Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 2, p 111-116
DOI 10.2478/v10136-009-0009-5
Kinetics of hydrolysis of organophosphate soman by cationic surfactant Resamin AE
Jiri Cabal, Jitka Micova, Kamil Kuca
Address: Jiri Cabal, Department of Toxicology, Faculty of Military Health Sciences, Trebesska 1575, 500 01 Hradec Kralove, Czech Republic
cabal@pmfhk.cz
Received 20th August 2009.
Published online 15th January 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Material and methods
Results and discussion
References
SUMMARY
This experiment tested the reactivity of the cationic surfactant Resamin AE
[N-(2-hydroxyethyl)-N-(2-hydroxy-4-oxa(C14÷22-alkyl))-N,N-dimethylammonium chloride] with organophosphonate soman. It was confirmed that soman is
incorporated in the micelle of Resamin AE and that the hydroxyl group or Resamin AE supports the hydrolysis of this organophosphonate. A further
increase in reactivity was achieved by the addition of hydrogen peroxide into the reaction mixture. In contrast, if the concentration of neutral
salts was increased, a decrease in the velocity of the hydrolysis was obtained. The hydrolytic efficacy of Resamin AE was also compared with that
of hexadecyldimethyl-(2-hydroxyethyl)-ammonium bromide. Resamin AE appeared to be a more efficacious hydrolytic agent than other similar compounds
due to its advantageous chemical structure.
KEY WORDS
cationic surfactants; micellar catalysis; soman; decontamination; Resamin AE
INTRODUCTION
Decontamination of chemical warfare agents (CWA)
is a very important process which would be necessary
if CWA were spread accidentally or intentionally
(Cabal et al. 2004, 2007, Waysbort et al. 2009). For
the last 50 years or so, attention has been paid to the
development of novel means of decontamination,
especially to nucleophiles able to act through the
hydrolytic reaction of surfactants with carboxylic or
phosphate esters. The structure of promising
surfactants able to support a hydrolytic reaction has
been identified and further modified (Cabal et al.
2003, Kuca et al. 2004). Mostly, however, simple
cationic surfactants have been used because of their
ability to concentrate anions with the properties of
nucleophile on their micellar surface (Ouarti et al.
2000, Tiwari et al. 2009a), and surfactants carrying
reactive nucleophile directly in their structure have
been prepared to improve the reactive properties of
micelles (Kotoucova et al. 2001, Kivala et al. 2006).
The hydrolytic efficacy of many compounds
containing the hydroxyl group, the oxime group or
cupric chelate has been tested (Bunton et al. 1983,
Scrimin et al. 1996, Cibulka et al. 1999, Cabal et al.
2007, Tiwari et al. 2009a, b). Surfactants bearing an
imidazol group have also been tested, but they were
synthesized only for the purposes mentioned above
and their usage does not range beyond the laboratory
level (Simanenko et al. 2004). Compounds with a
similar structure can also be found among surfactants
produced on an industrial scale mainly as
disinfectants or softeners of fabrics (Kuca et al. 2005,
2007).
When novel hydrolytically active surfactants are
designed there is a need to consider their spatial
arrangement; that of the detergent should be
considered in relation to the substrate, which should
be broken down, and in relation to its location in the
micelle.
Resamin AE belongs to the group of quaternary
detergents which have a hydroxyl group, and its
structure (Fig. 1) is unusual due to the location of the
hydroxyl groups. This spatial arrangement of
Resamin AE could be the great advantage of this
detergent. The first group is placed in the main and
the second in the adjacent alkyl chain. Based on this
setup, the first hydroxyl is located in the water phase
and the second hydroxyl group is located under the
surface of the micelles.
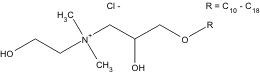
Fig. 1. Structure of Resamin AE.
In this paper, we describe the reactivity of the
surfactant Resamin AE with the nerve agent soman
with the aim of characterizing the influence regulating
its reactivity in relation to its possible further use in
actual decontamination mixtures of the foam or
emulsion type.
MATERIAL AND METHODS
Chemicals
The chemicals used in this study were purchased as
follows: sodium hydroxide (Dr. Jan Kulich, Hradec
Kralove, Czech Republic), potassium chloride
(Lachema, Brno, Czech Republic), hydrogen peroxide
(Peroxides, Sokolov, Czech Republic), propan-2-ol
(Merck, Darmstadt, Germany), Resamin AE - 30%
water solution (Chemotex, Decin, Czech Republic).
Soman was obtained from the Military Repair Facility
VOZ 072 Zemianske Kostolany (Slovak Republic).
Equipment used
For all experiments the following pieces of equipment
were used: automatic titrator Radiometer RTS 822
(Radiometer, Kopenhagen, Danmark), termostat U1
(VEB Prufgerate Werk, Medingen, Germany) and a
device for measurement of the surface tension by the
ring method which was constructed at the Military
Medical Academy Hradec Kralove.
Determination of kinetic parameters of the
reactions of soman in the micellar environment
The kinetics of hydrolytic reactions of soman was
monitored by an automatic titrator in pH-stat mode.
The consumption of 0.01 M NaOH necessary for a
constant pH of the hydrolytic reaction producing
hydrogenfluoridic acid and methylphosphonic acid
rising from the cleavage of soman was recorded. The
reaction was usually monitored at constant pH 9 and
temperature 25 °C ± 0.1 °C. 20 ml of a solution
containing all the required substances except soman
was poured into the titration vessel of the pH stat.
After the stabilization of the temperature and pH, a
reaction was started by adding 0.1 ml of 0.05 M
soman solution. The reaction was monitored for seven
halftimes or longer.
Determination of critical micelle concentration of
surfactants
The determination was carried out by the Du Nouy
ring method under conditions determined in the
OECD manual (OECD 1981). Solutions of the
surfactants were placed in a crystallisation cup with
a volume of 100 ml and diameter 75 mm. A ring with
a diameter of 33 mm was used for the measurement.
Math processing of raw experimental data
The calculation of rate constant
Data on the consumption of the titration solution over
time were transferred from the plotter of the pH-stat
to the MS Excel program. The observed rate constant
was calculated by the procedure described by
Guggenheim (1926) using a modification introduced
by Zajicek and Radl (1979). This procedure is based
on acceptance of the following equation:
At+t = At*exp(kobs*t) + An*(1-exp(-kobs*t)) (1)
where:
At+t = consumption of titration solution at time t+t
At = consumption of titration solution at time t
exp = base of natural logarithm
kobs = observed rate constant
t = time distance between two successive
measurements
An = consumption of titration solution in infinite time
This relationship is a linear equation with slope
exp(kobs*t) and intersection An*(1-exp(-kobs*t)).
Data obtained from one measurement (10 min) were
arranged in two columns of MS Excel so that the
second column was shifted one row up. This shift
presents one value t. Data prepared in such a way
were displayed in an XY Scatter chart. A trend line
was interlaid through the displayed points with a
printout of the coefficients regression equation. The
slope obtained was used for the calculation of the rate
constant kobs from the term exp(kobs*t).
Calculation of limit values of rate constant of
hydrolysis of soman by surfactant
Rate constants of the hydrolysis of soman by
surfactants were calculated by means of the Menger
and Portnoy equation (1967). This equation was set
up so as to include the data from the premicellar
region of the surfactant concentration in the
calculation:
kCt = ko + (((((-ko + kmax)*K/N* ((10^ (Ct))-CMC)) /
(1 + K/N*((10^(Ct))-CMC)))^2)^0,5) + ((-ko +
kmax)*K/N*((10^(Ct))-CMC))/(1+K/N*((10^(Ct))
-CMC))) /2 (2)
where:
kCt = first order rate constant for molar concentration
of surfactant Ct
ko = first order rate constant of hydrolysis in
premicellar region of surfactant concentration
kmax = limit rate constant for Ct =
K/N = fraction of association constant of substrate
and aggregation number of surfactant
Ct = logarithm of molar concentration of surfactant
CMC = critical micelle concentration of surfactant
Calculation of critical micelle concentrations -
measuring by the ring method
The data on the dependence of the surface tension on
the concentration of surfactant were processed by a
program for nonlinear regression using the
Syzszkowski equation in semilogarithmic expression
(Syzszkowski 1908). The equation was set up so as to
include in the calculation the data from the region
where the surface tension was not dependent on the
concentration of the surfactant:
Uct = Umin + (((((Umax-Umin)-A*LOG(1 + B*
(10^Ct)))^2)^0,5) + ((Umax-Umin)-A*LOG(1 + B*
(10^Ct)))) /2 (3)
where:
UCt = surface tension by concentration of surfactant
Ct
Umin = minimal attainable surface tension
Umax = surface tension of solven
A = constants defining kind of surfactant
B = constant defining position of surfactant in
homologous sequence by magnitude of hydrophobic
alkyl
Ct = logarithm of molar concentration of surfactants
The values of constants obtained from equation
(3) were introduced in another equation (4) to obtain
the logarithm of critical micelle concentration
LOG (CMC) = LOG((10^((Umax-Umin )/A)-1)/B) (4)
RESULTS AND DISCUSSION
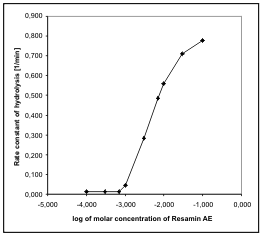
Fig. 2. Dependence of the rate of hydrolysis of soman on
the concentration of Resamin AE (pH9, I = 0.01M KCl,
t = 25 °C).
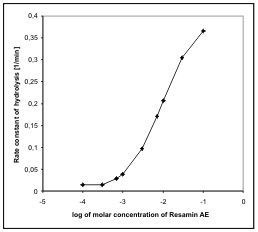
Fig. 3. Dependence of the rate of hydrolysis of soman on
the concentration of N-(2-hydroxyethyl)-
hexadecyldimethylammonium bromide (C16OH1) (pH9,
I = 0.01M KCl, t = 25 °C).
Table 1. Comparison of the parameters of the hydrolysis
of soman by Resamin AE and surfactant C16OH1
| Resamin AE |
C16OH1 |
| ko |
1/min |
0.014 |
0.014 | | kmax |
1/min |
0.81 |
0.40 | | K/N |
L/mol |
237 |
103 | | CMC |
mol/L |
8.30E-04 |
3.40E-04 |
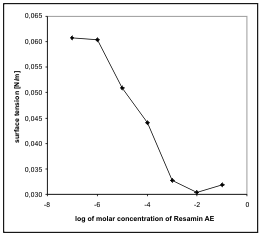
Fig. 4. Dependence of surface tension on the
concentration of Resamin AE measured by the ring
method.
Table 2. Calculated parameters of the Szyszkowski
equation for Resamin AE.
| Parameter |
| | A |
- 0.012 | | B |
- 300 000 | | CMC |
mol/L 0.0015 |
Table 3. Dependence of the rate of hydrolysis of soman
by Resamin AE on pH (t = 25 °C, Ctenz = 0.01M, I =
0.01M KCl).
| pH |
kobs (1/min) | | 8 |
0.084 | | 9 |
0.593 | | 10 |
1.900 |
Table 4. Influence of ionic strength on the rate of
hydrolysis of soman by Resamin AE (t = 25 °C, Ctenz =
0.01M, pH9).
| CKCI
mol/L |
kobs
1/min | | 0.000 |
1.090 | | 0.001 |
0.866 | | 0.010 |
0.638 | | 0.100 |
0.277 | | 0.500 |
0.109 |
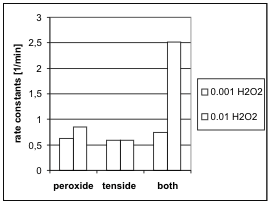
Fig. 5. Influence of hydrogen peroxide on rate of
hydrolysis of soman by Resamin AE (t = 25 °C, Ctenz =
0.01M, pH9, I = 0.01 M KCl).
The dependence of the rate constant of soman
hydrolysis on the concentration of the surfactant is
characterized by a typical shape, with a short plateau
of premicellar concentration and subsequent
progressive phase up to the maximal rate constant
(Fig. 2). Therefore, this dependence can be described
by a Menger-Portnoy equation. The parameters
calculated are shown in Table 1.
A comparison of the parameters of Resamin AE
and C16OH1 (Fig. 3) shows that the rate of reaction
with Resamin AE is double that of C16OH1. This
difference is probably caused by the better structural
disposition of Resamin AE for the soman hydrolysis.
The calculated values of critical micellar
concentrations (Table 1) have the same magnitude of
order and, therefore, they do not influence the
reactivity of surfactants. Parameter K/N is a fraction
of the association constant of the substrate and
aggregation number of the surfactant. Constant K
describes the affinity of the substrate to the micellar
phase and constant N describes how many molecules
of the surfactant are needed to create one micelle. The
instrumentation used in this study does not make it
possible to determine these parameters separately.
With regard to the similarity of the structure of
both surfactants, it is possible that the difference in
the values of K/N is caused by the difference in the
affinity of the substrate to the micelles of both
surfactants. The critical micellar concentration of
Resamin AE was also measured by the ring method in
distilled water only, and is only twice higher than the
CMC in a solution with pH9 and ionic strength 0.01
M KCl. It is evident that the changes of solution
properties have no influence on the aggregation
ability of Resamin AE.
On the other hand, a change of pH is an essential
parameter remarkably changing the rate of hydrolytic
reaction. The values of pKa of both hydroxyl groups
in Resamin AE were predicted with the help of the
program pKaDB (ACDlab, USA). The value of pKa
for the hydroxyl group localized on the
2-hydroxyethyl group of the surfactant was 12.7 and
for the hydroxyl group localized on the hydrophobic
alkyl of the surfactant it was 11.9. The values of pKa
do not explain the strong dependence of rate constants
on pH in the range of pH 8-10. The results of the
kinetic measurements give us evidence for
considerably lower (9-10) values of pKa. This
discrepancy can be caused by the properties of the
Stern layer of micelles, where the acidobasic
properties of the reaction environment are strongly
changed. Unfortunately, our program is not able to
take this fact into account.
The next parameter tested was the influence of the
electrolytes on the rate of hydrolysis where the
concentration of KCl was found to be especially
important. This influence can be described by the
equation: kobs = 0.29 * log CKCl + 0.016. The
phenomenon described - the so called "salt effect" -
has been demonstrated also by other authors
(Zakharova et al. 1993).
The last measurement was focused on the
influence of other nucleophiles in the reaction, where
hydrogen peroxide was used. It is known that the
micelles of cationic surfactants are able to concentrate
negatively charged ions on their surface. If ions are
nuclephilically active, it is possible, that this higher
concentration influences the rate of hydrolysis. This
hypothesis was confirmed by the results as presented
in Fig. 5.
In conclusion, the data obtained in this study
confirmed that Resamin AE may be an effective
component of blends designed for the
decontamination of organophosphorus warfare agents.
ACKNOWLEDGEMENT
This work was also supported by Czech Ministry of
Defence project No. OVUOFVZ200803.
REFERENCES
Bunton CA, De Buzzaccarini F, Hamed FH: Nucleophilic aromatic substitution in microemulsions of a hydroxyethyl surfactant. J Org Chem 48:2461-2465,
1983.
Cabal J, Kassa J, Severa J: A comparison of the decontamination efficacy of foam-making blends based on cationic and nonionic tensides against
organophosphorus compounds determined in vitro and in vivo. Hum Exp Toxicol 22:507-14, 2003.
Cabal J, Kuca K, Micova J: Kinetics of decompositition of organophosphate Fenitrothion by decontaminating foam-making blends. J Appl Biomed
5:167-170, 2007.
Cabal J, Kuca K, Sevelova L, Dohnal V: Cyclodextrines as functional agents for decontamination of the skin contamined by nerve agents. Acta Medica
(Hradec Kralove) 47:115-118, 2004.
Cibulka R, Hampl F, Martinu T, Mazac J, Totevova S, Liska F: Metal ion chelates of lipophilic alkyl diazinyl ketoximes as hydrolytic catalysts.
Collect Czech Chem Commun 64:1159-1179, 1999.
Guggenheim EA: On the determination of the velocity constant of a unimolecular reaction. Phil Mag 2:538-543, 1926.
Kivala M, Cibulka R, Hampl F: Cleavage of 4-nitrophenyl diphenyl phosphate by isomeric quaternary pyridinium ketoximes - How can structure and
lipophilicity of functional surfactants influence their reactivity in micelles and microemulsions? Coll Czech Chem Commun 71:1642-1658,
2006.
Kotoucova H, Cibulka R, Hampl F, Liska F: Amphiphilic quaternary pyridinium ketoximes as functional hydrolytic micellar catalysts - does the
nucleophilic function position influence their reactivity? J Mol Catal A - Chem 174:59-62, 2001.
Kuca K, Kivala M, Dohnal V: General method for the quaternization of N,N-dimethyl benzylamines with long chain n-alkylbromides. J Appl Biomed
2:195-198, 2004.
Kuca K, Dohnal V, Bielavska M, Cabal J: Determination of benzalkonium bromide homologues in desinfection products using high-performance liquid
chromatography. Anal Lett 38:673-682, 2005.
Kuca K, Musilek K, Hanusova P, Hrabinova M, Marek J, Stodulka S, Jun D: Preparation of the benzalkonium salts differing in the length of the side
alkylating chain. Molecules 12:2341-2347, 2007.
Menger FM, Portnoy CE: On the chemistry of reactions proceeding inside molecular aggregates. J Am Chem Soc 89:4698-4703, 1967.
OECD Guidelines for the Testing of Chemicals, Test No. 115: Surface Tension of Aqueous Solutions. OECD, Paris 1981.
Ouarti N, Marques A, Blagoeva I, Ruasse MF: Optimization of micellar catalysis of nucleophilic substitutions in buffered cetyltrimethylammonium
salt solutions. 1. Buffers for the 9-10 pH range. Langmuir 16:2157-2163, 2000.
Scrimin P, Ghirlanda G, Tecilla P: Comparative reactivities of phosphate ester cleavage by metallomicelles. Langmuir 12:6235-6241, 1996.
Simanenko YS, Karpichev EA, Prokop'eva TM: Functional detergents containing an imidazole ring and typical fragments of alpha-nucleophiles
underlying micellar systems for cleavage of esters of phosphorus acid. Russ J Org Chem 40:206-218, 2004.
Szyszkowski B: Experimentelle Studien der Fiber Kapillaren Eigenschaften der Wasserigen Losungen von Fettsauren. Z Phys Chem 64:385-415,
1908.
Tiwari S, Kolay S, Ghosh KK, Kuca K, Marek J: Kinetic study of the reactions of p-nitrophenyl acetate and p-nitrophenyl benzoate with oximate
nucleophiles. Int J Chem Kinet 41:57-64, 2009a.
Tiwari S, Ghosh KK, Marek J, Kuca K: Comparative study of nucleophilic efficacy of pralidoxime towards phosphorus, sulphur and thiophosphorus based
esters. React Kinet Catal Lett 2009b, In press.
Waysbort D, McGarvey DJ, Creasy WR, Morrissey KM, Hendrickson DM, Durst HD: A decontamination system for chemical weapons agents using a liquid
solution on a solid sorbent. J Hazard Mater 161:1114-1121, 2009.
Zajicek M, Radl Z: Katalyticky vliv kationaktivniho tenzidu na hydrolyzu fosfonatu. In Sbornik Vyzkumneho ustavu 070, Brno 1979, pp. 115-129 (In
Czech).
Zakharova LY, Fedorov SB, Kudryavtseva LA, Bel'skii VE, Ivanov BE: The effect of electrolytes on the reaction rates and acid-base equilibria in
ionic micelles. Russ Chem Bull 42:1329-1333, 1993.
|
BACK
|






