Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 1, p 23-33
DOI 10.2478/v10136-009-0004-x
Antidiabetic and antioxidative effects of hydro-methanolic extract of sepals of Salmalia malabarica in streptozotocin induced diabetic rats
Debasis De, Kausik Chatterjee, Kazi Monjur Ali, Suvra Mandal, Bikashranjan Barik, Debidas Ghosh
Address: Debidas Ghosh, Department of Bio-Medical Laboratory Science and Management, Vidyasagar University, Midnapore-721102,
West Bengal, India
debidas_ghosh@yahoo.co.in
Received 9th May 2009.
Revised 12th August 2009.
Published online 4th December 2009.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
References
SUMMARY
Natural products with antidiabetic activities provide important sources for the development of new drugs in the treatment of diabetes mellitus.
This present work focuses on the antidiabetic activity of a hydro-methanolic (2:3) extract of the sepals of Salmalia malabarica on the blood
glucose, the carbohydrade metabolic enzyme, oxidative stress, glycated haemoglobin and transaminase activity in streptozotocin (STZ) induced
diabetic rats. Diabetic rats show a significant diminution in the activities of hexokinase, glucose-6-phosphate dehydrogenase and an elevation in
the activity of glucose-6-phosphatase in the liver and skeletal muscle. Administration of hydro-methanolic extract of the sepals of Salmalia
malabarica to diabetic rats resulted in a significant recovery in the parameters concerned. In the liver and kidney, the activities of catalase
(CAT) and peroxidase (Px) were decreased significantly and levels of conjugated diene (CD) and thio-barbituric acid reactive substance (TBARS) were
increased significantly in diabetic rats which recovered significantly after administration of hydro-methanolic extract of S. malabarica.
Serum glutamate oxaloacetate transaminase (SGOT) and serum glutamate pyruvate transaminase (SGPT) activities which are increased in diabetes were
restored by the extract. Glycated haemoglobin (HbA1C) levels were resettled significantly in the extract treated group compared to the
diabetic group. The antidiabetic activity of the extract was supported after a comparison with glibenclamide, a standard antidiabetic drug.
KEY WORDS
Salmalia malabarica; antidiabetic; carbohydrade metabolic enzyme; oxidative stress; transaminases
INTRODUCTION
Diabetes is the most common of the endocrine
disorders and poses a serious challenge to health care
worldwide. It is projected that by 2010, at least
239 million people will be affected by diabetes
(Mandrup-Poulsen 1998). Diabetes mellitus is
becoming the third killer of mankind after cancer and
cardiovascular disease due to its high prevalence,
morbidity and mortality (Li et al. 2004).
Diabetes has been shown to decrease the activities
of enzymes in the glycolytic and pentose phosphate
pathways, while increasing the activities of the
glucogenic, glycogenolytic and lipolytic pathways
(Basu et al. 2005, Ramis et al. 2006). Oxidative stress
induced by chronic hyperglycaemia has been
associated with dysfunction and apoptosis of several
cell types, including pancreatic beta cells (Wu et al.
2004), neurons and glial cells (Russel et al. 2002,
Vincent et al. 2004). Oxidative stress results from
overproduction of reactive oxygen species coupled
with insufficient antioxidant capacity. The oral
hypoglycaemic drugs have many side effects such as
nausea, vomiting, cholestic jaundice, agranulocytosis,
aplastic and haemolytic anaemia, generalized
hypersensitivity reactions, dermatological reactions
and lactic acidosis (Murad et al. 2009).
Several drugs such as biguanides, glibenclamide
and sulfonylureas are presently available to reduce
hyperglycaemia in diabetes mellitus but these drugs
also have side effects and thus searching for a new
class of compounds is essential to overcome these
problems (Kamaeswara et al. 2001).
Diabetes mellitus was known in ancient times, and
some medicinal plants have been used for its control
in traditional medicine (Andrade-Cetto and Heinrich
2005, Mukherjee et al. 2006). The efficacy of plants
for the management of diabetes requires confirmation
and the WHO (World Health Organization 1980) has
recommended the assessment of traditional plant
remedies. Some of the work in this field, including
our own previous work, has noted the remarkable
antidiabetic effects of some plant parts (Maiti et al.
2005, Mallick et al. 2006, Deshmukh et al. 2008,
Mandal et al. 2008, Senthilkumar and Subramanian
2008).
Salmalia malabarica is a large and tall deciduous
tree belonging to the family of 'Malvaceae'. It is
beneficial in acne and skin eruptions (Kumar et al.
2008), and also possesses antibacterial and antifungal
properties (Sharma and Patel 2009). From an
ethnobotanical survey we came to know that the
sepals of this flower are also used as an antidiabetic
medicine in tribal communities, especially in our
country, but there are no scientific reports on the
antidiabetic activity of S. malabarica and the present
investigation was conducted to explore the
possibilities. Its efficacy was compared with that of
glibenclamide, a standard antidiabetic drug.
MATERIALS AND METHODS
Plant materials
The sepals of S. malabarica of the family
"Malvaceae" were collected from Midnapur, District,
Paschim, Midnapur, West Bengal, India, in the
months of January to April, 2008. The materials were
identified by a taxonomist in the Botany Department,
of Vidyasagar University, Midnapur and the voucher
specimen marked BIO-MED-S.M-01, was deposited
in the Department of Botany, Vidyasagar University.
Preparation of hydro-methanolic (2:3) extract of
sepals of Salmalia malabarica
Fresh sepals were dried in an incubator for 2 days at
37 °C, crushed separately in an electric grinder and
then pulverized. From this powder, 50 g was
suspended in 80 ml of water and 120 ml methanol
(2:3) and kept in an incubator at 37 °C for 72 h. The
slurry was stirred intermittently for 2 h and left
overnight. The mixture was then filtered, and the
filtrate was dried by a low pressure rotary evaporator.
The residue was collected, suspended in water in a
fixed dose and used for treatment.
Chemicals
Streptozotocin (STZ) was obtained from Spectrochem
Pvt. Ltd (India). All other chemicals used were of
analytical grade obtained from E. Merck, Mumbai or
HIMEDIA, Mumbai, India or purchased from
Sigma-Aldrich Diagnostic Ltd. USA. Kits for the
various enzyme assays were purchased from Crest
Biosystems, Goa, India.
Selection of animal and animal care
Twenty six matured normoglycaemic wistar strain
male albino rats, 3 months of age weighing about
120 ± 10 g were chosen for this experiment. The
animals were acclimatised to our laboratory
conditions for a period of 15 days prior to the
experiment. They were housed at an ambient
temperature of 25 ± 2 °C with a 12 h light : 12 h dark
cycle. They had free access to standard food and
water, and the principles of laboratory animal care
and particular instructions given by our institutional
ethical committee were followed throughout the
experiment, which lasted for 28 days.
Induction of diabetes mellitus
Twenty rats, fasted for 12 hours were subjected to a
single intramuscular injection of streptozotocin (STZ)
at a dose of 4 mg/0.1 ml of citrate buffer/100 g body
weight (b.w.)/rat; after 24 h of STZ injection this
produced type 1 diabetes (i.e. having a fasting blood
glucose level of more than 250 mg/dl but less than
350 mg/dl). This level of fasting blood glucose has
been selected here as it represented a moderate
diabetic state (Joussen et al. 2001). Subsequently, six
days were allowed to stabilize the diabetes and after
that eighteen of the rats meeting the above criteria
were selected for this experiment. Six
normoglycaemic rats were included in the control
group and rats of this group were subjected to a single
intramuscular injection of citrate buffer only to keep
all the animals in same condition in relation to the
injection process.
Measurement of fasting blood glucose level
At the time of grouping of the animals, their fasting
blood glucose (FBG) level was measured. After every
six days of treatment (on every 7th day), FBG was
further recorded in all the animals of all groups.
Blood was collected from the tail vein or from an
orbital puncture and the FBG level was measured by
a single touch glucometer.
Animal treatment
Twenty-four rats were divided into the following four
equal groups. The experiment took place over a
period of 28 days.
Group I (Control group): normoglycaemic rats of this
group were subjected to forced feeding of 0.5 ml
distilled water/100 g b. w./rat/day for 21 days.
Group II (Diabetic group): forced feeding of 0.5 ml
distilled water/100 g b. w./rat/day for 21 days by
gavage.
Group III (Diabetic + S. malabarica extract): diabetic
rats were forcefully fed by gavage with
hydro-methanolic (2:3) extract of sepals of
S. malabarica at a dose of 20 mg/0.5 ml distilled
water/100 g b. w./rat/day from the 7th day of
streptozotocin injection for the next 21 days in a
fasting state.
Group IV (Diabetic + glibenclamide): rats of this
group were administered by gavage with commercial
glibenclamide at a dose of 0.06 mg/0.5 ml water/100
gm b. w./rat/day from the 7th day of streptozotocin
injection for next 21 days in a fasting state.
The extract was administered to the animals of
group III and glibenclamide to the animals of
group-IV early in the morning and in a fasting state
i.e. 11-12 hrs after feed delivery. Starting from day 1
of extract administration to the diabetic rats, the
fasting blood glucose levels in all groups were
measured by a single touch glucometer at six day
intervals. On the 29th day after the start of the
experiment (considering the day of STZ injection as
the first day of experiment), all the animals were
sacrificed by decapitation after recording the final
body weight. Blood was collected from the dorsal
aorta by a syringe. A portion of the blood was used
for the separation of serum by centrifugation at
3000 g for 5 min for the estimation of serum toxicity
study. The liver, kidney and skeletal muscle were
dissected out and stored at -20 °C for biochemical
analysis. Blood was used for the quantification of
haemoglobin and glycated haemoglobin.
Biochemical estimations
The activities of carbohydrate metabolic enzymes
such as glucose-6-phosphate dehydrogenase,
hexokinase, and glucose-6-phosphatase were assayed
by the methods of Langdon (1966), Chou and Wilson
(1975) and Swanson (1955) respectively. The liver
and skeletal muscle glycogen levels were measured
bio-chemically according to Sadasivam and
Manickam (1996). An estimation of lipid
peroxidation was performed from a concentration of
thiobarbituric acid reactive substance (TBARS) and
conjugated diene (CD) according to Okhawa et al.
(1979) and Slater (1984). A biochemical estimation of
antioxidative enzyme activities such as catalase
(CAT) and peroxidase (Px) were measured according
to Beers and Sizer (1952), Sadasivam and Manickam
(1996) respectively. Haemoglobin and glycated
haemoglobin levels were measured according to
Balasubramaniam and Malathi (1992), and Chandalia
et al. (1980) respectively. The activities of serum
glutamate oxaloacetate transaminase (SGOT) and
serum glutamate pyruvate transaminase (SGPT) were
measured by specific kits. The activities of these
enzymes were expressed as relative units (Henry et al.
1960).
Statistical analysis
Data were presented as mean ± S.E.M. All the data
were evaluated statistically using one-way analysis of
variance (ANOVA) followed by a multiple
comparison two tail 't' test by using the Origin
Lab (Ver. 6.0) software at the significance level 2 =
0.05.
RESULTS
Blood glucose level
Diabetes induced by STZ resulted in a significant
elevation in blood glucose in comparison to the
control group. After the administration of
hydro-methanolic (2:3) extract of sepals of
S. malabarica or glibenclamide to the diabetic
animals for 21 days, a significant recovery of blood
glucose level was noted at a level close to the control
level. There was an insignificant difference in the
level of fasting blood glucose between the extract
treated group and the glibenclamide treated group
(Table 1).
Table 1. Effect of hydro-methanol (2:3) extract of S. malabarica on fasting blood glucose level in streptozotocin induced diabetic male
albino rat. Data are expressed as Mean S.E.M; n = 6, ANOVA followed by multiple comparisons two tail 't' test. Values with superscripts like a,
b, c in each vertical column differ from each other significantly.
| Time (min) | Groups
Fasting blood glucose level (mg/dl) | | 1st day
(The day of
STZ injection) |
7th day (The
day of extract
treatment) |
14th day |
21st day |
28th day | | Control |
73.21 ± 4.48a |
72.12 ± 4.9a |
75.83 ± 4.7a |
78.02 ± 4.6a |
74.48 ± 5.1a | | Diabetic |
76.58 ± 5.2a |
339.32 ± 5.8b |
336.54 ± 6.2b |
338.00 ± 5.9b |
341.52 ± 6.5b | | Diabetic +
S. malabarica extract |
78.36 ± 4.5a |
348.31 ± 4.8b |
187.67 ± 5.2c |
124.61 ± 5.3c |
98.62 ± 4.9c | | Diabetic + glibenclamide |
76.87 ± 3.8a |
346.31 ± 3.5b |
179.38 ± 3.49c |
119.46 ± 5.1c |
95.92 ± 4.3c |
Glycogen level in tissue
The quantity of glycogen both in liver and skeletal
muscle was decreased significantly in the diabetic
group compared with the control group.
Administration of hydro-methanolic extract of sepals
of S. malabarica or glibenclamide to the diabeticanimals for 21 days resulted in a significant
elevation in the levels of glycogen in the liver and
skeletal muscle towards the control level. The level of
this parameter showed no significant difference
between the extract treated group and the
glibenclamide treated group (Fig. 1).
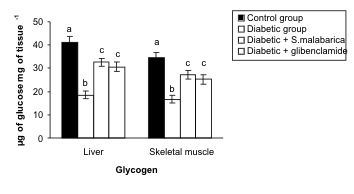
Fig. 1. Resettelment in the levels of glycogen in liver and skeletal muscle after administration of hydro-methanolic extract of sepals of S.
malabarica in STZ-induced diabetic male albino rat. Data are expressed as Mean ± S.E.M; n = 6, ANOVA followed by multiple comparison
two-tail 't'-test. Bars with different superscripts like a, b, c differ from each other significantly.
Carbohydrate metabolic enzymes
The streptozotocin induced diabetic animal displayed
a significant elevation in glucose-6-phosphatase
activity along with a diminution in the activities of
glucose-6-phosphate dehydrogenase and hexokinase
in the liver and skeletal muscle in comparison to the
controlgroup. Administration of the plant extract or
glibenclamide to the diabetic animals resulted in
significant protection and the levels of these
parameters were resettled towards the control group.
There was no significant difference in the levels of
these parameters between the extract treated group
and the glibenclamide treated group (Figs. 2-4).
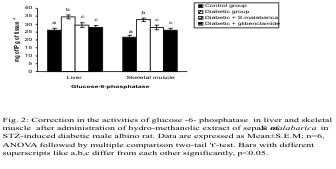
Fig. 2. Correction in the activity of glucose-6-phosphatase in liver and skeletal muscle after administration of
hydro-methanolic extract of sepals of S. malabarica in STZ-induced diabetic male albino rat. Statistics and symbols as in Fig.
1.
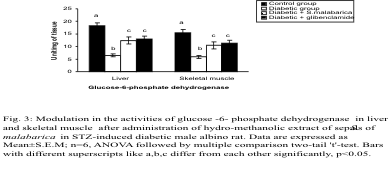
Fig. 3. Modulation in the activities of glucose -6-phosphate dehydrogenase in liver and skeletal muscle after administration
of hydro-methanolic extract of sepals of S. malabarica in STZ-induced diabetic male albino rat. Statistics and symbols as
in Fig. 1.

Fig. 4. Recovery in the activities of hexokinase in liver and skeletal muscle after administration of hydro-methanolic
extract of sepals of S. malabarica in STZ-induced diabetic male albino rat. Statistics and symbols as in Fig. 1.
Activities of CAT and Px
The activities of CAT and Px in the liver and kidney
were decreased significantly in the diabetic group in
comparison with the control group. After the
administration of hydro-methanolic (2:3) extract of
sepals of S. malabarica or glibenclamide to STZ-treated diabetic rat, the activities of the above
enzyme were restored towards the control level.
Activities of both the said enzymes did not differ
significantly between the extract treated group and
the glibenclamide treated group (Figs. 5-6).
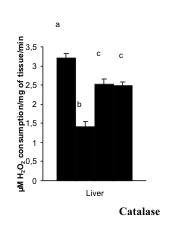 <
<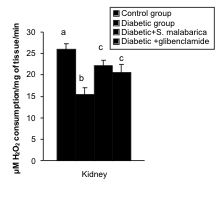
Fig. 5. Correction in the activities of catalase in liver and kidney after administration of hydro-methanolic extract of sepals
of S. malabarica in STZ-induced diabetic rat. Statistics and symbols as in Fig. 1.
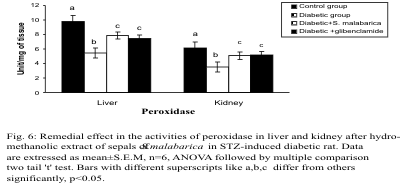
Fig. 6. Remedial effect in the activities of peroxidase in liver and kidney after administration of hydro-methanolic extract
of sepals of S. malabarica in STZ-induced diabetic rat. Statistics and symbols as in Fig. 1.
Levels of CD and TBARS
The levels of CD and TBARS in the liver and kidney
were increased significantly in the diabetic group
when compared to the control group. Significant
recovery was noted in the levels of the above parameters after administration of the plant part
extract or glibenclamide to the diabetic rats. The
levels of these parameters differed insignificantly
between the extract treated group and glibenclamide
treated group (Figs. 7-8).
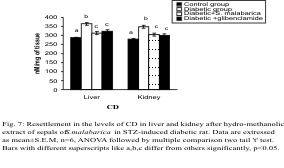
Fig. 7. Resettlement in the levels of CD in liver and kidney after administration of hydro-methanolic extract of sepals of
S. malabarica in STZ-induced diabetic rat. Statistics and symbols as in Fig. 1.
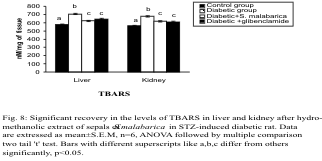
Fig. 8. Significant recovery in the levels of TBARS in liver and kidney after administration of hydro-methanolic extract
of sepals of S. malabarica in STZ-induced diabetic rat. Statistics and symbols as in Fig. 1.
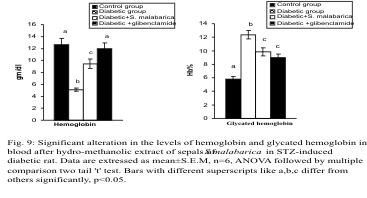
Fig. 9. Significant alteration in the levels of hemoglobin and glycated hemoglobin in blood after administration of
hydro-methanolic extract of sepals of S. malabarica in STZ-induced diabetic rat. Statistics and symbols as in Fig. 1.
Levels of hemoglobin and glycated hemoglobin
The haemoglobin level was decreased significantly
and the glycated haemoglobin (HbA1C) level was
increased significantly in the diabetic group in
comparison with the control group. After treatment
with the plant extract or glibenclamide, the levels of haemoglobin and glycated haemoglobin were
resettled towards the control group. The haemoglobin
level differed significantly whereas the glycated
haemoglobin level differed insignificantly between
the extract treated and glibenclamide treated groups
(Fig. 9).
Activities of GOT and GPT in Serum
The activities of GOT and GPT in serum were
increased in the diabetic group compared to the
control group. After administration of the plant extract or glibenclamide there was a significant
recovery in the levels of these parameters which
showed no significant difference between the extract
treated and glibenclamide treated groups (Fig. 10).
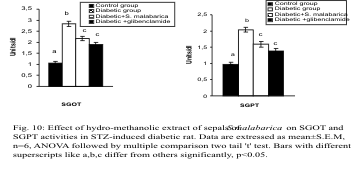
Fig. 10. Effect of hydro-methanolic extract of sepals of S. malabarica on SGOT and SGPT activities in STZ-induced diabetic rat.
Statistics and symbols as in Fig. 1.
DISCUSSION
The present study was conducted to investigate the
antihyperglycaemic and the antioxidative activities of
the hydro-methanolic (2:3) extract of sepals of
S. malabarica in STZ-induced diabetic male albino
rats. The streptozotocin-induced diabetic rat was
selected as an experimental model because it is the
best model for study of the effect of the
antidiabetogenic agent (Veeramani et al. 2008); it is
also in continuity with our previous work (Maiti et al.
2005, Mallick et al. 2006, 2007a, b, 2009, Mandal et
al. 2008).
Catalase and peroxidase play an important role in
preventing the cells from oxidative injury. Enzymatic
antioxidants like CAT and Px catalyze the reduction
of hydrogen peroxides and protect the tissues against
reactive hydroxyl radicals (Bukan et al. 2003). A
decrease in the activities of the above enzymes in
STZ-induced diabetic rats in vital metabolic tissues
such as liver and kidney was as reported by others
(Kyuichi and Takuji 2006, Kaviarasan et al. 2008)
and our own previous work (Mallick et al. 2006,
2007b, Mandal et al. 2008) which may be due to the
deleterious effects of free radicals on enzymes
(Kinalski et al. 2000). The elevation in the levels of
TBARS and CD, the products of free radicals, in the
metabolic tissues in the diabetic state also supports
the above mechanism and is consistent with previous
observations (Jung et al. 2006). Administration of this
plant extract or glibenclamide to diabetic rats
increased the activities of CAT and Px, supported
here by the quantification of the production of free
radicals, a marker of oxidative stress (Phillips et al.
2004).
For the assessment of the antidiabetic potency of
the plant extract, we measured the activity of hepatic
glucose-6-phosphatase, an important enzyme for
glycogenolysis (Aiston et al. 2003). In a similar way,
glucose-6-phosphate dehydrogenase and hexokinase
are two enzymes under the positive control of insulin
(Kruszynska et al. 1998). In the diabetic state the
activities of these three enzymes were altered as
reported by others (Rajasekaran et al. 2004) and by
our own publication (Mallick et al. 2006). The plant
extract is able to recover this enzymatic biosensor
significantly compared to glibenclamide which may
be due to the recovery of insulin. Another possibility
is the correction of oxidative injury by the plant
extract as free radicals have detrimental effects on the
enzyme system (Zama et al. 2007).
A low level of glycogen in the liver and skeletal
muscle is another parameter indicating diabetes (Luis
et al. 2001). The recovery in the glycogen level in the
plant extract group may be due to the correction in
plasma insulin, as proposed by others using other
plant materials (Shirwaikar et al. 2006).
The antidiabetic activity of S. malabarica has
been further supported here by the measurement of
the glycated haemoglobin level as the diabetic state
elevates this level; as reported by others (Denise et al.
2008) and by our own previous work (Mallick et al.
2007b, 2009). The correction of glycated
haemoglobin may be due to a correction in the
glucose level through plasma insulin.
There was no toxicity of this extract proved by
SGOT and SGPT activities, as these enzymes are
themselves important sensors for toxicity assessment
(Ghosh and Suryawanshi 2001).
From the above results, the antidiabetic efficacy of
the plant extract may be explained in two ways. One
is the indirect pathway through which the
phytomolecule may stimulate the existing beta cell or
regenerate the cell for the recovery in serum insulin
along with protection of oxidative injury. Another is
the direct way where the phytoingredients present
there may inhibit enzymes such as glucosidase that
may interfere with the glucose production in the
gastro intestinal tract from complex carbohydrates.
The actual mechanism may be reported after future
research.
ACKNOWLEDGEMENT
This study was supported by a grant from UGC Major
Research Project having project no. 34-494/2008 (SR)
to Prof. Debidas Ghosh.
REFERENCES
Aiston S, Andersen B, Agius L: Glucose 6-phosphate regulates hepatic glycogenolysis through inactivation of phosphorylase. Diabet 52:1333-1339,
2003.
Andrade-Cetto A, Heinrich M: Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J Ethnopharmacol 99:325-348, 2005.
Balasubramanium P, Malathi A: Comparative study of hemoglobin estimated by Drabkins and Sahli’s methods. J Postgrad Med 38:8-9, 1992.
Basu R, Chandramouli V, Dicke B, Landau B, Rizza R: Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as
gluconeogenesis. Diabetes 54:1942-1948, 2005.
Beers RF, Sizer IW: Spectrophotometric method for measuring the breakdown of hydrogen peroxidase by catalase. J Biol Chem 195:133-140,
1952.
Bukan N, Sancant B, Yuvaz O, Koca C, Tuthun F, Ozcelikay AT, Altan N: Lipid peroxidation and scanvenging enzyme levels in the liver of
streptozotocin induced diabetes rats. Indian J Biochem Biophysics 40:447-450, 2003.
Chandalia HB, Sadikot S, Bhargava DK, Krisnaswamy PR: Estimation of glycosylated hemoglobin by a simple chemical method. J Assoc Phys Ind
29:285-286, 1980.
Chou AC, Wilson JE: Carbohydrate metabolism. In Wood WA (ed.): Methods in Enzymology Vol. XLII, Academic Press, New York 1975, pp. 20-21.
Denise AE, Richard WN, Edward CF, Larry AN: Glycosylated hemoglobin concentration for assessment of glycemic control in diabetic cats. J Veterin
Intern Med 11:161-165, 2008.
Deshmukh TA, Yadav BV, Badole SL, Bodhankar SL, Dhaneshwar SR: Antihyperglycaemic activity of alcoholic extract of Aerva lanata (L.) A. L.
Juss. Ex J. A. Schultes leaves in alloxan induced diabetic mice. J Appl Biomed 6:81-87, 2008.
Ghosh S, Suryawanshi SA: Effect of Vinca rosea extracts in treatment of alloxan diabetes in male albino rats. Indian J Exp Biol 39:748-759,
2001.
Henry RJ, Chiamori M, Gonub OJ, Berkman S: Revised spectrophotometric methods for the determination of glutamate oxaloacetic transaminase, glutamic
pyruvate transaminase and lactic acid dehydrogenase. Am J Clin Pathol 34:381-398, 1960.
Joussen AM, Huang S, Poulaki V, Comphausenk K, Beeckaen WD, Kirchhof B, Adamis AP: In vivo retinal gene expression in early diabetes. Invest
Opthalmol Vis Sci 42:3047-3050, 2001.
Jung CH, Zhou S, Ding GX, Kim JH, Hong MH, Shin YC, Kim GH, Ko SC: Antihyperglycemic activity of herb extract on streptozotocin induced diabetic
rats. Biosci Biotechnol Biochem 70:2556-2569, 2006.
Kamaeswara RB, Giri R, Kesavulu MM, Apparao CH: Effect of oral administration of bark of Pterocarpus santalinus L. on blood glucose level in
experimental animals. J Ethnopharmacol 74:69-74, 2001.
Kaviarasan K, Kalaiarasi P, Pugalendi V: Antioxidant efficacy of flavonoid-rich fraction from Spermacoce hispida in hyperlipidemic rats. J
Appl Biomed 6:165-176, 2008.
Kinalski M, Sledziewski A, Telejko B, Zarzychi W, Kinalska I: Lipid eroxidation and scavenging enzyme activity in streptozotocin induced diabetes.
Acta Diabetologica 37:179-183, 2000.
Kruszynska YT, Mulford MI, Baloga J, Yu JG, Olefsky JM: Regulation of skeletal muscle hexokinase II by insulin in nondiabetic and NIDDM subjects.
Diabetes 47:1107-1113, 1998.
Kumar A, Baboota S, Agarwal SP, Ali J, Ahuja A: Treatment of acne with special emphasis on herbal remedies. Expert Rev Dermatol 3:111-122,
2008.
Kyuichi T, Takuji T: Studies on oxidative stress in liver disease: Important future trends in liver research. Med Mol Morphol 39:22-27,
2006.
Langdon RG: Glucose-6-phosphate dehydrogenase from erythrocytes. In Wood WA (eds.): Methods in Enzymology. Vol. IX. Academic Press, New York 1966,
pp. 126-131.
Li WL, Zheng HC, Bukuru J, De Kimpe N: Natural medicines used in the traditional Chinese medicinal system for therapy of diabetes mellitus. J
Ethnopharmacol 92:1-21, 2004.
Luis DMCBF, Lambert B, Sasha N, Ghazala R, Palmer TN, Fournier PA: Effect of streptozotocin-induced diabetes on glycogen resynthesis in fasted rats
post high intensity exerscise. Am J Physiol Endocrinol Metab 280:83-91, 2001.
Maiti R, Das UK, Ghosh D: Attenuation of hyperglycemia and hyperlipidemia in streptozotocin induced diabetic rats by aqueous extract of seed of
Tamarindus indica. Biol Pharm Bull 28:1172-1176, 2005.
Mallick C, Maiti R, Ghosh D: Comparative study on antihyperglycemic and antihyperlipidemic effect of separate and composite extract of seed of
Eugenia jambolana and root of Musa paradisiaca in streptozotocin induced diabetic male albino rat. Iran J Pharmacol Therap 5:27-33, 2006.
Mallick C, Chatterjee K, GuhaBiswas M, Ghosh D: Antihyperglycemic effects of separate and composite extract of root of Musa paradisiaca and
leaf of Coccinia indica in streptozotocin induced diabetic male albino rat. Afr J Tradit Complement Altern Med 4:362-371, 2007a.
Mallick C, Chatterjee K, Mandal U, Ghosh D: Antihyperglycemic, Antilipidperoxidative and Antioxidative effects of Musa paradisiaca and
Coccinia indica in streptozotocin-induced diabetic rat. Ethiop Pharmacol J 25:9-22, 2007b.
Mallick C, De D, Ghosh D: Correction of protein metabolic disorders by composite extract of Musa paradisiaca and Coccinia indica in
streptozotocin-induced diabetic albino rat: An approach through the pancreas. Pancreas 38:322-329, 2009.
Mandal S, Barik B, Mallick C, De D, Ghosh D: Therapeutic effect of ferulic acid, an ethereal fraction of seed of Syzygium cumini against
streptozotocin-induced diabetes on male rat. Methods Find Exp Clin Pharmacol 30:121-128, 2008.
Mandrup-Poulsen T: Recent advances in diabetes. Brit Med J 316:1221-1225, 1998.
Mukherjee PK, Maiti K, Mukherjee K, Houghton PJ: Leads from Indian medicinal plants with hypoglycemic potentials. J Ethnopharmacol 106:1-28,
2006.
Murad MH, Yglesias FC, Wang AT, Sheidaee N, Mullan RJ, Elamin MB, Erwin PJ, Montori VM: Drug induced hypoglycemia. A systemic review. J Clin
Endocrinol Metab 98:741-745, 2009.
Okhawa H, Ohishi N, Yagi K: Assay for lipid peroxidation in animal tissues thiobarbituric acid reaction. Anal Biochem 95:351-358, 1979.
Phillips M, Cataneo RN, Cheema T, Greenberg J: Increased breath biomarkers of oxidative stress in diabetes mellitus. Clinica Chimica Acta
344:189-194, 2004.
Ramis JM, Salinas R, Garcia-Sanz JM, Moreiro J, Proeza AM, Liado I: Deport and gender-related differences in the lipolytic pathway of adipose
tissue from several obese patients. Cell Physiol Biochem 17:173-180, 2006.
Rajasekaran S, Sivagnanam K, Ravi K, Subramanian S: Hypoglycemic effect of Aloe vera on streptozotocin induced diabetes in experimental
rats. J Med Food 7:61-66, 2004.
Russel JW, Golovoy D, Vincent A M, Mahendru P, Olzmann JA, Mentzer A, Feldman EL: High glucose-induced oxidative stress and mitochondrial
dysfunction in neurons. FASEB J 16:1738-1748, 2002.
Sadasivam S, Manickam A: Carbohydrates. In Sadasivam S, Manickam A (eds.): Methods in Biochemistry. New Age International Pvt. Ltd., New Delhi
1996, pp. 11-12.
Sadasivam S, Manickam A: Peroxidase. In: Methods in Biochemistry. New Age International: New Delhi 1996, pp. 108-110.
Senthilkumar GP, Subramanian SP: Biochemical studies on the effect of Terminalia chebula on the levels of glycoproteins in
streptozotocin-induced experimental diabetes in rats. J Appl Biomed. 6:105-115, 2008.
Sharma A, Patel VK: In vitro screening of the antibacterial activity and identification of bio-active compounds from plants against selected
Vibrio spp. Pathogens Turk J Biol 33:1-8, 2009.
Shirwaikar A, Rajendran K, Barik R: Effect of aqueous bark extract of Garuga pinnata Roxb. In streptozotocin-nicotinamide induced type-II
diabetes mellitus. J Ethnopharmacol 107:285-290, 2006.
Slater TI: Overview of methods used for detecting lipid peroxidation. Methods Enzymol 105:283-293, 1984.
Swanson MA: Glucose-6-phosphatase from liver. In Colowick SP, Kaplan NO (eds.): Methods in Enzymology. Vol. II. New York, Academic Press. 1955, pp.
541-543.
Veeramani C, Pushpavalli G, Pugalendi KV: Antihyperglycemic effect of Cardiospermum halicacabum Linn. leaf extract on STZ-induced diabetic
rats. J Appl Biomed 6:19-26, 2008.
Vincent AM, Russel JW, Low P, Feldman EI: Oxidative stress in pathogenesis of diabetic neuropathy. Endocr Rev 25:612-628, 2004.
Wu L, Nicholson W, Knobel SM, Steffner RJ, May JM, Piston DW, Powers AC: Oxidative stress is a mediator of glucose toxicity in insulin-secreting
pancreas islet cell lines. J Biol Chem 289:126-134, 2004.
WHO: WHO Expert Committee on Diabetes Mellitus, Second Report. Technical Report Series no. 646, Geneva WHO, pp. 66, 1980.
Zama D, Meraihi Z, Tebibel S, Benayssa W, Benayache F, Benayache S, Vlietinck AJ: Chlorpyrifos-induced oxidative stress and tissue damage in the
liver, kidney, brain and fetus in pregnant rats: The protective role of the butanolic extract of Paronychia argentea L. Indian J Pharma
39:145-150, 2007.
|
BACK
|





 <
<





