Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 1, p 41-46
DOI 10.2478/v10136-009-0006-8
The effect of S-nitrosocaptopril and S-nitroso-N-acetyl-D,L- penicillamine on blood glucose concentration and
haemodynamic parameters
Miroslav Pohanka, Vitezslav Vlcek, Kamil Kuca, Hana Bandouchova, Jiri Pikula
Address: Miroslav Pohanka, Centre of Advanced Studies, Faculty of Military Health Sciences, University of Defense, Trebesska 1575, 500 01 Hradec
Kralove, Czech Republic
rau@atlas.cz
Received 16th July 2009.
Revised 11th September 2009.
Published online 26th November 2009.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Material and Methods
Results and discussions
Conclusions
References
SUMMARY
Sorption isotherms were estimated for two model organophosphorus pesticides - methamidophos and paraoxon-ethyl - and four typical Central European
soils endangered by these pesticides: haplic Chernozems, Cambisols, Luvisols, and Fluvisols. A photometric microplate assay based on the
recognition capability of the enzyme acetylcholinesterase toward organophosphorous pesticides was used for the construction of sorption isotherms.
The sorption capacity of each soil was then determined and it was found that the soils with the highest content of humic acid (haplic Chernozems
and haplic Luvisols) sorbed pesticides most. Pesticides in concentrations over the sorption capacity were easily removed from soil by
water.
KEY WORDS
organophosphates; methamidophos; paraoxon; sorption isotherms; capacity
INTRODUCTION
Organophosphates are a large group of compounds
well known because of their toxicity pathway based
on the inhibition of enzyme acetylcholinesterase (AChE). The inhibition results in a partial or total dysfunction of neurosynapses in the body
(Goel and
Aggarwal 2007). Two groups of organophosphates
are commonly known: pesticides (e.g. chlorfevinphos,
dichlorvos, paraoxon, parathion, and propoxur) and
nerve agents (e.g. sarin, soman, tabun, and VX)
(Barthold and Schier 2005).
The sorption of pesticides in soil is influenced by
multiple parameters, including factors such as organic
carbon, the consistency of the soil (texture), pH and
metal content (e.g. iron, aluminium) (Johnson and
Sims 1993, Seybold et al. 1994, Fujisu and Urano
2001, Sheng et al. 2005, Jarvis et al. 2007). Humic
acids are considered one of the more important
fractions of organic carbon influencing the properties
of soils (Delgado et al. 2003, Gondar et al. 2005) and
an approachable way of presenting sorption
characteristics seems to be by means of the
distribution coefficient Kd (Coquet 2003). Soils from
New Zealand (Baskaran et al. 1996) and Brazil
(Spadotto and Hornsby 2003), for example, have been
investigated in this way.
An estimation of the sorption of pesticides is
typically based on chromatographic techniques such
as high performance liquid chromatography (HPLC),
used, for example by Spadotto and Hornsby (2003).
However there are other techniques available, such as
the analysis of the 14C labelled pesticide (Baskaran et
al. 1996). Our contemporary effort is aimed not only
at the investigation of the sorption capacities of
selected soils but also at the performance of the
assays engaged with the use of AChE. The
above-mentioned assay could thus be used in several
ways; biosensor (Pohanka et al. 2008a, b, 2009a) and
photometric (Pohanka et al. 2008c) assays may be
mentioned as examples, but, its use for pedological
purposes has not yet been described. We decided to
perform a photometric assay using 96-wells
microplates for the data amplification and
demonstration of the method.
MATERIAL AND METHODS
Soil sampling
Soil samples were sieved through a 5 mm mesh
commonly used for fresh soil samples, and were
air-dried at room temperature. Sieving through a
2 mm mesh, more typical for analyses of dry soil
samples, was performed only to determine a number
of physical, physical-chemical and chemical
properties of the uppermost soil layer. Four profiles
representative of soils in Central Europe and Czech
Republic (from 75%) were sampled. Man's influence
is widespread, with cultivation over a long period and
one soil sample is from a flood-plain forest with its
original type of vegetation.
The soils studied are haplic Chernozems (S1),
haplic Cambisols (S2), haplic Luvisols (S3), and
haplic Fluvisols (S4). The soils were divided
according to FAO (2006).
Soils
S1 Locality Prace (N 49o 8.333´, E 16o 45.444´):
haplic Chernozems (ha-CH), Climatic region by
Köppen's Classification is Cfb (temperate broadleaf
deciduous forest). Silty-clay loam Soil. S2 Locality
Nove Mesto na Morave (N 49o 32.840´, E 16o
4.877´): haplic Cambisols (ha-CM), Climatic region
by Koppen's Classification is Dfb (boreal climates).
Loam Soil. S3 Locality Zelesice (N 49o 7.194´, E 16o
35.743´): haplic Luvisols (ha-LV), Climatic region by
Koppen's Classification is Cfb (temperate broadleaf
deciduous forest). Silt loam Soil. S4 Lokality Ivan (N
48o 55.533´, E 16o 34.033´): haplic Fluvisols (ha-FL),
Climatic region by Koppen's Classification is Cfb
(temperate broadleaf deciduous forest). Clay loam
Soil.
Soil analysis
Air-dried soil samples were analysed. The particle
size distribution was measured by the sedimentation
method (Day 1965, Franzmeier et al. 1977). Cation
exchange capacity (CEC) and Base saturation were
measured by the Mehlich method (Mehlich 1948,
Deller 1981), solid organic matter (SOM) by a
modified version of the Walkley-Black method
(Walkley and Black 1934); humic to fluvic acid ration
(HA/FA) were measured according to the Kononova
and Belcikova's (1961) method, and pH (in KCl) by
ISO 10390:2005.
Pesticides sorption
Paraoxon-ethyl and methamidophos compounds (both
of analytical standard, pesticides content over 98%)
were obtained from Labor Dr. Ehrenstorfer-Schafers
(Augsburg, Germany). The pesticides were diluted in
deionized water to obtain the final concentration
10-5-10-10 M. 1 g of soil sample was suspended with
1 ml of the pesticide solution and kept in a fridge for
24 hours of incubation. Deionized water was treated
in the same way as the pesticide for the purpose of
negative controls. Centrifugation 3.000 x g for
10 minutes was used for obtaining the liquid phase
(further sample) from the soil - pesticide mixture.
Acetylcholinesterase-based assay
Measurements were carried out in standard conditions
for temperature and pressure (SATP). Buffered
lyophilized human recombinant AChE (2.000 U/mg)
was purchased from Sigma-Aldrich (Czech Republic
branch). AChE was diluted with deionized water up
to the final activity 1 U/ml. Polystyrene 96-well
microplates (Gama, Ceske Budejovice, Czech
Republic) typically used for immunological studies
(Pohanka 2009b) were used throughout the
AChE-based experiments. The photometric
evaluation was carried out according to Pohanka et al.
(2008b).
The sample was injected with an amount of 10 microl
per well. 40 l of acetylthiocholine chloride
(ATChCl) 1 mM with 1 mM Ellman's reagent:
5,5´-dithiobis (2-nitrobenzoic acid) (further DTBN) in
phosphate buffered saline (PBS) per well was injected
after thirty minutes pre-incubation of AChE with the
sample. Absorbance was measured at 412 nm using a
microplate reader MRX (Dynatech Laboratories,
Chantilly, USA) after another 10 minute-period of
pre-incubation. ATChCl with DTBN in PBS alone
served as a blank.
AChE inhibition by the pesticide from the sample
could be described using a percentage scale (I):
Ai
I = (1 - ------------) x 100
An
(Eq. 1)
The Ai indicates absorbance shift (against blank)
provided by AChE effected by the pesticide from the
samples; An is the absorbance shift caused by intact
AChE when the negative control is used in the same
manner as the pesticide-containing sample.
RESULTS AND DISCUSSIONS
Characterization of soils
The most important parameters of the sampled
profiles are summarized in Table 1. The profiles
typically occurring in Central Europe were selected,
while the exact localities were selected at random.
The haplic Chernozems, Cambisols and Luvisols
(S1-S3) are typical soils of arable land where the
application of pesticides by farmers may be expected.
The haplic Fulvisols (S4) represent the soil
endangered by contamination after rain and/or floods.
The fine earth was characterized at the start of the
experiments (Table 2). The most important
parameters for estimation of pesticides sorption by
soils were considered to be organic material, and
humic and fulvic acids. The correlation between
above-mentioned parameters and AChE-based assay
is described below.
Performance of AChE-based assay
The photometric assay based on AChE was found to
be reliable in pesticide analysis. No significant
interference caused by soil particles was observed
when pure water was used instead of the pesticide.
The proposed assay could be considered very
intriguing for pedology because the low consumption
of reagents results in low costs per each analysis: an
estimation of the material costs per each analysis is
lower than ten euro cents. The low cost of the whole
device is an undisputed advantage when we consider
the price and costs of the more traditional devices
such as chromatography or mass spectroscopy. The
simultaneous analysis of a large number of samples
(one plate includes 96 wells) is another advantage.
Complete graphic presentations of the percentage
of inhibition vs. pesticide concentration (sorption
isotherm) for samples eluted from soil by water are
shown in Fig. 1. The elected concentration (mol/l)
used in the axis description corresponds to molar
sorption per one kilogram of soil (mol/kg) for our
experimental setup.Complete graphic presentations of the percentage
of inhibition vs. pesticide concentration (sorption
isotherm) for samples eluted from soil by water are
shown in Fig. 1. The elected concentration (mol/l)
used in the axis description corresponds to molar
sorption per one kilogram of soil (mol/kg) for our
experimental setup.
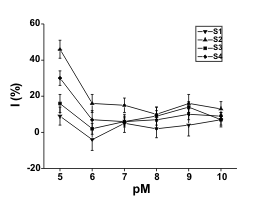
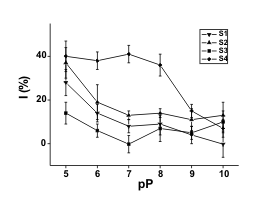
Fig. 1. Sorption isotherms for organophosphorus
pesticides and soils (S1-S4). Description of axes is
following: (x-axis) inverse logarithm of pesticide paraoxon
(pP) or methamidophos (pM) molar concentration; (y-axis)
percentage of inhibition. Used soils are marked in the
graphs. Means ± standard deviation are indicated (n = 4).
Table 1: General characteristics of the profiles sampled
| Profile |
Soil classificationa |
Altitude
[m] |
Slope
[%] |
Vegetation or
land use |
Substratum |
Soil climateb | | Precipita-tion [mm] |
Tempera-ture [° C] | | S1 |
haplic Chernozems |
240 |
5 |
Arable land |
Loess |
490 |
8.7 | | S2 |
haplic Cambisols |
636 |
5 |
Arable land |
Syenite |
581 |
6.6 | | S3 |
haplic Luvisols |
210 |
9 |
Arable land |
Loess |
490 |
8.7 | | S4 |
haplic Fluvisols |
170 |
< 1 |
Flood-plain
forest |
Fluvial
deposits |
482 |
9.2 |
a - According to FAO [7]
b - Annual quantities (data from the Czech Hydrometeorological Institute), temperature average at 2 m above earth
Table 2: Selected properties of the fine earth
| Sample |
Depth
[cm] |
Particle size distribution
[%] |
pH
(KCl) |
CEC
[cmol/kg] |
Base
saturation
[%] |
SOM
[%] |
HA/FA | | Clay |
Silt |
Sand |
| S1 |
5-25 |
17.6 |
50.4 |
32.0 |
6.7 |
288.0 |
87 |
3.28 |
1.25 | | S2 |
5-25 |
26.1 |
42.0 |
31.9 |
5.0 |
165.7 |
55 |
2.90 |
0.51 | | S3 |
5-25 |
21.3 |
55.1 |
23.6 |
7.2 |
218.2 |
100 |
1.46 |
0.84 | | S4 |
5-25 |
29.2 |
36.5 |
34.3 |
6.0 |
330.5 |
87 |
4.24 |
0.79 |
Soil organic matter (from Soil Organic Carbon * 1.724)
HA/FA: Humic (HA) and fulvic acid (FA)
A similarity between the sorption of
methamidophos and paraoxon can be observed for
soils S1-S3. However, soil S4 provided a different
shape of sorption isotherm for paraoxon.
Methamidophos and paraoxon sorption amounted to
approximately 10-6 mol/kg for all tested soils and 10-7
mol/kg for soils S1-S3, respectively. A sharp increase
in percentage of inhibition occurred when the amount
of methamidophos or paraoxon used exceeded this
level. The soils S2 and S4 had a lower influence on
the percentage of inhibition and probably less affinity
of soil particles to pesticides, than the others. S1 and
S3 soils remained partially effective in
methamidophos and paraoxon retention. We could not clearly explain this fact. These soils may be
characterized by higher pH and HA/FA in comparison
with the last two soils. Although there were some
expectations about the parameters effecting the
sorption capacity (Seybold et al. 1994), correlation
with the SOM and CEC was not clearly confirmed.
Because of near neutral or only slightly acidic pH, we
may exclude the influence of pH on the stability of
pesticides. The most important parameter seems to
have been the content of HA. We could see a
correlation between HA and C contents and explain
the expected influence of the C content in this way, as
already proposed in references: Karickhoff et al. 1979,
Yaron et al. 1985, Johnson and Sims 1993, but when
soil S4 and paraoxon were used, unexpected data
were observed. Sorption of paraoxon on S4 soil was
minimal in comparison with any other sorption
isotherm. Sorption maximum for S4 soil was less than
10-9 mol/kg when paraoxon was used. The data in
Fig. 1 point at a sharp increase in the percentage of
inhibition - up to 40%; however, this effect is caused
by a slow reaction between the pesticide and serine
hydroxyl in the enzymatic active centre. Prolongation
of the pre-incubation interval up one hour caused a
better distribution of inhibition. The percentage of
inhibition increased up to 80% (10-5 M paraoxon; data
not shown). The low sorption of paraoxon is probably
caused by its lipophilic characteristics since the S4
soil contains a lot of dissociable organic groups. This
idea is supported by the highest CEC and SOM from
applied soils (Table 2). We consider low sorption of
paraoxon in S4 soil as a serious menace since
paraoxon could be easily eluted into drinking water
supplies, especially after floods or rains. The
described menace is amplified due to the occurrence
of S4 in the tidal lands. The sorption capacities of the
other soils are incomparably higher. However,
pesticides could be eluted from it under other
conditions.
The overall sorption capacity of soils is due to the
specific structure of the soil matrix. The surface of
soil particles is extensive because of their minute size.
Though the average size of silt and clay (Goossens
and Buck 2009) is approximately ten to one hundred
times greater than typical nanoparticles (Panyala et al.
2008, Tejral et al. 2009), some specific parameters
such as sorption on catalyzed oxidation would be
expected.
CONCLUSIONS
An AChE-based assay was successfully performed
for the evaluation of organophosphates in soils and
the estimation of sorption capacity and sorption
isotherms in four soils typically found in Central
Europe. Two model organophosphates, i.e. paraoxon
and methamidophos, were selected as important
pesticides widely available on the market. The
described assay proved to be very reliable and seems
to be suitable for routine applications. The sorption
isotherms and sorption capacities were obtained using
the AChE-based assay only. Results were comparable
with the studies found in available databases.
ACKNOWLEDGEMENT
Support of the Ministry Defence of the Czech
Republic for the Project FVZ0000604 is gratefully
acknowledged.
REFERENCES
Barthold CL, Schier JG: Organic phosphorus compounds - nerve agents. Crit Care Clin 21:673-689, 2005.
Baskaran S, Bolan NS, Rahman A, Tillman RW: Pesticide sorption by allophanic and non-allophanic and soils of New Zealand. NZ J Agric Res
39:297-310, 1996.
Coquet Y: Variation of pesticide sorption isotherm in soil at the catchment scale. Pest Manag Sci 59:69-78, 2003.
Day PR: Particle fractionation and particle-size analysis. In Black CA (ed.) Methods of Soil Analysis, Part 1: American Society of Agronomy, Inc.,
Madison, Wisconsin, pp. 545-567, 1965.
Delgado R, Martin-Garcia JM, Oyonarte C, Delgado G: Genesis of the terrae rossae of the Sierra Gador (Andalusia, Spain). Eur J Soil Sci 54:1-16,
2003.
Deller B: Determination of exchangeable acidity carbonate ions and change of buffer in triethanoloamine-buffered solutions perkolated through soil
samples containing carbonates. Commun Soil Sci Plant 12:161-161, 1981.
FAO 2006. World Reference Base for Soil Resources. World Soil Resources Report 103, FAO, Rome.
Franzmeier DP, Steinhardt GC, Crum JF, Norton LD: Soil Characterization in Indiana: I. Field and Laboratory Procedures. Research Bulletin
943:13-14, 1977.
Fujisu Y, Urano K: Adsorption of pesticides and their biodegraded products on clay minerals and soils. J Health Sci 47:429-432, 2001.
Goel A, Aggarwal P: Pesticide poisoning. Natl Med J India 20:182-191, 2007.
Gondar D, Lopez R, Fiol S, Antelo JM, Arce F: Effect of soil depth on acid properties of humic substances extracted from an ombrotrophic peat bog
in northwest Spain. Eur J Soil Sci 56:793-801, 2005.
Goossens D, Buck B: Dust dynamics in off-road vehicle trails: measurements on 16 arid soil types, Nevada, USA. J Environ Manage 90:3458-3469,
2009.
Jarvis N, Larsbo M, Roulier S, Lindahl A, Persson L: The role of soil properties in regulating non-equilibrium macropore flow and solute transport
in agricultural topsoils. Eur J Soil Sci 58:282-292, 2007.
Johnson RM, Sims JT: Influence of surface and subsoils properties on herbicide sorption by atlantic and coastal plain soils. Soil Sci 155:339-348,
1993.
Karickhoff SW, Brown OS, Scott TA: Sorption of hydrophobic pollutants on natural sediments and soils. Water Res 13:241-248, 1979.
Kononova MM, Belcikova NP: A rapid analysis of humus composition in mineral soil. Pochvovedenie 10:75-87, 1961.
Mehlich, A: Determination of cation- and anion-exchange properties of soil. Soil Sci 66:429-445, 1948.
Panyala NR, Pena-Mendez EM, Havel J: Silver or silver nanoparticles: a hazardous threat to the environment and human health? J Appl Biomed
6:117-129, 2008.
Pohanka M, Skladal P: Electrochemical biosensors - principles and applications. J Appl Biomed 6:57-64, 2008a.
Pohanka M, Kuca K, Jun D: Optimization of acetylcholinesterase immobilization onto screen printed platinum electrode. J Appl Biomed 6:27-30,
2008b.
Pohanka M, Jun D, Kuca K: Photometric microplates assay for estimation of paraoxon inhibited acetylcholinesterase reactivation efficacy. J Enzyme
Inhib Med Chem 23:781-784, 2008c.
Pohanka M, Musilek K, Kuca K: Progress of biosensors based on cholinesterase inhibition. Curr Med Chem 16:1790-1798, 2009a.
Pohanka M: Monoclonal and polyclonal antibodies production - preparation of potent biorecognition element. J Appl Biomed 7:115-121, 2009b.
Seybold CA, McSweeney K, Lowery B: Atrazine adsorption in sandy soils of Wisconsin. J Environ Qual 23:1291-1297, 1994.
Sheng GY, Yang YN, MinSheng H, Kai Y: Influence of pH on pesticide sorption by soil containing wheat residue-derived char. Environ Pollut
134:457-463, 2005.
Spadotto CA, Hornsby AG: Soil sorption of acidic pesticides: modeling pH effects. J Environ Qual 32:949-956, 2003.
Tejral G, Panyala NR, Havel J: Carbon nanotubes: toxicological impact on human health and environment. J Appl Biomed 7:1-13, 2009.
Walkley A, Black IA: An examination of the Degtjareff method for determining organic carbon in soils: Effect of variations in digestion conditions
and of inorganic soil constituents. Soil Sci 63:251-263, 1934.
Yaron B, Gerstl Z, Spencer WF: Behaviour of herbicides in irrigated soils. Adv Soil Sci 3:121-211, 1985.
|
BACK
|



