Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 1, p 11-18
DOI 10.2478/v10136-009-0002-z
Biomedically relevant chemical constituents of Valeriana officinalis
Jiri Patocka, Jiri Jakl
Address: Jiri Patocka, Faculty of Health and Social Studies, University of South Bohemia in Ceske Budejovice, Czech Republic
prof.patocka@gmail.com
Received 24th July 2009.
Revised 15th October 2009.
Published online 1st December 2009.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Botany
Ethnobotany
Medicinal use
Constituents - chemistry and pharmacology
Mechanism of action
Animal behaviour tests
Toxicology
Conclusions
References
SUMMARY
Valerian is used to treat sleeping disorders, restlessness and anxiety, but it seemsonly to work when taken over long periods (several weeks). Some
studies have demonstrated that valerian extracts interact with the GABA and benzodiazepine receptors. Valerian is also used traditionally to treat
gastrointestinal pain and spastic colitis. There are no long term safety studies. Valerian contains over 150 chemical constituents and many of them
are physiologically active, mainly pyridine alkaloids, some organic acids and terpenes, especially the so called valepotriates, esterified
iridoid-monoterpenes. As valepotriates may be potential mutagens, valerian should only be used after consultation with a physician. Valerian
medication is sometimes recommended as first line treatment when the benefit-risk relation requires it and is often indicated as transition
medication during the discontinuation processes involving bromazepam, clonazepam and diazepam, among others.
KEY WORDS
Valeriana officinalis; valerian; chemical constituents; alkaloids; terpenes; valepotriates
INTRODUCTION
The herbal medicine valerian, the dried root of the
plant Valeriana officinalis L., has been used as a
medicinal herb since at least the time of ancient
Greece and Rome. Its phytotherapeutical properties
were described by Hippocrates as sedative and
anti-anxiety. Galen prescribed it as a remedy for
insomnia. Related species of the Valerianaceae family,
were used in traditional Chinese and Indian Ayurvedic
medicine (Jarema 2008).
Valerian extracts became popular in the United
States and Europe in the mid-1800s and continued to
be used by both physicians and the lay public until it
was widely replaced by prescription sedative drugs.
Other common uses included the treatment of
headaches, anxiety, palpitations, irritable or spastic
bowel, menstrual cramps, high blood pressure,
epilepsy and childhood behavior problems and
learning (Klich 1975). During World War I, valerian
was used to prevent and treat shell shock in frontline
troops, and it was used during World War II to help
calm civilians subjected to air raids (Mowrey 1986). In
1998, valerian was the 10th most popular herbal
remedy sold in the United States (Fugh-Berman and
Cott 1999).
The mechanism of action of valerian in general,
and in particular as a mild sedative, remains
unknown. The aim of this article is to describe the
biomedically active chemical constituents of valerian,
and to review, its chemistry, biochemistry,
pharmacology and toxicology.
BOTANY
The family Valerianaceae comprises 10 genera and
about 300 species (Simpson 2006), or the Valeriana
genus is of the family Caprifoliaceae and comprises
about 200 species (Judd et al. 2002). The
Valerianaceae are mostly distributed worldwide and
consist of herbs, rarely shrubs, with opposite leaves,
a sympetalous, spurred corolla, 1-4 stamens, and a
tricarpellate, inferior ovary with 1 functional locule
and a single, apical ovule, the fruit is an achene, with
a pappuslike calyx in some members. The economic
uses include some cultivated ornamentals (e.g.
Centranthus) and minor edible, medicinal, or essential
oil plants.
ETHNOBOTANY
Valeriana officinalis is native to Europe and Asia and
has naturalized in eastern North America. This tall
perennial prefers moist woodlands; it has been
extensively cultivated in northern Europe. Most of the
European supply is grown in Holland. It is cultivated
in low lying, damp sandy humus with a lime fertilizer.
It is harvested in the late fall and dried. V. officinalis
is the species used in Europe. The genus contains
over 250 species, with many more subspecies.
V. fauriei is used in traditional Chinese and Japanese
medicine (Huang 1999, Hikino et al. 1971, 1972a, b).
V. capensis is used in African traditional medicine
(Iwu 1993), V. edulis is used in Mexico and
V. wallichii is used in India (Schulz et al. 1997).
MEDICINAL USE
The roots of V. officinalis known as valerian have a
long history of use as a sedative medicine in Europe.
Valerian is a mild sedative and sleep-promoting agent
that is often used as a milder alternative or a possible
substitute for stronger synthetic sedatives, such as the
benzodiazepines, in the treatment of nervous states
and anxiety-induced sleep disturbances (Miyasaka et
al. 2006). Presently, valerian extracts are available as
dietary supplements, which are primarily comprised of
dried root or extracts from the root, formulated into
tablets or soft gelatin capsules. Each dose contains
between approximately 50 mg and approximately
1 gram of dried root or extract. The use of these
dietary supplements is extensive, with an estimated
210 million doses sold annually in the United States
and 125 million doses sold annually in Europe
(Grunwald 1995).
It has been recommended for epilepsy but that is
not supported by research (Spinella 2001). The current
indications for valerian are restlessness, insomnia,
nervousness, and tension (Tariq and Pulisetty 2008).
Large doses are known to cause withdrawal symptoms
when stopped (Garges et al. 1998), as it, as well as
most all sleep aids, may result in dependency. Those
with liver disease are advised not to use valerian
(Cohen and Del Torro 2008). While shown to be an
effective remedy for the reduction of anxiety, it has
also been reported to cause headaches and night terrors
in some individuals. This is due to the fact that some
people lack a digestive conversion property necessary
to effectively break down valerian. In these
individuals, valerian can cause agitation (Dennehy et
al. 2005).
One study found that valerian tends to sedate the
agitated person and stimulate the fatigued person,
bringing about a balancing effect on the system
(Muller and Klement 2006).
It is not fully understood which constituents of
V. officinalis, and/or of the other heretofore
unidentified members of the Valerianaceae family, are
responsible for the sedative and/or anxiolytic action of
valerian extracts. Nevertheless, the valepotriates
(iridoids) as well as valerenic acid, a sesquiterpenoid
compound, and the derivatives of valerenic acid are
present in valerian extracts. Of these components, the
valepotriates and valerenic acids are generally
considered to contribute to the sedative action of
valerian extracts, but have not been clearly and
positively identified as such (Hendriks et al. 1981).
CONSTITUENTS - CHEMISTRY AND PHARMACOLOGY
Valerian contains over 150 chemical constituents and
many of them are physiologically active (Jiang et al.
2007). There is substantial variation in the chemical
constituents in plants from different sources and
growing conditions, processing methods and storage
conditions but the differences are small (Wagner et al.
1972). In order to guarantee the quality of the drug,
producers have standardized production of the plant
extracts (Gutierrez et al. 2004).
The known pharmacologically active compounds
detected in valerian extract are alkaloids, terpenes,
organic acids and its derivatives, valepotriates and
flavones. It is generally accepted that the valepotriates
are the compounds responsible for the sedative
activity of the Valerianaceae.
Alkaloids
Alkaloids are present in amounts of 0.01-0.05% and
there are also terpene alkaloids present (Duke 1985).
The principal valerian alkaloids are actinidine,
chatinine, valerianine, valerine, alpha-methyl pyrryl
ketone and naphthyridin methyl ketone (Torssell and
Wahlberg 1967, Franck et al. 1970, Janot et al. 1979).
The structures of some valerian alkaloids are shown
in Fig. 1.
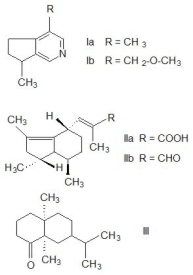
Fig. 1. The structures of principal compounds present in
volatile essential oil of Valeriana officinalis. Valerian
alkaloids actinidine (Ia) and valerianine (Ib), valerenic acid
(IIa), its aldehyde valerenal (IIb) and terpene valeranone
(III).
Actinidine (Ia) is a steam-volatile monoterpenoid
pyridine alkaloid with a cyclopenta[c]pyridine
skeleton found in the essential oil of valerian root
(Johnson and Waller 1971) and Actinidia polygama
(silver vine) (Sakan 1967). Actinidine is a cat
attractant, with similar effects as nepetalactone, the
active compound found in catnip (Nepeta cataria).
The alkaloid actinidine has been identified as an anal
gland product of two species of dolichoderine ants in
the genus Conomyrma (Wheeler et al. 1977) and is
also a pheromone for a variety of insects and an
important component of the defensive secretions of
rove beetles of various staphylinid species (Bellas et
al. 1974). Biosynthesis of actinidine results from
lysine and quinolinic acid as precursors (Auda et al.
1967). Actinidine is a psychoactive alkaloid that
interferes with the GABA-ergic metabolism; it is an
agonist on benzodiazepine receptors and thus exhibits
an allosteric modulation of the
GABA-receptor-proteins.
Chatinine was isolated from valerian by
Waliszewski (see Baby et al. 2005) but its biological
properties have not been studied; similarly poor
information exists about valerianine (Ib) and valerine although there are pyridine alkaloids close to
actinidine (Janot et al. 1979). Alpha-methyl pyrryl
ketone has been studied in Germany as a CNS active
compound (Sandor et al. 1970). Synthetic
naphthyridinones similar in structure to natural
naphthyridyl methyl ketone were patented as potential
drugs for the treatment of schizophrenia (Clark et al.
2005, Favor et al. 2006, Johnson et al. 2006). Since the
pharmacological properties of valerian alkaloids have
been studied separately only sporadically, it is difficult
to say how these participate in the medical effects of
V. officinalis.
Organic acids and terpenes
These compounds are present in the volatile essential
oil which represents 0.2-2.8% of the dry weight of the
root. The essential oils are not only present in the
subrerranean parts of the plants but also in the aerial
parts (Funke and Friedrich 1975). Terpenes are
chemically characterized as monoterpenes and
sesquiterpenes. Most considerable are valeric,
isovaleric, valerenic, isovalerenic and
acetoxyvalerenic acids, bornyl acetate, bornyl
isovalerenate, 1-pinene, 1-comphene, 1-borneol,
terpineol, valeranone and cryptofauronol. Some of the
oil components were suggested to have sedative
properties. Isovaleric acid and bornyl isovalerate are
compounds responsible for the characteristic aroma of
valerian.
Valeric acid, or n-pentanoic acid - straight-chain
fatty acid - has a very unpleasant odor. It is interesting
that the volatile esters of valeric acid tend to have
pleasant odors and are used in perfumes and
cosmetics. This is also true of isovaleric acid,
3-methylbutanoic acid. Both acids have
non-significant pharmacological and toxicological
properties and share the drug's odor only.
Nevertheless, it was recently found that isovaleric acid
reduces ATPase activity in the synaptic membranes of
the cerebral cortex and it is probably involved in the
pathophysiology of the neurological dysfunction of
isovaleric acidemic patients (Ribeiro et al. 2007).
Valerenic acid (IIa) and its aldehyde valerenal (IIb) are pharmacologically active monoterpenes. It
has been suggested that valerian acts via
gamma-aminobutyric acid (GABA) mechanisms
(Cavadas et al. 1995) and previous studies have
shown binding of valerian extract to GABA receptors,
but the functional effect of the binding has not been
demonstrated. Data from the study of Yuan et al.
(2004) suggest that the pharmacological effects of
valerian extract and valerenic acid are mediated
through modulation of GABAA receptor function.
Thus, the pharmacological effects of valerian extract
and valerenic acid are mediated through modulation
of the GABAA receptor function. Thus, valerian may
potentiate the sedative effects of anaesthetics and
other medications that act on GABA receptors, and
presurgical valerian use may cause a
valerian-anaesthetic interaction. Valerenic acid was
recently identified as a GABAA receptor modulator
(Trauner et al. 2008) and is known to penetrate into
the central nervous system transcellularly by passive
diffusion (Neuhaus et al. 2008). Dietz et al. (2005)
showed that valerenic acid is also a partial agonist of
the 5HT receptor with the strong binding affinity to
the 5-HT(5a) receptor, but only weak binding affinity
to the 5-HT(2b) and the serotonin transporter.
Valerenic acid, acetylvalerenolic acid and valerenal
were active as inhibitors of NF-B at a concentration
of 100 g/ml. Acetylvalerenolic acid reduced NF-B
activity to 4%, whereas valerenic acid (3) reduced
NF-B activity to 25% (Jacobo-Herrera et al. 2006).
Valeranone (III) was tested as a medical drug in
hyperkinetic behaviour disorders (Gupta and Virmani
1968). Valeranone was pharmacologically
investigated in animal experiments of its sedative,
tranquilizing and antihypertensive properties but the
activity of valeranone was lower than those of the
standard substances used (Ruker et al. 1978). The
structures of principal compounds present in volatile
essential oil are shown in Fig. 1. Other volatile
terpenes are bornyl isovalerate, bornyl acetate, bornyl
formate, eugenyl isovalerate, isoeugenyl isovalerate,
and many terpenic alcohols, aldehydes, ketones and
esters (Hikino et al. 1965).
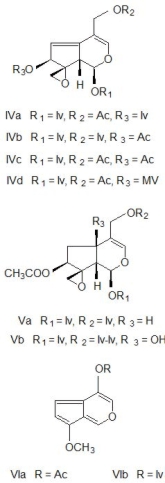
Fig. 2. The structures of principal compounds of Valeriana officinalis, valepotriates and their degradation
products, baldrinals. Diene valepotriates: valtrate (IVa),
isovaltrate (IVb), 7-desisovaleroyl-7-acetyl-valtrate (IVc)
and homovaltrate (IVd), and monoene valepotriates:
didrovaltrate (Va) and isovaleoxyhydroxydidrovaltrate (Vb).
Degradation products of valtrates are: baldrinal (VIa) and
homobaldrinal (VIb).
Abbreviations: Ac = acetyl, Iv = isovaleryl, Iv-Iv =
isovaleryloxyisovaleryl, MV = beta-methylvaleryl.
Valepotriates
Valepotriates are esterified iridoid-monoterpenes.
Their name is derived from the
valeriana-epoxy-triester, because these are triesters of
polyhydroxycyclopenta-(c)-pyrans with carboxylic
acids: acetic, valeric, isovaleric, alpha-isovaleroxy-isovaleric, beta-methylvaleric,
beta-acetoxy-isovaleric, beta-hydroxyisovaleric and
beta-acetoxy-beta-methylvaleric acid (Thies 1969). It is a
major component consisting of 50-80% active
compounds. Valepotriates are divided into two clases:
monoene and the diene derivatives. The structures of
some valepotriates are shown in Fig. 2. The principal
diene valepotriates are valtrate (IVa), isovaltrate (IVb), 7-desisovaleroyl-7-acetylvaltrate (IVc) and
7-homovaltrate (IVd), and the main monoene
derivatives are didrovaltrate (Va) and isovaleroxyhydroxydidrovaltrate (Vb). The amount
of valepotriates varies widely between species. The
underground parts contain normally higher amount of
valepotriates than the other parts of the plant (Violon
et al. 1984).
In an in vivo and in vitro investigation of
valepotriates and valeranone on guinea-pig ileum
smooth muscle preparations it was found that
dihydrovalerate and valeranone were able to relax
stimulated smooth muscle preparations with a potency
comparable to that of papaverine. Moreover, it was
shown that these valeriana compounds induced
smooth muscle relaxation via a musculotropic action,
which is also known to be the case for papaverine
(Hazelhoff 1984).
The valepotriates isovaltrate and valtrate, as well
as valeranone caused a suppression of rhythmic
contractions in a closed part of the guinea-pig ileum
in vivo. The same compounds relaxed potassium
stimulated contractures and inhibited BaCl2
contractions in guinea-pig ileum preparations in vitro.
Valeranone and didrovaltrate were about equipotent
to papaverine in inhibiting BaCl2 contractions.
Guinea-pig stomach fundic strips stimulated by
carbachol were also relaxed by these substances.
Potassium stimulated smooth muscle cells were also
relaxed by the valeriana compounds (Hazelhoff et al.
1982).
Valepotriates are very unstable compounds and
are sensitive to temperature, acids and alkali. Their
degradation products are known as baldrinals. Major
decomposition products of the valepotriates are the
baldrinal (VIa) and homobaldrinal (VIb). Baldrinals
reduced the spontaneous motor activity of light-dark
synchronized mice (Veith et al. 1986).
MECHANISM OF ACTION
Because of valerian's historical use as a sedative,
anti-convulsant, migraine treatment and pain reliever,
most basic science research has been directed at the
interaction of valerian constituents with the GABA
neurotransmitter receptor system (Trauner et al.
2008). The mechanism of action of valerian in
general, as a mild sedative in particular, remains
unknown (Wheatley 2005). Valerian extracts and
some of its constituents, mainly valerenic acid, appear
to have some affinity for the GABAA receptor, but the
exact mechanism of action is not yet known. Benke et
al. (2009) have described a specific binding site on
GABAA receptors with nM affinity for valerenic acid
and valerenol, common constituents of valerian. Both
agents enhanced the response to GABA at multiple
types of recombinant GABAA receptors. A point
mutation in the beta2 or beta3 subunit of recombinant
receptors strongly reduced the drug response. In vivo,
valerenic acid and valerenol have anxiolytic activity
with high potencies in the elevated plus maze and the
light/dark choice test in wild type mice. In beta3
point-mutated mice the anxiolytic activity of valerenic
acid was absent. Thus, neurons expressing beta3
containing GABAA receptors are a major cellular
substrate for the anxiolytic action of valerian extracts
(Benke et al. 2009). Valerenic acid is a GABAA
receptor modulator (Trauner et al. 2008). Substances
such as valerenic acid and its derivatives
acetoxyvalerenic acid and hydroxyvalerenic acid have
to cross the blood-brain barrier and interact with this
receptor in the brain (Neuhaus et al. 2008). Transport
of these terpenic acids was compared with the
permeability of the GABAA modulator diazepam,
which is known to penetrate into the central nervous
system transcellularly by passive diffusion. It was
hypothesized that the investigated terpenes from
V. officinalis can probably only pass through the
blood-brain barrier by a still unknown transport system
and not transcellularly by passive diffusion (Neuhaus
et al. 2008).
ANIMAL BEHAVIOUR TESTS
Hazelhoff (1984), in his dissertation, shows that the
valerian preparations and compounds contained in
V. officinalis extract cause a significant reduction in
thelocomotor activity of mice, whereas of the
valepotriates only didrovaltrate was found to be active
in this test model (Hazelhoff 1984).
The effect of a mixture of valepotriates on the
elevated plus-maze performance of diazepam
withdrawn rats was evaluated by Andreatini and Leite
(1994).
The rats were chronically (28 days) treated with
diazepam (doses increased up to 5.0 mg/kg) and then
treated with a control solution for 3 days to induce a
withdrawal syndrome. Chronically vehicle-treated rats
were used as control. The abstinent animals treated
with the vehicle showed a significant decrease in the
percentage of time spent in the open arms when
compared with the control animals. Diazepam and
valerian 12.0 mg/kg reversed this anxiogenic effect.
Valerian 6.0 mg/kg did not show any difference in
relation to the other group.
TOXICOLOGY
Numerous studies have indicated that aqueous and
alcoholic extracts of V. officinalis are a little toxic and
have high LD50 values. For example valeranone has
LD50 for mice at i.p. administration 580 mg/kg
(Holzl 1997). A unique case of overdose where the
patient had ingested almost 25 g of powdered V.
officinalis root in capsule form, demonstrated only
mild symptoms (Willey et al. 1995) which included
fatigue, abdominal cramps and tremor; all of the
symptoms disappeared within 24 hours.
The clinical evidence indicates that valerian is a
relatively safe substitute for the benzodiazepines as a
mild tranquilizer. It was traditionally contraindicated
in pregnancy, but until recently there were no studies
to warrant this warning. An Australian study (Yao et
al. 2007) on female rats which were orally dosed with
a valerian extract daily on either gestation days 1-8 or
8-15 indicated that valerian had no adverse effects on
fertility or foetal development.
Literature reports have suggested that valerian
induces genotoxicity in vitro (ECV304 cells) by a
reactive oxygen species-mediated mechanism
(Hui-lian et al. 2003); however, there are no reports
on its genotoxicity and/or the epigenetic mechanism
in vivo (Al-Majed et al. 2006).
Genotoxicity has been reported for both baldrinal
and homobaldrinal, the decomposition products of
valtrate and isovaltrate. These compounds showed
direct mutagenic effects in vitro in the AMES assay
and the SOS-chromo-test (Hude et al. 1986). Studies
on the effects of baldrinals on haemopoietic cells in
vitro (Braun et al. 1986), indicating decreased liver
function.
CONCLUSIONS
Valerian (Valeriana officinalis) is widely known for
its use as a sedative and an anti-anxiety drug in folk
medicine. The root of valerian is used most
commonly for its sedative and hypnotic properties in
patients with insomnia, and less commonly as an
anxiolytic. The chemical composition of valerian
includes sesquiterpenes of the volatile oil (including
valeric acid), iridoids (valepotriates), alkaloids, and
free amino acids. Although the sesquiterpene
components of the volatile oil are believed to be
responsible for most of valerian's biologic effects, it
is likely that all of the active constituents of valerian
act in a synergistic manner to produce a clinical
response. Valerian is a safe herbal choice for the
treatment of mild insomnia and has good tolerability.
REFERENCES
Al-Majed AA, Al-Yahya AA, Al-Bekairi AM, Al-Shabanah OA, Qureshi S: Studies on the cytological and biochemical effects of valerian in somatic and
germ cells of Swiss albino mice. Food Chem Toxicol 44:1830-1837, 2006.
Andreatini R, Leite JR: Effect of valepotriates on the behavior of rats in the elevated plus-maze during diazepam withdrawal. Eur J Pharmacol
260:233-235, 1994.
Auda H, Waller GR, Eisenbraun EJ: Biosynthesis of methylcyclopentane monoterpenoids. 3. Actinidine. J Biol Chem 242:4157-4160, 1967.
Baby R, Cabezas M, Castro E, Filip R, Walsoe de Reca NE: Quality control of medicinal plants with an electronic nose. Sensors Actuators B Chemical
106:24-28, 2005.
Bellas TE, Brown WV, Moore BP: The alkaloid actinidine and plausible precursors in defensive secretions of rove beetles. J Insect Physiol
20:277-280, 1974.
Benke D, Barberis A, Kopp S, Altmann KH, Schubiger M, Vogt KE, Rudolph U, Mohler H: GABA(A) receptors as in vivo substrate for the
anxiolytic action of valerenic acid, a major constituent of valerian root extracts. Neuropharmacology 56:174-181, 2009.
Braun R, Dieckmann H, Machut M, Echarti C, Maurer HR: Studies on the effects of baldrinal on hemopoietic cells in vivo, on the metabolic
activity of the liver in vivo, and on the content in proprietry drugs. [Article in German] Planta Med 52:446-450, 1986.
Cavadas C, Araujo I, Cotrim MD, Amaral T, Cunha AP, Macedo T, Ribeiro CF: In vitro study on the interaction of Valeriana officinalis
L. extracts and their amino acids on GABAA receptor in rat brain. Arzneimittelforschung 45:753-755, 1995.
Clark J, Davis J, Favor DA, Fay L, Franklyn L, Heneger K, Johnson D, Zhijian Z: [1,8]Naphthyridin-2-ones and related compounds for the treatment of
schizophrenia. US Patent WO2005019215, March 3, 2005.
Cohen DL, Del Torro Y: A case of valerian-associated hepatotoxicity. J Clin Gastroenterol 42:961-962, 2008.
Dennehy CE, Tsourounis C, Horn AJ: Dietary supplement-related adverse events reported to the California Poison Control System. Am J Health Syst
Pharm 62:1476-1482, 2005.
Dietz BM, Mahady GB, Pauli GF, Farnsworth NR: Valerian extract and valerenic acid are partial agonists of the 5-HT5a receptor in vitro.
Brain Res Mol Brain Res 138:191-197, 2005.
Duke JA: CRC Handbook of Medicinal Herbs. CRC Press, Boca Raton 1985.
Favor DA, Johnson DS, Repine JT, White AD: Isoquinoline [1,8]naphthyridin-2-ones and related compounds for treatment os schizophrenia. US Patent
WO2006090272, August 31, 2006.
Franck B, Petersen U, Huper F: Valerianine, a tertiary monoterpene alkaloid from valerian. Angew Chem Int Ed Engl 9:891, 1970.
Fugh-Berman A, Cott JM: Dietary supplements and natural products as psychotherapeutic agents. Psychosom Med 61:712-728, 1999.
Funke ED, Friedrich H: Valepotriates in the aerial parts of some more valerianaceae species. [Article in German] Planta Med 28:215-224,
1975.
Garges HP, Varia I, Doraiswamy PM: Cardiac complications and delirium associated with valerian root withdrawal. JAMA 280:1566-1567, 1998.
Grunwald J: The European Phytomedicines Market-Figures, Trends Analyses. Herbal Gram, Austin 1995.
Gupta PD, Virmani V: Clinical trial of jatamansone (syn: Valeranone) in hyperkinetic behaviour disorders. Neurol India. 16:168-173, 1968.
Gutierrez S, Ang-Lee MK, Walker DJ, Zacny JP: Assessing subjective and psychomotor effects of the herbal medication valerian in healthy volunteers.
Pharmacol Biochem Behav 78:57-64, 2004.
Hazelhoff B: Phytochemical and pharmacological aspects of valerian compounds. With special reference to valepotriates. Dissertation, Thesis,
University of Groningen, Groningen 1984.
Hazelhoff B, Malingre TM, Meijer DK: Antispasmodic effects of valeriana compounds: an in-vivo and in-vitro study on the guinea-pig
ileum. Arch Int Pharmacodyn Ther 257:274-287, 1982.
Hendriks H, Geertsma HJ, Th. M. Malingre TM: The occurence of valeranone and crypto-fauronol in the essential oil of Valeriana offcinalis L.
s.l. collected in the northern part of The Netherlands. Pharm Worls Sci 3:1316-1320, 1981.
Hikino H, Hikino Y, Kato H, Takeshita Y, Takemoto T: Constituents of wild Japanese valerian root. 1. Yakugaku Zasshi 91:766-769, 1971.
Hikino H, Ono M, Takemoto T: Constituents of wild Japanese valerian root. 2. Yakugaku Zasshi 92:479-481, 1972a.
Hikino H, Hikino Y, Nakamara R, Ono M, Takemoto T: Constituents of wild Japanese valerian root. 3. Yakugaku Zasshi 92:498-502, 1972b.
Hikino H, Takeshita Y, Hikino Y, Takemoto T: Structure of fauronyl acetate and cryptofauronol. Chem Pharm Bull (Tokyo) 13:631-632, 1965.
Holzl J: The pharmacology and therapeutics of valeriana. In: Houghton PJ (ed.): Valerian. The Genus Valeriana. Harwood Acad Publ Amsterdam 1997.
pp. 55-74.
Huang KC: The Pharmacology of Chinese Herbs. Boca Raton: CRC Press, 1999.
Hude von der W, Scheutwinkel-Reich M, Braun R: Bacterial mutagenicity of the tranquilizing constituents of Valerianaceae roots. Mutat Res
169:23-27, 1986.
Hui-lian W, Dong-fang Z, Zhao-feng L, Yang L, Qian-rong L, Yu-zhen W: In vitro study on the genotoxicity of dichloromethane extracts of
valerian (DEV) in human endothelial ECV304 cells and the effect of vitamins E and C in attenuating the DEV-induced DNA damages. Toxicol Appl
Pharmacol 188:36-41, 2003.
Iwu MM: Handbook of African Medicinal Plants. Boca Raton: CRC Press, 1993.
Jacobo-Herrera NJ, Vartiainen N, Bremner P, Gibbons S, Koistinaho J, Heinrich M: NF-kappaB modulators from Valeriana officinalis. Phytother
Res 20:917-919, 2006.
Janot MM, Guilhem J, Contz O, Venera G, Cionga E: Contribution to the study of valerian alcaloids (Valeriana officinalis L.): actinidine and
naphthyridylmethylketone, a new alkaloid. Ann Pharm Fr 37:413-420, 1979.
Jarema M: Herbal drug treatment. Neuro Endocrinol Lett 29, Suppl 1:93-104, 2008.
Jiang X, Zhang JC, Liu YW, Fang Y: Studies on chemical constituents of Valeriana officinalis. [Article in Chinese]. Zhong Yao Cai.
30:1391-1393, 2007.
Johnson DS, Singer JM, White AD: [1,8]Naphthyridin-2-ones and related compounds with keto or hydroxyl linkers for the treatment of schizophrenia.
US Patent WO2006090273, August 31, 2006.
Johnson RD, Waller GR: Isolation of actinidine from Valeriana officinalis. Phytochemistry 10:3334-3335, 1971.
Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ: Plant Systematics, a Phylogenetic Approach. Sinauer Associates, Sunderland,
2002.
Klich R: Behavior disorders in childhood and their therapy. Med Welt 26:1251-1254, 1975.
Miyasaka LS, Atallah AN, Soares BG: Valerian for anxiety disorders. Cochrane Database Syst Rev 18:CD004515, 2006.
Mowrey DB: The Scientific Validation of Herbal Medicine. Keats Pub., New Cannan 1986, p. 316.
Muller SF, Klement S: A combination of valerian and lemon balm is effective in the treatment of restlessness and dyssomnia in children.
Phytomedicine 13:383-387, 2006.
Neuhaus W, Trauner G, Gruber D, Oelzant S, Klepal W, Kopp B, Noe CR: Transport of a GABAA receptor modulator and its derivatives from
Valeriana officinalis L. s. l. Across an in Vitro Cell Culture Model of the Blood-Brain Barrier. Planta Med 74:1338-1344,
2008.
Ribeiro CA, Balestro F, Grando V, Wajner M: Isovaleric acid reduces Na+, K+-ATPase activity in synaptic membranes from cerebral cortex of young
rats. Cell Mol Neurobiol 27:529-540, 2007.
Rucker G, Tautges J, Sieck A, Wenzl H, Graf E: Isolation and pharmacodynamic activity of the sesquiterpene valeranone from Nardostachys jatamansi
DC. [Article in German] Arzneimittelforschung. 28:7-13, 1978.
Sakan T: Matatabi (Actinidia polygama Miq.)-isolation and structure of its biologically active components [Article in Japanese] Tanpakushitsu
Kakusan Koso 12:2-9, 1967.
Sandor P, Kovach AG, Horvath KB, Szentpetery GB, Clauder O: Pharmacological studies on the effect of synthetic alpha-methyl-pyrryl-ketone on the
central nervous system and blood circulation. [Article in German] Arzneimittelforschung 20:29-32, 1970.
Simpson MG: Plant Systematics. Elsevier, Amsterdam, 2006.
Schulz V, Hansel R, Tyler VE: Rational Phytotherapy: A Physicians' Guide to Herbal Medicine. Berlin. Springer, p. 306, 1997.
Spinella M: Herbal medicines and epilepsy: The potential for benefit and adverse effects. Epilepsy Behav 2:524-532, 2001.
Tariq SH, Pulisetty S: Pharmacotherapy for insomnia. Clin Geriatr Med 24:93-105, 2008.
Thies PW: On the chromomgenic behavior of valepotriate. 5. Report on the active substances
of Valerian. [Article in German] Arzneimittelforschung. 19:319-322, 1969.
Torssell K, Wahlberg K: Isolation, structure and synthesis of alkaloids from Valeriana officinalis L. Acta Chem Scand 21:53-62,
1967.
Trauner G, Khom S, Baburin I, Benedek B, Hering S, Kopp B: Modulation of GABAA receptors by valerian extracts is related to the content
of valerenic acid. Planta Med 74:19-24, 2008.
Veith J, Schneider G, Lemmer B, Willems M: The influence of some degradation products of valepotriates on the motor activity of light-dark
synchronized mice. [Article in German] Planta Med 52:179-183, 1986.
Violon C, Dekegel D, Vercruysse A: Relation between valepotriate content and differentiation level in various tissues from Valerianeae. J Nat Prod
47:934-940, 1984.
Wagner H, Schaette R, Horhammer L, Holzl J: Dependence of the valepotriate and essential oil content in Valeriana officinalis L. on various
exogenous and endogenous factors. Arzneimittelforschung 22:1204-1209, 1972.
Wheatley D: Medicinal plants for insomnia: a review of their pharmacology, efficacy and tolerability. J Psychopharmacol 19:414-421, 2005.
Wheeler JW, Olagbemiro T, Nash A, Blum MS: Actinidine from defensive secretions of dolichoderine ants. J Chem Ecol 3:241-244, 1977.
Willey LB, Mady SP, Cobaugh DJ, Wax PM: Valerian overdose: a case report. Vet Hum Toxicol 37:364-365, 1995.
Yao M, Ritchie HE, Brown-Woodman PD: A developmental toxicity-screening test of valerian. J Ethnopharmacol 113:204-209, 2007.
Yuan CS, Mehendale S, Xiao Y, Aung HH, Xie JT, Ang-Lee MK: The gamma-aminobutyric acidergic effects of valerian and valerenic acid on rat brainstem
neuronal activity. Anesth Analg 98:353-358, 2004.
|
BACK
|



