Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 1, p 35-40
DOI 10.2478/v10136-009-0005-9
In vitro screening of blood-brain barrier penetration of clinically used acetylcholinesterase reactivators
Jana Zdarova Karasova, Petr Stodulka, Kamil Kuca
Address: Donovan McGrowder, Department of Pathology, Faculty of Medical Sciences, University of the West Indies, Mona Campus, Kingston, Jamaica
karasova@pmfhk.cz
Received 23rd June 2009.
Revised 10th September 2009.
Published online 8th December 2009.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
Conclusion
References
SUMMARY
In this in vitro study, using the HPLC method, we determined the ability of acetylcholinesterase (AChE) reactivators, used clinically, to
penetrate the blood-brain barrier (BBB). We evaluated pralidoxime, HI-6, obidoxime, trimedoxime and methoxime - reactivators varying in the
position of the oxime group on the pyridinium ring and linker connecting the pyridinium rings. Our results indicated that pralidoxime, a
monoquaternary AChE reactivator, was the oxime with the most penetration. Molecular weight seems to be the most important factor for passive
transport through the BBB. From the structural perspective, the connecting linker also plays a key role in the ability of the reactivators to
penetrate the CNS. In this case, the simple and short linker is favorable for permeation of these compounds. The location of the oxime group on the
pyridine ring may also influence passive transport into the brain; the best position of the oxime group seems to be position four.
KEY WORDS
blood-brain barrier; CNS penetration; HI-6; obidoxime; HPLC; oxime
INTRODUCTION
The basis of the current standard treatment of
organophosphate (OP) poisoning is the administration
of cholinesterase reactivators (Eyer 2003, Musilek et
al. 2007). These include standard oximes with a
similar basic structure but differing in the number of
pyridinium rings, in the position of the oxime group
on the pyridinium ring and in the linker connecting
the pyridinium rings (Kuca et al. 2006). Some mono-
and also bisquaternary pyridinium oximes are more or
less frequently used in clinical practice. Pralidoxime,
obidoxime, trimedoxime, methoxime and HI-6 are
typical members of this family (Kuca et al. 2007).
The mechanism of their action is hydrolytical
cleaving of the OP from acetylcholinesterase (AChE;
3.1.1.7), restoring its enzymatic function. This
reactivation of the inhibited enzyme is dependent on
the type of OP and, on the reactivator used (Bajgar
2004, Zdarova Karasova et al. 2009).
Reactivation of AChE in the peripheral and also in
the central nervous system (CNS) is very important
for the survival of an organism poisoned with OP.
The question of their penetration through the blood
brain barrier (BBB) as well as the possibility of their
achievement of effective brain concentration is under
discussion (Bajgar et al. 2007a).
There is direct and indirect evidence for the ability
of oximes to penetrate the BBB. The indirect
evidence is based on AChE reactivation in the brain
following OP intoxication (Bajgar et al. 1972, Kassa
et al. 2007, Zdarova Karasova et al. 2008). The direct
evidence for presence of oximes in the brain has been
demonstrated by Sakurada et al. (2003) using
microdialysis detection of pralidoxime. Similar
observations have been described by other authors
(Falb and Erdmann 1969, Cassel et al. 1997, Lorke et
al. 2007, Petroianu et al. 2007).
The main aim of this study is to predict the extent
of BBB penetration by standard AChE reactivators.
Immobilized artificial membrane (IAM)
chromatography was utilized for the assessment of
these pharmacokinetic properties of the different
oximes (Yoon et al. 2006). The method was validated
on a set of 21 structurally varying therapeutics and
subsequently applied to clinically used
monoquaternary (pralidoxime) and bisquaternaly
AChE reactivators (obidoxime, trimedoxime, HI-6
and methoxime).
MATERIALS AND METHODS
Chemicals
Atenolol, beta-estradiol, caffeine, cefuroxime,
chlorpromazine, cimetidine, corticosterone,
desipramine, enalapril, hydrocortisone, ibuprofen,
imipramine, lomefloxacin, loperamide, nadolol,
piroxicam, progesterone, promazine, propranolol, and
testosterone were purchased from Sigma Aldrich
(Steinheim, Germany). Acetonitrile gradient grade
LiChrosolv was purchased from Merck (Darmstadt,
Germany). KH2PO4, Na2HPO4, KCl, and NaCl were
purchased from Lachema (Neratovice, The Czech
Republic). AChE reactivators were synthesized
earlier in our laboratory (Musilek et al. 2006, Kuca et
al. 2008). Water was reverse osmosis pure.
Apparatus
The HPLC system consisted of a P200 gradient pump
(Spectra-Physics Analytical, Fremont, USA),
a 7125 injection valve - 10 microl loop (Rheodyne,
Cotati, USA), an UV1000 detector (Spectra-Physics
Analytical, Fremont, USA) and a CSW
Chromatography Station 1.5 software (DataApex,
Praha, Czech Republic).
Chromatographic condition
Conditions for prediction (analysis)
An IAM.PC.DD 2 (150 x 4.6 mm; 12 microm) column
(Regis Technologies, Morton Grove, USA) was used
for analysis. The mobile phase was 80% PBS and
20% acetonitrile (v/v) with pH adjusted to 5.5 and 7.0
using Na2HPO4. The phosphate-buffered saline (PBS)
was prepared with 2.7 mM KCl, 1.5 mM KH2PO4,
137 mM NaCl, and 8.1 mM Na2HPO4. It was
delivered isocratically at a flow-rate of 1 ml/min. The
absorbance was measured at 210 nm. All
chromatograms were obtained at 37 °C.
Conditions for samples
For the analyses a 125 x 3 mm I.D. Purospher RP-18e
(5 m) column (Merck, Darmstadt, Germany) was
used. The mobile phase was 24% acetonitrile and
76% water (v/v), containing 5 mM octane-1-sulfonic
acid sodium salt, 5 mM tetramethylammonium
chloride. It was delivered isocratically at a flow-rate
of 1 ml/min. The absorbance was measured at UVmax
of each reactivator. All chromatograms were obtained
at 24 °C.
RESULTS
The most important coefficient for the determination
of IAM partition is kIAM (IAM capacity factor), which
was calculated as
kIAM = (tr - t0)/t0
where tr>is the retention time of the drug and t0is the
hold up time of the column.
In this study, the kIAM was determined for
twenty-one reference drugs. The kIAM values were
determined with a mobile phase of pH 7.4, although
Yoon has recommended using a mobile phase of pH
5.5 because it provided better results. Our experiment
was, however, carried out with a mobile phase of a
higher pH because of the need to establish an
environment similar to that in human body. This
change of pH range may haved markedly changed the
state of the chemical ionization of the drugs.
Chemical ionization is a very important factor which
may in turn significantly change the possibility of
molecule penetrating through the BBB.
According to Yoon et al. (2006) the assortment of
drugs which can cross the BBB (CNS+) and those
which do not penetrate into the brain (CNS-) was
chosen based on kIAM corrected by the molecular
weight (MW). The assortment of pH 7.4 was most
successful with the power function of the molecular
weight set at 4 (Yoon et al. 2006). The designated
formula was:
X = kIAM/MW4 × 1010
In addition a calculation was made of the
predicted constants of the synthesized compounds -
the partition coefficient (LogP), the molecular polar
surface area (PSA) and the molecular weight (MW).
In respect of LogP, it can be clearly seen that all
substances are more soluble in water than in octanol.
Fig. 1 illustrates the correlation between log P and log
P and kIAM/MW4, the correlation coefficient (r2) being
0.6677 at pH 7.4.
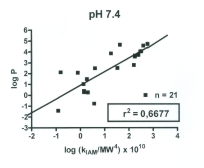
Fig. 1. Correlation between log P and kIAM/MW4
determined at the mobile phase pH of 7.4.
The PSA (the sum of surfaces of polar atoms:
oxygens, nitrogens and attached hydrogens, in a
molecule) is a parameter very useful for the
prediction of drug transport properties (Zhu et al.
2002). The PSA has been previously shown to
correlate with human intestinal absorption (Palm et al.
1998, Clark 1999). When PSA is applied to a larger
and more diverse compound set, however, outliers
become more frequent (Zhu et al. 2002). In this study,
a good correlation was observed between PSA and
kIAM/MW4 with a correlation of 0.7199 at the mobile
phase of pH of 7.4 (Fig. 2).
The CNS-drugs showed evident inability to bind
to the phosphatidylcholine column and have X values
less than 0.50, whereas the CNS+ drugs bound much
better and their x values were distinctively higher
than 1.00.
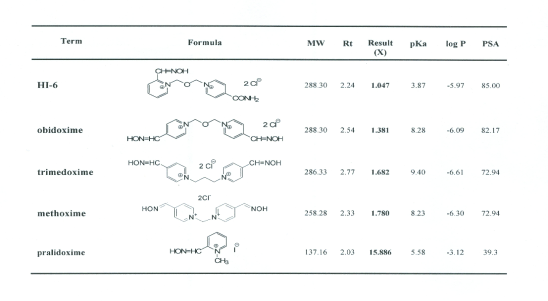
The utility of the optimized prediction method was
examined for five reactivators of AChE, commonly
used in therapy, which differ in their chemical
structure (Table 1). At Fig. 3 is a HPLC
chromatogram of the commonly used reactivators
(pralidoxime, obidoxime, trimedoxime, HI-6,
methoxime) with different retention times.
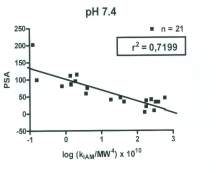
Fig. 2. Correlation between polar surface area (PSA)
and kIAM/MW4 determined at the mobile phase pH of
7.4.
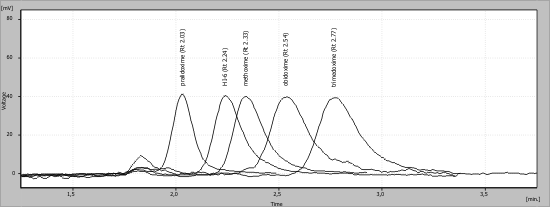
Fig. 3. HPLC chromatogram of the clinically used reactivators (pralidoxime, obidoxime, trimedoxime, HI-6, methoxime)
with retention times.
Table 1. The group of tested reactivators, their results.
DISCUSSION
There are many questions still to be answered which
are focused on the presence of reactivators in the brain.
It has generally been accepted that oximes as
quaternary compounds are not able to penetrate the
BBB (Kassa et al. 2008). As already written above,
there is much direct and indirect evidence for the
ability of oximes to pass through into the brain (Falb
and Erdmann 1969, Cassel et al. 1997, Lorke et al.
2007, Petroianu et al. 2007).
The AChE reactivation is very important in CNS,
because there are so many changes in the physiology of
the brain after OP intoxication (Kuca and Kassa 2004).
They cause a strong cholinesterase inhibition with
subsequent changes in the level of neurotransmitters
including acetylcholine and catecholamines (Bajgar et
al. 2007b). Also recorded in CNS were changes in
membrane permeability, in the influence of BBB
permeability and in metabolic imbalances (changes in
the brain energy metabolism during soman
intoxication, influence of the oxidative metabolism and
ATP level in the brain) (Gupta 2004).
The pontomedullar area, where respiration is
regulated (controlled by cholinergic neurons) is of
particular importance. Depression of the central
respiratory control centres in the pontomedullar area is
considered as a primary event leading to death
(Goswany et al. 1994, Sungur and Guven 2001, Kubin
and Fenik 2004). When the AChE reactivation is
present in this area, a good therapeutic effect is
observed. The survival of intoxicated animals is
correlated with AChE activity in the pontomedullar
area (Bajgar et al. 2007b).
According to our hypothesis, the results obtained in
our study proved a dependency between CNS
penetration and the structure of various reactivators.
The less penetrative bisquaternary compounds were
HI-6 and obidoxime. Oxime HI-6, is a very promising
antidote against the broad spectrum of OP (Kuca et al.
2009). Obidoxime is effective against tabun and
pesticide intoxications. There are three differences in
the chemistry of these clinically used oximes. The
position of the oxime group on the pyridinium ring is
the first (obidoxime - 4, HI-6 - 2 position). Then,
obidoxime has two oxime groups of HI-6 instead of
one. Finally, the second obidoxime oxime group is
replaced by the carbamoyl group in the HI-6 structure.
If these results are compared with those of
trimedoxime, it can clearly be seen that the difference
in the connecting linker also influences BBB
penetration. The oxygen in the linker between two
pyridinium rings is not conducive to BBB permeation.
The best bisquaternary structure from our point of view
is methoxime which has two oxime groups in the
position four on the pyridinium rings and very short
linker without oxygen.
It is known that the most important parameters of
influence in BBB passive penetration are MW and the
presence of sufficient liposolubility (Bellawance et al.
2008). This was confirmed also in our study. The
oxime with lowest MW (monoquaternary AChE
reactivator, pralidoxime) was the best penetrating
structure (Zdarova Karasova et al. 2010). Our results
were indirectly confirmed in other in vivo studies,
(Lorke et al. 2007, 2008, Petroianu et al. 2007, Kalasz et al. 2009). The monoquaternary oximes
penetrated 10 times more than bisquaternary
compounds.
On the basis of our results we can predict the more
permeating oxime structures. This knowledge may be
useful in the synthesis of more effective AChE
reactivators.
CONCLUSION
In conclusion, we have tested an HPLC method with
UV detection for the prediction of five clinically used
AChE reactivators. According to our results molecular
weight seems to be the most important factor for
passive transport throught the BBB. Secondly, based
on results obtained, even small changes in the chemical
structure of oxime (connecting linker, location of
oxime group on the pyridinium ring and also
substitution of oxime group) are important in
influencing the extent of brain penetration.
ACKNOWLEDGEMENT
Authors would like to thank to the Ministry of Health
(Czech Republic) - "The Evaluation of Penetration of
Different Acetylcholinesterase Reactivators through
Blood-Brain Barrier". Thanks are due to Mrs. M.
Hrabinova for skilled technical assistance.
REFERENCES
Bajgar J: Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem 38:151-216,
2004.
Bajgar J, Jakl A, Hrdina V: The influence of obidoxime on acetylcholinesterase activity in different parts of the mouse brain following
isopropylmethyl phosphonofluoridate intoxication. Eur J Pharmacol 19:199-202, 1972.
Bajgar J, Fusek J, Kuca K, Bartosova L, Jun D: Treatment of organophosphate intoxication using cholinesterase reactivators: facts and fiction. Mini
Rev Med Chem 7:461-466, 2007a.
Bajgar J, Kuca K, Fusek J, Karasova J, Kassa J, Cabal J, Blaha V: Inhibition of blood cholinesterases following intoxication with VX and its
derivatives. J Appl Toxicol 27:458-463, 2007b.
Bellawance MA, Blanchette M, Fortin D: Recent advances in blood-brain barrier disruption as a CNS delivery strategy. AAPS J 10:166-177,
2008.
Cassel G, Karlsson L, Waara L, Ang KW, Goransson-Nyberg A: Pharmacokinetics and effects of HI 6 in blood and brain of soman-intoxicated rats: a
microdialysis study. Eur J Pharmacol 30:43-52, 1997.
Clark DE: Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena: Prediction of intestinal
absorption potential. J Pharm Sci 88:807-814, 1999.
Eyer P: The role of oximes in the management of organophosphorus pesticide poisoning. Toxicol Rev 22:165-190, 2003.
Falb A, Erdmann WD: Penetration of 14C-obidoxime through the so-called blood-brain barrier of mice and rats. Arch Toxicol 24:123-132, 1969.
Goswany R, Chaudhuri A, Mahashur AA: Study of respiratory failure in organophosphate and carbamate poisoning. Heart Lung 23:466-472, 1994.
Gupta RC: Brain regional heterogeneity and toxicological mechanisms of organophosphates and carbamates. Toxicol Mech Methods 14:103-143,
2004.
Kalasz H, Szoko E, Tabi T, Petroianu GA, Lorke DE, Omar A, Alafifi S, Jasem A, Tekes K: Analysis of Pralidoxime in Serum, Brain and CSF of Rats.
Med Chem 5:237-241, 2009.
Kassa J, Karasova J, Vasina L: The evaluation of neuroprotective efficacy of newly developed oximes (K 074, K 075) and currently available oximes
(obidoxime, HI-6) in cyclosarin-poisoned rats. J Appl Toxicol 27:621-630, 2007.
Kassa J, Jun D, Karasova J, Bajgar J, Kuca K: A comparison of reactivating efficacy of newly developed oximes (K074, K075) and currently available
oximes (obidoxime, HI-6) in soman, cyclosarin and tabun-poisoned rats. Chem Biol Interact 175:425-427, 2008.
Kubin L, Fenik V: Pontine cholinergic mechanisms and their impact on respiratory regulation. Respir Physiol Neurobiol 143:235-249, 2004.
Kuca K, Kassa J: Oximes-induced reactivation of rat brain acetylcholinesterase inhibited by VX agent. Hum Exp Toxicol 23:167-171, 2004.
Kuca K, Jun D, Musilek K: Structural requirements for acetylcholinesterase reactivators. Mini Rev Med Chem 6:269-277, 2006.
Kuca K, Jun D, Bajgar J: Currently used cholinesterase reactivators against nerve agent intoxication: Comparison of their effectivity in
vitro. Drug Chem Toxicol 30:31-40, 2007.
Kuca K, Stodulka P, Hrabinova M, Hanusova P, Jun D, Dolezal B: Preparation of oxime HI-6 (dichloride and dimethanesulphonate)-antidote against
nerve agents. Def Sci J 58:399-404, 2008.
Kuca K, Musilek K, Jun D, Pohanka M, Zdarova Karasova J, Novotny L, Musilova L: Could oxime HI-6 really be considered as "broad-spectrum" antidote?
J Appl Biomed 7:143-149, 2009.
Lorke DE, Hasan MY, Nurulain SM, Sheen R, Kuca K, Petroianu GA: Entry of two new asymmetric bispiridium oximes (K-27 and K-48) into the rat brain:
comparison with obidoxime. J Appl Toxicol 27:482-490, 2007.
Lorke DE, Kalasz K, Petroianu GA, Tekesz K: Entry of oximes into the brain: A review. Curr Med Chem 15:743-753, 2008.
Musilek K, Lipka L, Racakova V, Kuca K, Jun D, Dohnal V, Dolezal M: New methods in synthesis of acetylcholinesterase reactivators and evaluation of
their potency to reactivate cyclosarin-inhibited AChE. Chem Pap 60:48-51, 2006.
Musilek K, Kuca K, Jun D, Dolezal M: In vitro reactivation potency of bispyridinium (E)-but-2-ene linked acetylcholinesterase reactivators
against tabun-inhibited acetylcholinesterase. J Appl Biomed 5:25-30, 2007.
Palm K, Luthman K, Ungell AL, Strandlung G, Beigi F, Lundahl P: Evaluation of dynamic polar molecular surface area as predictor of drug absorption:
comparison with other computational and experimental predictors. J Med Chem 41:5382-5392, 1998.
Petroianu GA, Lorke DE, Hasan YM, Adem A, Sheen R, Nurulain SM, Kalasz H: Paraoxon has only a minimal effect on pralidoxime brain concentration in
rats. J Appl Toxicol 27:350-357, 2007.
Sakurada K, Matsubara K, Shimizu K, Shiono H, Seto Y, Tsuge K, Yoshino M, Sakai I, Mukoyama H, Takatori T: Pralidoxime iodide (2-PAM) penetrates
across the blood brain barrier. Neurochem Res 28:1401-1407, 2003.
Sungur M, Guven M: Intensive care management of organophosphate insecticide poisoning. Crit Care 5:211-215, 2001.
Yoon HCh, Kim SJ, Shin BS, Lee KCh, Yoo SD: Rapid screening of blood-brain barrier penetration of drugs using the immobilized artificial memebrane
phosphatidylchline column chromatography. J Biomol Screen 11:13-20, 2006.
Zdarova Karasova J, Kassa J, Jung Y-S, Musilek K, Pohanka M, Kuca K: Effect of Several new and currently available oxime cholinesterase
reactivators on tabun-intoxicated rats. Int J Mol Sci 9:2243-2252, 2008.
Zdarova Karasova J, Bajgar J, Novotny L, Kuca K: Is a high dose of Huperzine A really suitable for pretreatment against high doses of soman? J Appl
Biomed 7:93-99, 2009.
Zdarova Karasova J, Stodulka P, Pohanka M, Kuca K (2010): In vitro screening of blood-brain barrier penetration of monoquaternary
acetylcholinesterase reactivators. Anal Lett - In press. DOI 10.1080/00032710903502082.
Zhu C, Jiang L, Chen TM, Hwang KK: A comparative study of artificial membrane permeability assay for high throughput profiling of drug absorption
potential. Eur J Med Chem 37:399-407, 2002.
|
BACK
|





