-electron transfer reactions; pulse radiolysis studies of 2, 2’-azinobis-(3-ethylbenzothiazoline-6-sulfonate). J. Chem. Soc. 24:805-812, 1982.
Yoshida H, Ishikawa T, Hosoai H, Suzukawa M, Ayaori M, Hisada T, Sawada S, Yonemura A, Higashi K, Ito T, Nakajima K, Yamashita T, Tomiyasu K, Nishiwaki M, Ohsuzu F, Nakamura H: Inhibitory effect of tea flavonoids on the ability of cells to oxidize low density lipoprotein. Biochem. Pharmacol. 58:1695–1703, 1999.
BACK



