Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 2, p 55-66
DOI 10.2478/v10136-009-0013-9
Gas plasmas and plasma modified materials in medicine
Sadiqali Cheruthazhekatt, Mirko Cernak, Pavel Slavicek, Josef Havel
Address: Josef Havel, Department of Chemistry, Faculty of Science, Masaryk University, Kotlarska 2, 611 37 Brno, Czech Republic
Havel@chemi.muni.cz
Received 8th February 2010.
Revised 25th March 2010.
Published online 19th April 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Plasma form of matter and methods for generation
Plasma treatment in medicine
Plasma modified materials in medicine
Conclusion
References
SUMMARY
The applications of gas plasma and plasma modified materials in the emerging fields of medicine such as dentistry, drug delivery, and tissue
engineering. Plasma sterilization of both living and non-living objects is safe, fast and efficient; for example plasma sterilization of medical
equipment quickly removes microorganisms with no damage to the tiny delicate parts of the equipment and in dentistry it offers a non-toxic, painless
bacterial inactivation of tissues from a dental cavity. Devices that generate plasma inside the root canal of a tooth give better killing efficiency
against bacteria without causing any harm to the surrounding tissues. Plasma modified materials fulfill the requirements for bioactivity in
medicine; for example, the inclusion of antimicrobial agents (metal nano particles, antimicrobial peptides, enzymes, etc.) in plasma modified
materials (polymeric, metallic, etc) alters them to produce superior antibacterial biomedical devices with a longer active life. Thin polymer films
or coating on surfaces with different plasma processes improves the adherence, controlled loading and release of drug molecules. Surface
functionalization by plasma treatment stimulates cell adhesion, cell growth and the spread of tissue development. Plasma applications are already
contributing significantly to the changing face of medicine and future trends are discussed in this paper.
KEY WORDS
plasma; sterilization; dentistry; surface functionalization; drug delivery; tissue engineering
Abbreviations
DBD, dielectric barrier discharge
B. cereus, Bacillus cereus
E. coli, Escherichia coli
NPs, nanoparticles
PA, porous alumina
PAA, polyacrylic acid
P. aeruginosa, Pseudomonas aeruginosa
PE, polyethylene
PEG, poly(ethylene glycol)
PHBV, poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid)
PIII, plasma immersion and ion implantation
PP, polypropylene
PSDVB, polystyrene-divinylbenzene
PU, polyurethanes
RF, radio frequency
S. aureus, Staphylococcus aureus
S. mutans, Streptococcus mutans
INTRODUCTION
Plasma is considered as the fourth state of matter and
it is the most abundant state in the universe. It exists
in a variety of forms among which is 'fire', known for
millions of years, since the early stone age. Plasma is
not a human invention, and is present in nature, as fire
in the sun, stars, in the tails of comets and as flashes
of lightning (Conrads and Schmidt 2000). In medicine
and biology 'plasma' refers to the non-cellular fluid
component of blood. The term was introduced into
physics by Irving Langmuir in 1928, because it
resembles the ionic liquids in biology and medicine.
The number of applications of plasma technology
in many fields including microelectronics, metallurgy,
polymer engineering, and biomedical engineering, is
growing rapidly. One of the advantages of this
technology is that surface properties such as hardness,
corrosion resistance and other chemical and physical
properties can be selectively modified without
affecting the bulk characteristics of the materials. The
use of synthetic materials in biomedical applications
has increased dramatically during the past few
decades. However some synthetic biomaterials, for
example polymeric implants, can, in biosystems,
cause problems, such as microbial growth and/or
adsorption of bioorganisms. The simple addition or
deposition of bioactive molecules to such materials
can offer less stability and uniformity than covalently
bonded species. In comparison with other methods for
surface modification (layer by layer deposition,
dipping, etc.) plasma surface modification offers a
shorter and more economical method for the covalent
attachment of bioactive molecules to the substrate
without obstructing the bulk properties (Chu et al.
2002, Oehr 2003). Thus plasma technology has great
importance in the development of new biomaterials.
In medicine direct plasma treatment for sterilization,
deactivating pathogens, blood clotting, wound
healing, cancer treatment, etc. is more effective than
any other method. Thus, plasma and plasma modified
materials play an important role in our daily live,
making it more convenient and healthier.
Several reviews of the biomedical application of
plasma and plasma treated materials have been
published (Fridman et al. 2008, Gomathi et al. 2008,
Laroussi 2008, Desmet et al. 2009), but to date, none
gives an overview of modern applications of plasma
and plasma modified materials in medicine; the aim
of this review is therefore to present a survey of
recent advances of plasma and plasma modified
materials in this field.
PLASMA FORM OF MATTER AND METHODS FOR GENERATION
On the application of sufficient heat, a solid material
transforms firstly into a liquid and then. at a higher
temperature, into a gas. As the energy supplied is
increased, the electrons receive sufficient energy to
separate from the atoms or molecules of gas and
become electrically conductive. In this way gas
undergoes a phase transition to a partially or
completely ionized gas, called the plasma state. Fig. 1
illustrates the phase transformations of matter by
changes in the energy of the system under processes
such as melting, vaporization, ionization, etc.
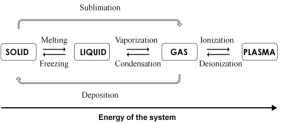
Fig. 1. Different states of matter.
Plasma consists of a mixture of positively and
negatively charged ions, electrons and neutral species
(atoms, molecules). It can be divided into two main
categories; hot plasma (near-equilibrium plasma) and
cold plasma (non-equilibrium plasma). Hot plasma
consists of very high temperature particles and they
are close to the maximal degree of ionization. Cold
plasma is composed of low temperature particles and
relatively high temperature electrons and they have a
low degree of ionization (Tendero et al. 2006). Cold
plasma can be further subdivided into low pressure
and atmospheric pressure cold plasma. Atmospheric
pressure cold plasma is the basis of one of the most
promising methods of achieving a more flexible,
reliable, less expensive and continuous method of
surface modification (Bogaerts et al. 2002). Different
forms of energy (thermal, electric current,
electromagnetic radiations, light from a laser, etc.) are
used to create the plasma regardless of the nature of
the energy source. Depending on the type of energy
supplied and the amount of energy transferred to the
plasma, the properties of the plasma change in terms
of electron density or temperature (Braithwaite 2000).
In common, man made, plasma, electrical energy is
usually injected into a system in a continuous manner
in order to avoid stoppage of the plasma discharge.
Plasma is most commonly produced by passing an
electric current through the gas. Different frequencies
of power sources - direct current, alternating current,
low frequency, radio frequency, microwave, etc. are
used for the generation of discharges such as
atmospheric and low pressure glow discharge, corona,
magnetron and dielectric barrier discharge (DBD)
(Conrads and Schmidt 2000, Denes and Manolache
2004). An example of a high frequency plasma jet
pencil is given in Fig. 2 (Klima et al. 1998, 2003,
2005) and plasma generated by a surface coplanar
barrier discharge in ambient air atmosphere is given
in Fig. 3. Plasma parameters must be designed
specifically for a given application as plasma sources
have their own peculiarities, advantages, and
disadvantages. The selection of a plasma source and
design for the production of novel material is a great
challenge for scientists and industry.
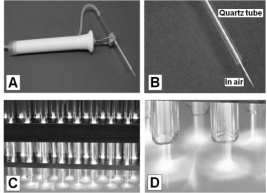
Fig. 2. (A) Plasma pencil device. (B) Magnified plasma
pencil torch glowing in a quartz tube and in air. (C) Multi
jet system. (D) Magnified multi jests modifying the surface.
Photo: M. Klima (reproduced with permission).

Fig. 3. Scheme of barrier discharge generation. (A)
Plasma of surface barrier discharge and (B) Illustration of
safety of the surface coplanar barrier discharge burning in
ambient air atmosphere.
PLASMA TREATMENT IN MEDICINE
Heat and high temperature (steam, hot metal objects)
have been used in medicine for a significant length of
time: in tissue removal, blood clotting, wound healing
and for the disinfection of both living and non-living
biomedical articles. Direct contact with hot metal will
affect the surrounding tissues in living organisms by
tissue adhesion, restarting of bleeding, charring of the
neighbouring tissues and causes damage to heat
sensitive biomedical articles. Treatment with low
temperature plasma provides an alternative method of
avoiding the difficulties associated with this ancient
method (Hayashi et al. 2006, Fridman et al. 2008),
because the ions and the neutral species in low
temperature plasma are relatively cold and do not
cause any thermal damage to articles which come in
contact with the plasma. This non-thermal behavior
recommends the use of gas plasma for the treatment
of heat sensitive materials including biological matter,
such as cells and tissues (Laroussi 2005). In recent
years, non-thermal atmospheric plasma effects have
been developed to extend the plasma treatment of
living tissue. These can be selective in achieving a
desired result for some living matter, while having
little or no effect on the surrounding tissue (Fridman
et al. 2008), and have found application in low heat
surface modification of polymers (Gomathi et al.
2008), clinical instrument sterilization, tissue
engineering and dental cavity treatment (Shenton and
Stevens 2001, Denes and Manolache 2004, Laroussi
2005). Many different types of plasma devices
including plasma pencils, radio frequency plasma
needles, direct current plasma brushes and plasma jets
have been developed for non-thermal atmospheric
pressure plasma generation (Laroussi et al. 2008, Nie
et al. 2009). A brief overview of gas plasma
applications in medicine is given in Fig. 4.
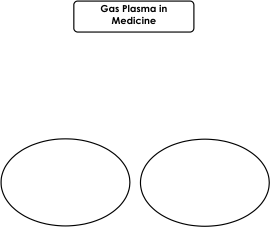
Fig. 4. Gas plasma uses in medicine.
Plasma sterilization
Plasma sterilization is a well established technology
in medicine. Plasma, in the form of fire, was used for
sterilization thousands of years ago. The sterilization
of living objects, such as human, animal, and plant
tissues is of much interest in medicine (Crow and
Smith 1995). Plasma sterilization works at the
atomic/molecular level and therefore it helps to reach
all surfaces, including the interior parts of medical
equipment (catheters, needles, syringes, etc.) and
other regions which are not accessible to fluid
disinfectants (Fridman et al. 2007). It has several
advantages (see Fig. 5) over commonly used
sterilization methods such as heat, chemical solutions,
or gas and radiation bombardment which cause
thermal, chemical, or irradiation damage to both
living and non-living objects. The parametric study of
plasma for sterilization is of importance in
understanding and controlling the deactivation of
microbes, because the main sterilizing factors are
strongly dependent on the plasma source type and/or
the plasma characteristics. Nowadays non-thermal
atmospheric pressure plasma is more frequently used
for the sterilization of both living and non-living
materials (Lerouge et al. 2001, Trompeter et al. 2002,
Xingmin et al. 2006, Fridman et al. 2008, Moreau et
al. 2008).
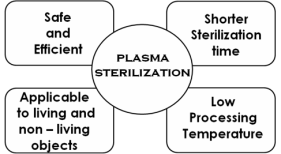
Fig. 5. Some advantages of plasma sterilization.
Sterilization of living materials
Several types of plasma device have been reported for
the sterilization of living animal and human tissues.
An electrically safe DBD plasma with a floating
electrode set up has been reported for the sterilization
of living tissue. This method provides complete tissue
sterilization within seconds, with no damage to skin
samples (Fridman et al. 2006). Recently a new DBD
non-thermal plasma at atmospheric pressure with
conical geometry structured electrodes was developed
for evaluating the bactericidal effect against
Pseudomonas aeruginosa (P. aeruginosa), Bacillus
cereus (B. cereus) and Escherichia coli (E.coli)
bacteria. The complete removal of these
microorganisms was effected within an exposure time
of 10 min for P. aeruginosa, and 15 min for E. coli
and B. cereus, respectively (Sohbatzadeh et al. 2009).
Low power radio frequency (RF) plasma at
atmospheric pressure with a helium flow is used for
the no damaging sterilization of living tissues. This
plasma has the capacity to kill different kinds of
bacteria: E. coli, P. aeruginosa and Staphylococcus
aureus (S. aureus) with a decimal reduction time of
1-2 minutes, while preserving the living cells of the
substrate (Martines et al. 2009). Gweon et al. (2009)
studied the sterilization mechanisms and the major
sterilization factors of RF plasma, with E. coli as the
target. They found that sterilization is more effective
(up to 40%) with 0.15% oxygen added to the helium
gas supply. Moon et al. (2009), generated a relatively
large area (110 mm x 25 mm) of RF discharges with
low current and low gas temperature at atmospheric
pressure to carry out treatment on living tissue. They
investigated possible electrical and thermal damage
and also the sterilization efficiency for living cell
treatment which was tested with microorganisms
inoculated on pork and human skin surfaces.
Sterilization of non-living materials
The sterilization of medical equipment is an important
procedure for disinfection in hospitals, and a number
of medical plasma sterilizer devices have been
introduced (Griffiths 1993, Herrmann et al. 1999, Lin
et al. 1999, Montie et al. 2000, Gaunt et al. 2006,
Laroussi et al. 2006).
The removal of protein residues from surgical
instruments is quite difficult and commonly used
sterilization and decontamination techniques can
cause major damage to the objects treated. Kylian et
al. (2008) developed a low pressure inductively
coupled (Ar/O2 mixture) plasma discharge for the
removal of model proteins from different substrate
materials ranging from metallic surfaces to polymeric
materials. Ar/O2 mixture represents a favorable option
compared to the discharges sustained in other gases or
gas mixtures, since it allows for the fast elimination
of proteins and killing of bacterial spores. Moreover,
the application of this mixture overcomes the
environmental and safety drawbacks of mixtures
containing fluorine which is found to be capable of
sterilizing and etching organic materials.
It is important to find appropriate plasma
sterilization conditions for modern polymeric medical
devices, because under some conditions sterilization
will destroy the surfaces by degradation of the chains
and produce some low molecular volatiles. Halfmann
et al. (2007) introduced double inductively coupled
low pressure plasma for sterilization of three
dimensional biomedical materials. The short
treatment time and low temperature allow for the
sterilization of heat sensitive materials such as ultra
high molecular weight polyethylene (PE) and
polyvinyl chloride (PVC). In the experimental study
of Miao and Jierong (2009), the germicidal effect of
E. coli on the surface of medical PVC in remote
oxygen plasma and the effective inactivation of the
E. coli by this plasma was observed. Compared with
direct oxygen plasma sterilization, remote plasma can
enhance the hydrophilic property and limits the
degradation of the PVC surface.
Plasma in dentistry
A number of methods, such as mechanical drilling,
laser techniques and chemical reagents have been
employed for the cleaning and disinfection of the
tissue in dental cavities or in root canals. However,
most of these methods have disadvantages such as
heating, the destruction of healthy tissues, and
undesirable side effects including a disagreeable taste
and staining by chemotherapeutic agents such as
chlorhexidine (Goree et al. 2006). Plasma bacterial
inactivation of tissues in a dental cavity or in a root
canal is of importance and a tissue saving method in
dentistry. The exposure of enamel to the plasma is
painless and the heating of the pulp is tolerable.
Furthermore, plasma is non-toxic and it does not
cause damage to the mineralized matrix of the tooth.
Several types of nonthermal atmospheric plasma
devices have been used for dental treatment (Sladek
et al. 2004). A low power, millimeter sized,
atmospheric pressure glow discharge plasma needle
was developed to kill Streptococcus mutans
(S. mutans) which is the main microorganism causing
dental caries. This plasma can effectively kill the
bacteria with a treatment time of ten seconds within
a solid circle of 5 mm diameter, demonstrating its site
specific treatment capabilities (Goree et al. 2006).
Atmospheric pressure DBD plasma needles with a
funnel shaped nozzle were used for the inactivation of
S. mutans. Oxygen was injected downstream in the
plasma afterglow region through a powered steel tube
(Zhang et al. 2009). Jiang et al. (2009), introduced a
safe and novel technique for endodontic disinfection
with a hollow electrode based, 100 ns pulsed plasma
dental probe. It generates a room temperature, tapered
cylindrical plasma plume in ambient atmosphere. The
plasma plume causes minimal heating of biological
materials and is safe to touch with bare hands without
causing a burning sensation or pain. Greater
sterilization depth and surface coverage were
achieved by optimizing the width and length of the
plasma plume. A non-thermal atmospheric pressure
helium plasma jet device was developed to enhance
the tooth bleaching effect of hydrogen peroxide. The
combination of the plasma with hydrogen peroxide
improves the bleaching efficacy by a factor of three
compared to sterilization by hydrogen peroxide alone
(Lee et al. 2009).
Due to the narrow channel shape geometry of the
root canal of a tooth, the plasma generated by some
devices is not efficient in delivering reactive agents
into the root canal for disinfection. Therefore, to have
a better killing efficacy, plasma has to be generated
inside the root canal. Recently Lu et al. (2009),
constructed a cold plasma jet device which can
generate plasma inside the root canal and which
efficiently kills Enterococcus faecalis (one of the
main types of bacteria causing failure of the root
canal) within several minutes.
PLASMA MODIFIED MATERIALS IN MEDICINE
The surface properties of materials play an essential
role in determining their biocompatibility, strongly
influence their biological response and determine
their long term performance in vivo (Chu et al. 2002).
Many synthetic biomaterials such as metals, alloys,
ceramics, polymers and composites have a different
environment from the natural environment consisting
of neighbouring cells or extra cellular matrix
components. So it is important to design biomaterials
with the right surface properties, especially chemical
binding properties to achieve the biocompatibility of
artificial biomaterial surfaces. For surface
modification in the medical field, very thin layers
with a thickness of some ten to hundred nanometers
are mainly required (Favia et al. 2008). The treatment
of the surfaces of materials with non-thermal plasma
can lead to surface activation and functionalization.
This creates unique surface properties often
unobtainable with conventional, solvent based
chemical methods. Thus plasma surface modification
can improve biocompatibility and biofunctionality.
Appropriate selection of the plasma source enables
the introduction of diverse functional groups on the
target surface to improve biocompatibility or to allow
subsequent covalent immobilization of various
bioactive molecules (Gupta and Anjum 2003, Oehr
2003, Denes and Manolache 2004). Polymers are
common medical materials because of their superior
properties such as easy processing, ductility, impact
load damping and excellent biocomparability
(Gomathi et al. 2008). A list of polymeric and
metallic plasma treated biomaterials and their uses is
given in Table 1.
Table 1. Plasma modified materials and their applications.
| Plasma modified materials |
Applications | | Polymers |
| | Polyethylene
Polypropylene
Polyvinylchloride
Polyurethanes |
Catheters, anti-microbial coatings, implants | | Polytetrafluoroethylene |
Implants, vascular grafts | | Poly(methyl methacrylate)
Silicone rubber |
Contact lenses, artificial corneas | | Poly(ethylene terephthalate)
Polystyrene |
Implants, tissue culture dishes | | Polylactic acid
Polyglycolic acid |
Sutures, drug delivery matrix | | Metals and alloys |
| | Ti
Ti-Ni alloys
Co-Cr alloys |
Implants | | Steel |
Stents |
Types of plasma surface modification processes
A number of plasma processes have been developed
to attain specific surface properties for biomaterials
and some are listed below
a) surface functionalization by gas plasma (O2, CO2,
N2, NH3, etc.);
b) formation of thin films by plasma polymerization;
c) inclusion of metal ions in the surface by plasma
induced ion implantation.
Analytical techniques such as optical microscopy, 3D
laser profiling, scanning electron microscopy, atomic
force microscopy, contact angle, X-Ray photoelectron
spectroscopy, static time of flight secondary ion mass
spectrometry and dynamic secondary ion mass
spectrometry have been used to characterize the
surface properties of plasma modified materials.
Antimicrobial materials
The biomaterials used for the treatment of diseases
and for implants must possess good antimicrobial
properties. So it is important to improve the
antibacterial properties of such materials by the
incorporation of antimicrobial agents in, or by the
application of surface coatings to the materials used.
The antimicrobial properties of metals and metal ions
(silver, copper, etc.) have been well known since
ancient times. Nowadays, metal nanoparticles (NPs)
are widely employed to improve the antimicrobial
activity of many synthetic biomaterials (Weir et al.
2008). This bactericidal effect of metal NPs has been
attributed to their small size and high surface to
volume ratio which allow them to interact closely
with microbial membranes. Metal NPs with
bactericidal activity can be immobilized and coated
onto surfaces which may find application in medical
instruments and devices (Kim et al. 2007, Ruparelia
et al. 2008). Plasma processes such as plasma
sputtering, plasma induced ion implantation and
plasma enhanced chemical vapor deposition among
others, are relatively simple and efficient methods for
the incorporation of such agents. In this way the
antimicrobial properties of the biomaterials made
from metals, polymers, and other materials have been
improved.
Metallic biomaterials
Copper is known to be active against bacteria and
fungi (Silver and Phung 1996, Noyce et al. 2006). An
antibacterial nanocomposite of copper containing
organosilicon thin films, has been successfully
synthesized on stainless steel using a mixed plasma
enhanced chemical vapor deposition-sputtering
deposition technique. The antimicrobial properties
were evaluated with a solution containing E. coli
microorganisms for 24 h, the E. coli concentration
decreased to the minimal detectable value. The
process parameters were optimized to control the
quantity of incorporated copper in the layer (Daniel et
al. 2009).
Silver ions are widely used as a bactericide in
catheters, burn wounds and dental work. The
incorporation of silver into implants is a most
promising method in reducing the infection rate,
while exhibiting low toxicity towards cells and
tissues. Some harmful effects of silver nanoparticles
and their toxicity for human health have been
reviewed (Panyala et al. 2008). The inhibitory effect
of silver on bacteria is generally believed to be caused
by silver reacting with thiol groups in protein which
induce the inactivation of the bacterial proteins (Rai
et al. 2009, Sharma et al. 2009). A plasma sprayed
nano-titania/silver coating was deposited on titanium
substrates for the prevention of bacterial infections.
The experimental results confirmed that the plasma
sprayed nano-titania/silver coating has good
bioactivity, cytocompatibility and antibacterial
properties, which makes it a promising application
against postoperative infections in the replacement of
hard tissues (Li et al. 2009). The inclusion of silver
into the chemical treatment of the surface of vacuum
plasma sprayed titanium coatings plays an important
role in inhibiting the proliferation of bacteria. The
treated titanium coatings exhibit a prominent
antibacterial effect against E. coli, P. aeruginosa and
S. aureus (Chen et al. 2009b). The antibacterial
properties of doped silver on biocompatible silica
based glass have also been studied. Firstly the glass
powders were coated on titanium alloy and stainless
steel substrates by a plasma spray process in air. In
vitro test results showed an antimicrobial action
against tested bacteria without disturbance of the
biocompatibility of the glass (Miola et al. 2009).
Stainless steel dental device plates were modified by
the plasma based fluorine and silver ion
implantation-deposition method. Due to the presence
of both fluoride and silver ions, the brushing abrasion
resistance of the deposited or mixing layer was
improved and the hydrophobic properties remained
even after brushing with a toothbrush. This
simultaneous fluoride and silver ion
implantation-deposition could provide a possible
antimicrobial property to medical and dental devices
(Shinonaga and Arita 2009).
A nanolayer biofilm of polyacrylic acid (PAA)
was uniformly coated on the surface of magnetic
nickel NPs using a dielectric barrier discharge glow
plasma fluidized bed. The PAA acting as an adhesion
layer was used to immobilize a certain concentration
of antimicrobial peptide (LL-37) to kill the bacteria
E. coli. The results indicated that the modified nickel
NPs immobilizing a certain concentration of LL-37
could kill the bacteria effectively (Chen et al. 2009a).
Polymeric biomaterials
Medical polymers are widely used in biomedical
applications because of their excellent mechanical
and biological properties. However, the infection in
medical polymers is a major clinical complication.
Recently plasma surface modification techniques
have been employed in the development of
anti-infective medical polymers for the biomedical
industry (Sodhi et al. 2001, Ji et al. 2007).
A comparative study has been carried out on
single and dual copper plasma immersion and ion
implantation (PIII) to produce an antibacterial surface
on polyethylene (PE). Compared with the single
copper PIII process, the dual plasma implantation
process (Cu/N2 PIII) can better regulate the copper
release rate and improve the long term antibacterial
properties of PE against E. coli and S. aureus (Zhang
et al. 2007). The improved antimicrobial activity of
plasma treated PE films after chemical
immobilization of an antimicrobial enzyme
(lysozyme) has also been investigated. Plasma
conditions and enzyme solution concentrations were
optimized for the effective immobilization on the PE
surface (Conte et al. 2008). A tunable antimicrobial
polypropylene (PP) surface with a controllable
strength against Pseudomonas putida and S. aureus
has been recently reported. Microwave plasma
reaction in the presence of maleic anhydride results in
the formation of acid groups on the surface of PP.
This modification of the plasma surface helps the
attachment of antibiotics such as penicillin V (PEN)
and gentamicin (GEN) to the modified PP surface
through the reaction of the acid group on the PP
surface and polyethylene glycol (PEG), diglycidyl
PEG respectively (Aumsuwan et al. 2009).
Drug delivery
Plasma surface modification provides sufficient
adherence to metallic and polymeric materials for the
binding of drug molecules. The bioabsorbable
materials can act as drug carriers by controlling the
release rate of the drug initially loaded in an
application for drug delivery systems. Fig. 6 shows
the schematic illustration of a drug molecule grafting
on an O2 plasma treated substrate.
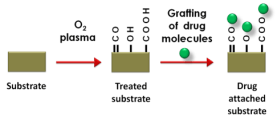
Fig. 6. Incorporation of drug molecules to plasma
treated substrate for drug delivery (O Drug molecule).
Nanoporous membranes have attracted
considerable interest for application in drug delivery.
The deposition of heptylamine plasma polymer
coatings onto porous alumina (PA) membranes has
been investigated with the aim of adjusting the
surface chemistry and the pore size of the membranes.
The structural (pore size) properties of PA
membranes can be altered systematically by adjusting
the deposition time during the polymerization
process. The resultant PA membranes with active
amino groups and controlled pore size are applicable
for molecular separation and drug delivery (Losic et
al. 2008). The polylactic acid ultrasound contrast
agent has significant importance in ultrasound
imaging and eventually in drug treatment for cancer.
It has an additional advantage, because ultrasound in
drug delivery may induce cavitations, increase cell
membrane permeability and facilitate drug release.
Plasma surface modification improves drug loading
for ultrasound-triggered drug delivery. Plasma
treatment appears to both sterilize and beneficially
modify the agent for increased doxorubicin
adsorption (Eisenbrey et al. 2009). The macroporous
structure of polystyrene-divinylbenzene (PS-DVB)
solid foams materials with high pore volume makes
them interesting for the design of new drug delivery
systems. The wettability of the highly hydrophobic
PS-DVB films was improved by a short post
discharge plasma treatment with different gases with
a view to opening new possibilities for the absorption
of hydrophilic compounds (Canal et al. 2009). The
surface functionalization of TiO2 nanotubes by
plasma polymerization generates a thin and
chemically reactive polymer film rich in amine
groups on top of the substrate surface. The tailoring
of surface functionalities on nanotube surfaces has
potential for significantly improving the properties of
this attractive biomaterial and promoting the
development of new biomedical devices such as drug
eluting medical implants with multiple functions
(orthopedic implants, dental, coronary stents). This
will provide an elegant route to the prevention of
infection, clotting control or to a decrease in
inflammation as a result of these implants (Vasilev et
al. 2010).
Tissue engineering
Artificial materials are of growing importance in
medicine and biology. A modern scientific
interdisciplinary field known as tissue engineering
has been developed to design artificial biocompatible
materials to substitute irreversibly damaged tissues
and organs. Cells can sense the physical properties
and chemical composition of these materials and
regulate their behavior accordingly (Bacakova et al.
2004). Cell affinity is the most important factor to be
considered when biodegradable polymeric materials
are utilized as a cell scaffold in tissue engineering. A
plasma technique can easily be used to introduce
desired functional groups or chains onto the surface
of materials, so it has a special application in
improving the cell affinity of scaffolds.
The copolymer, poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) (PHBV) has been intensely
studied as a tissue engineering substrate. Plasma
treatment of PHBV films increases the nano
roughness pattern and results in a moderate
hydrophilicity on the film surface. This
physicochemical change modifies the behaviour of
the vero cells by stimulating cell adhesion, cell
growth and spreading, etc. (Lucchesi et al. 2008).
Poly(methylmethacrylate) films were modified by the
application of glow discharge oxygen plasma. An
increase in the hydrophilicity and surface free energy
and an increase in the plasma power and application
time was observed. This improves the surface
properties of the implants (at the molecular level) in
order to enhance the cell attachment to the materials
(Ozcan et al. 2008).
Plasma treatment with acrylic acid is an attractive
way of introducing carboxylic groups to a
polyurethane (PU) surface and subsequently of
immobilizing natural or synthetic molecules carrying
amino groups in their structure, through the formation
of amide bonds. The plasma treatment allows a
monolayer of PAA, which is then functionalized with
a biomacromolecule. The PU treated with
macromolecules is a good candidate as a cell
substrate. In particular, functionalization with poly
(L-lysine) performs extremely well in the activation
of cellular processes and shows optimum cell
proliferation with increasing time (Sartori et al. 2008).
A variety of extracellular matrix protein components
such as gelatin, collagen, laminin and fibronectin
could be immobilized onto the plasma treated surface
to enhance cellular adhesion and proliferation.
Electrospun nanofibres composed of polyglycolic
acid, poly-L-lactic acid or poly(lactic-co-glycolic
acid) were modified with carboxylic acid groups
through plasma glow discharge with oxygen and gas
phased acrylic acid. Such hydrophilized nanofibres
were shown to enhance fibroblast adhesion and
proliferation without compromising physical and
mechanical bulk properties (Yool et al. 2009). Starch
based scaffolds treated by argon plasma were shown
to be a good support when used in bone tissue
engineering. Higher proliferation rates, because of the
novel protein surface interaction by plasma treatment
were observed on the scaffolds (Santos et al. 2009).
CONCLUSION
Modern plasma tools employed in medical treatments
are found to be more efficient and flexible in use. The
plasma sterilization of both living and non-living
objects offers non destructive removal of the
microorganisms in a shorter treatment time. The use
of different types of plasma jets in dentistry offers a
painless treatment for the cleaning of dental cavities.
The incorporation of the antimicrobial agents to
plasma treated polymeric and metallic material
enhances superior antimicrobial activity which
significantly increases the convenience of these
objects in medicine. Surface functionalization of
artificial biomaterials (implants and scaffolds) by
plasma treatment illustrates an improved rate of drug
loading and controlled release even long term. This
surface modification technique helps the introduction
of bioactive species on the scaffolds, and promotes
cell adhesion and proliferation which play an
important role in tissue development. Thus the plasma
treatment of materials represents an unusually
convenient and versatile technique for surface
activation and functionalization, which creates unique
surface properties, often not obtainable by other
methods. Plasma applications and plasma modified
materials in medicine are undergoing fast
development and plasma-medicine is becoming an
important part of modern health care.
ACKNOWLEDGEMENT
Financial aid from the research grant of the Academy
of Science of the Czech Republic (Project KAN
101630651), project MSM0021622411 and LC 06035
are greatly acknowledged.
REFERENCES
Aumsuwan N, McConnell MS, Urban MW: Tunable antimicrobial polypropylene surfaces: Simultaneous attachment of penicillin (Gram +) and gentamicin
(Gram -). Biomacromolecules 10:623-629, 2009.
Bacakova L, Filova E, Rypacek F, Svorcik V, Stary V: Cell adhesion on artificial materials for tissue engineering. Physiol Res 53(Suppl. 1):S35-S45,
2004.
Bogaerts A, Neyts E, Gijbels R, Mullen J: Gas discharge plasmas and their applications: Review. Spectrochim Acta Part B 57:609-658, 2002.
Braithwaite NSJ: Introduction to gas discharges. Plasma Sources Sci Technol 9:517-527, 2000.
Canal C, Gaboriau F, Vilchez A, Erra P, Celma MG, Esquena J: Topographical and wettability effects of post-discharge plasma treatments on
macroporous polystyrene-divinylbenzene solid foams. Plasma Process Polym 6:686-692, 2009.
Chen G, Zhou M, Chen S, Guohua L, Yao J: Nanolayer biofilm coated on magnetic nanoparticles by using a dielectric barrier discharge glow plasma
fluidized bed for immobilizing an antimicrobial peptide. Nanotechnology 20:465706, 2009.
Chen Y, Zheng X, Xie Y, Ji H, Chuanxian D: Antibacterial properties of vacuum plasma sprayed titanium coatings after chemical treatment. Surf Coat
Technol 204:685-690, 2009.
Chu PK, Chena JY, Wanga LP, Huang N: Plasma-surface modification of biomaterials. Mater Sci Eng R Rep 36:143-206, 2002.
Conrads H, Schmidt M: Plasma generation and plasma sources. Plasma Sources Sci Technol 9:441-454, 2000.
Conte A, Buonocore GG, Sinigaglia M, Lopez LC, Favia P, Agostino R, Del Nobile MA: Antimicrobial activity of immobilized lysozyme on plasma-treated
polyethylene films. J Food Prot 71:119-125, 2008.
Crow S, Smith JH: Gas plasma sterilization: Application of space-age technology. Infect Control Hosp Epidemiol 16:483-487, 1995.
Daniel A, Pen LC, Archambeau C, Reniers F: Use of a PECVD-PVD process for the deposition of copper containing organosilicon thin films on steel.
Appl Surf Sci 256:82-85, 2009.
Denes FS, Manolache S: Macromolecular plasma-chemistry: An emerging field of polymer science. Prog Polym Sci 29:815-885, 2004.
Desmet T, Morent R, Geyter N, Leys C, Schacht E, Dubruel P: Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface
modification: A Review. Biomacromolecules 10:2351-2378, 2009.
Eisenbrey JR, Hsu J, Wheatley MA: Plasma sterilization of poly lactic acid ultrasound contrast agents: surface modification and implications for
drug delivery. Ultrasound Med Biol 35:1854-1862, 2009.
Favia P, Sardella E, Lopez L, Laera S, Milella A, Pistillo B, Intranuovo F, Nardulli M, Gristina R, D'Agostino R: Plasma assisted surface
modification processes for biomedical materials and devices. NATO ASI Ser A 10:203-225, 2008.
Fridman G, Peddinghaus M, Ayan H, Fridman A, Balasubramanian M, Gutsol A, Brooks A, Friedman G: Blood coagulation and living tissue sterilization by
floating-electrode dielectric barrier discharge in air. Plasma Chem Plasma Process 26:425-442, 2006.
Fridman G, Brooks AD, Balasubramanian M, Fridman A, Gutsol A, Vasilets VN, Ayan H, Friedman G: Comparison of direct and indirect effects of
non-thermal atmospheric-pressure plasma on bacteria. Plasma Process Polym 4:370-375, 2007.
Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A: Applied plasma medicine. Plasma Process Polym 5:503-533, 2008.
Gaunt LF, Beggs CB, Georghiou GE: Bactericidal action of the reactive species produced by gas-discharge nonthermal plasma at atmospheric pressure: A
review. IEEE Trans Plasma Sci 34:1257-1269, 2006.
Gomathi N, Sureshkumar A, Neogi S: RF plasma-treated polymers for biomedical applications. Curr Sci 94:1478-1486, 2008.
Goree J, Liu B, Drake D, Stoffels E: Killing of S. mutans bacteria using a plasma needle at atmospheric pressure. IEEE Trans Plasma Sci
34:1317-1324, 2006.
Griffiths N: Low-temperature sterilization using gas plasmas. Med Device Technol 4:37-40, 1993.
Gupta G, Anjum N: Plasma and radiation-induced graft modification of polymers for biomedical applications. Adv Polym Sci 162:35-61, 2003.
Gweon B, Kim DB, Moon SY, Choe W: Escherichia coli deactivation study controlling the atmospheric pressure plasma discharge conditions. Curr
Appl Phys 9:625-628, 2009.
Halfmann H, Bibinov N, Wunderlich J, Awakowicz P: A double inductively coupled plasma for sterilization of medical devices. J Phys D Appl Phys
40:4145-4154, 2007.
Hayashi N, Guan W, Tsutsui S, Tomari T, Hanada Y: Sterilization of medical equipment using radicals produced by oxygen/water vapor RF plasma. Jpn J
Appl Phys 10B 45:8358-8363, 2006.
Herrmann HW, Henins I, Park J, Selwyn GS: Decontamination of chemical and biological warfare agents using an atmospheric pressure plasma jet. Phys
Plasmas 6:2284-2289, 1999.
Ji J, Zhang W: Bacterial behaviors on polymer surfaces with organic and inorganic antimicrobial compounds. J Biomed Mater Res Part A 10:448-453,
2007.
Jiang C, Chen MT, Gorur A, Schaudinn C, Jaramillo DE, Costerton JW, Sedghizadeh PP, Vernier PT, Gundersen MA: Nanosecond pulsed plasma dental probe.
Plasma Process Polym 6:479-483, 2009.
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH: Antimicrobial effects of silver
nanoparticles. Nanomed Nanotechnol Biol Med 3:95-101, 2007.
Klima M, Janca J, Kapicka V, Slavicek P, Saul P: Zpusob vytvareni fyzikalne a chemicky aktivniho prostredi plazmovou tryskou a plazmová tryska.
Patent CZ 147698, 12. 5. 1998.
Klima M, Janca J, Kapicka V, Slavicek P, Saul P: The method of making a physically and chemically active environment by means of a plasma jet and
the related plasma jet. Patent US 6, 525, 481, 2003; Patent EP 1077021, 2005. Prior 12. 5. 1998.
Kylian O, Rauscher H, Gilliland D, Bretagnol F, Rossi F: Removal of model proteins by means of low-pressure inductively coupled plasma discharge. J
Phys D Appl Phys 41:095201, 2008.
Laroussi M: Low lemperature plasma-based sterilization: Overview and state-of-the-art. Plasma Process Polym 2:391-400, 2005.
Laroussi M: The biomedical application of plasma: A brief history of the development of a new field of research. IEEE Trans Plasma Sci 36:1612-1614,
2008.
Laroussi M, Tendero C, Lu X, Alla S, Hynes WL: Inactivation of bacteria by the plasma pencil. Plasma Process Polym 3:470-473, 2006.
Laroussi M, Hynes W, Akan T, Lu X, Tendero C: The plasma pencil: A Source of hypersonic cold plasma bullets for biomedical applications. IEEE Trans
Plasma Sci 36:1298-1299, 2008.
Lee HW, Kim GJ, Kim JM, Park JK, Lee JK, Kim GC: Tooth bleaching with nonthermal atmospheric pressure plasma. J Endod 35:587-591, 2009.
Lerouge S, Wertheimer MR, Yahia LH: Plasma sterilization: A review of parameters, mechanisms, and limitations. Plasmas Polym 6:175-188,
2001.
Li B, Liua X, Menga F, Chang J, Dinga C: Preparation and antibacterial properties of plasma sprayed nano-titania/silver coatings. Mater Chem Phys
118:99-104, 2009.
Lin SM, Swanzy, Jacobs PT: Method of hydrogen peroxide plasma sterilization. U S patent 5 876 666, 1999.
Losic D, Cole MA, Dollmann B, Vasilev K, Griesser HJ: Surface modification of nanoporous alumina membranes by plasma polymerization. Nanotechnology
19:245704, 2008.
Lu X, Cao Y, Yang P, Xiong Q, Xiong Z, Xian Y, Pan Y: An RC plasma device for sterilization of root canal of teeth. IEEE Trans Plasma Sci 37:668-673
2009.
Lucchesi C, Ferreira BMP, Duek EAR, Santos AR. Jr, Joazeiro PP: Increased response of vero cells to PHBV matrices treated by plasma. J Mater Sci
Mater Med 19:635-643, 2008.
Martines E, Zuin M, Cavazzana R, Gazza E, Serianni G, Spagnolo S, Spolaore M, Leonardi A, Deligianni V, Brun P, Aragona M, Castagliuolo I, Brun P: A
novel plasma source for sterilization of living tissues. New J Phys 11:115014, 2009.
Miao H, Jierong C: Inactivation of Escherichia coli and properties of medical poly(vinyl chloride) in remote-oxygen plasma. Appl Surf Sci
255:5690-5697, 2009.
Miola M, Ferraris S, Nunzio SD, Robotti PF, Bianchi G, Fucale G, Maina G, Cannas M, Gatti S, Masse A, Brovarone CV, Verne E: Surface silver-doping
of biocompatible glasses to induce antibacterial properties. Part II: Plasma sprayed glass-coatings. J Mater Sci Mater Med 20:741-749, 2009.
Montie C, Kelly WK, Roth R: Overview of research using the one-atmosphere uniform glow discharge plasma for sterilization of surfaces and materials.
IEEE Trans Plasma Sci 28:41-50, 2000.
Moon Y, Kim DB, Gweon B, Choe W, Song HP, Jo C: Feasibility study of the sterilization of pork and human skin surfaces by atmospheric pressure
plasmas. Thin Solid Films 517:4272-4275, 2009.
Moreau M, Orange N, Feuilloley MGJ: Non-thermal plasma technologies: New tools for bio-decontamination. Biotechnol Adv 26:610-617, 2008.
Nie QY, Cao, Ren CS, Wang DZ, Kong MG: A two-dimensional cold atmospheric plasma jet array for uniform treatment of large-area surfaces for plasma
medicine. New J Phys 11:115015, 2009.
Noyce JO, Michels H, Keevil CW: Potential use of copper surfaces to reduce survival of epidemic methicillin-resistant Staphylococcus aureus
in the healthcare environment. J Hosp Infect 63:289-297, 2006.
Oehr C: Plasma surface modification of polymers for biomedical use. Nucl Instrum Methods Phys Res B 208:40-47, 2003.
Ozcan C, Zorlutuna P, Hasirci V, Hasirci N: Influence of oxygen plasma modification on surface free energy of PMMA films and cell attachment.
Macromol Symp 269:128-137, 2008.
Panyala NR, Pena-Mendez EM, Havel J: Silver or silver nanoparticles: A hazardous threat to the environment and human health? J Appl Biomed
6:117-129, 2008.
Rai M, Yadav A, Gade A: Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76-83, 2009.
Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S: Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta
Biomaterialia 4:707-716, 2008.
Santos MI, Pashkuleva I, Alves CM, Gomes ME, Fuchs S, Unger RE, Reisab RL, Kirkpatrick CJ: Surface-modified 3D starch-based scaffold for improved
endothelialization for bone tissue engineering. Mater Chem 19:4091-4101, 2009.
Sartori S, Rechichi A, Vozzi G, D'Acunto M, Heine E, Giusti P, Ciardelli G: Surface modification of a synthetic polyurethane by plasma glow
discharge: Preparation and characterization of bioactive monolayers. React Funct Polym 68:809-821, 2008.
Sharma VK, Yngard RA, Lin Y: Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv Colloid Interface Sci 145:83-96,
2009.
Shenton MJ, Stevens GC: Surface modification of polymer surfaces: Atmospheric plasma versus vacuum plasma treatments. J Phys D Appl Phys
34:2761-2768, 2001.
Shinonaga Y, Arita K: Surface modification of stainless steel by plasma-based fluorine and silver dual ion implantation and deposition. Dent Mater
28:735-742, 2009.
Silver S, Phung LT: Bacterial heavy metal resistance: New surprises. Annu Rev Microbiol 89:753-789, 1996.
Sladek REJ, Stoffels E, Walraven R, Tielbeek PJA, Koolhoven RA: Plasma treatment of dental cavities: A feasibility study. IEEE Trans Plasma Sci
32:1540-1543, 2004.
Sodhi RNS, Sahi VP, Mittelman MW: Application of electron spectroscopy and surface modification techniques in the development of anti-microbial
coatings for medical devices. J Electron Spectros Relat Phenomena 121:249-264, 2001.
Sohbatzadeh F, Colagar AH, Mirzanejhad S, Mahmodi S: E. coli, P. aeruginosa, and B. cereus bacteria sterilization using afterglow of
non-thermal plasma at atmospheric pressure. Appl Biochem Biotechnol 160:1978-1984, 2009.
Tendero C, Tixier C, Tristant P, Desmaison J, Leprince P: Atmospheric pressure plasmas: A review. Spectrochim Acta Part B 61:2-30, 2006.
Trompeter FJ, Neff WJ, Franken O, Heise M, Neiger M, Shuhai L: Reduction of Bacillus subtilis and Aspergillus niger spores using
nonthermal atmospheric gas discharges. IEEE Trans Plasma Sci 30:1416-1423, 2002.
Vasilev K, Poh Z, Kant K, Chan J, Michelmore A, Losic D: Tailoring the surface functionalities of titania nanotube arrays. Biomaterials 31:532-540,
2010.
Weir E, Lawlor A, Whelan A, Regan F: The use of nanoparticles in anti-microbial materials and their characterization. Analyst 133:835-845,
2008.
Xingmin S, Yukang Y, Yanzhou S, Wang Y, Fengling P, Yuchang Q: Experimental research of inactivation effect of low-temperature plasma on bacteria.
Plasma Sci Technol 8:569-572, 2006.
Yool HS, Kim TG, Park TG: Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv Drug Delivery Rev
61:1033-1042, 2009.
Zhang W, Zhang Y, Ji J, Yan Q, Huang A, Chu PK: Antimicrobial polyethylene with controlled copper release. J Biomed Mater Res Part A 83:838-844,
2007.
Zhang X, Huang J, Liu X, Peng L, Guo L, Lv G, Chen W, Feng K, Yang S: Treatment of Streptococcus mutans bacteria by a plasma needle. J Appl
Phys 105:1063, 2009.
|
BACK
|







