Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 2, p 81-86
DOI 10.2478/v10136-009-0012-x
The incidence of beta-defensin-1, 2, 3 in human healthy and chronically inflamed nasal and tonsillar mucosa
Hana Pacova, Jaromir Astl, Jindrich Martinek
Address: Hana Pacova, Inst. of Histology and Embryology, First Faculty of Medicine, Charles University, Albertov 4, 128 01 Prague 2, Czech Republic
Hpac@seznam.cz
Received 26th August 2009.
Published online 23rd March 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Material and Methods
Results
Discussion
References
SUMMARY
The nasal and tonsillar mucosa are exposed to massive incursions of pathological microorganisms. One of the mechanisms known to prevent an invasion of pathogens is an endogenous synthesis of antimicrobial peptides, which include human beta-defensins-1, 2, 3 (HBD-1, 2, 3).
The aim of this study was to demonstrate the occurrence of HBD-1, 2 and 3 in the human nasal mucosa and palatine tonsils in healthy tissues and during chronic inflammation (nasal polyposis with and without the colonization of Staphylococcus aureus and chronic tonsillitis) and to evaluate their incidence under varying conditions.
Another target was to compare the occurrence of human beta-defensins in these two different entities; that is, in the nasal mucosa and in the palatine tonsil.
It was assumed that the incidence of HBD-1, 2, 3 was lower in tonsils than in nasal mucosa; however, inflamed samples of tonsils and nasal mucosa showed no difference in the production of HBD-1, 2, 3. The presence of all three subfamilies of HBD was significantly lower in nasal polyps with Staphylococcus aureus positive than in the negative control.
KEY WORDS
beta-defensins; nasal mucosa; palatine tonsil; chronic inflammation
INTRODUCTION
Both the nasal and tonsillar mucosa are exposed to
massive incursions of pathological microorganisms
present in inhaled air and swallowed with food.
Therefore it is important for the nasal and tonsillar
mucosa to have effective mechanisms to fight against
these pathogens. These defense mechanisms include
epithelial integrity, lymphoid cells within the mucosa,
mucociliary apparatus and antimicrobial substances in
the lining fluid of nasal mucosa and epithelial
integrity, lymphoid tissue and the secreted
antimicrobial substances of the tonsils. If one or
several of these mechanisms fail, pathogens can enter
the body through the nasal or tonsillar mucosa.
Among the antimicrobial substances produced by
the epithelial cells are the human beta-defensins-1, 2 and
3 (HBD-1, 2, 3). beta-defensins are endogenous
antimicrobial peptides which are cationic peptides,
and their function is to produce lysis of the plasmatic
membrane and changes in the permeability of the
plasmatic membrane. They are important components
of innate immunity against bacteria, fungi and
viruses. They are involved in the early immune
response against pathogens. The HBD-1 is expressed
constitutively and cannot be up-regulated by bacteria,
fungi or other inflammatory stimuli ( Dunsche et al.
2001, Po-Hsu and Shee-Yie 2004, Kluver et al.
2006); the expression of the others (HBD-2, 3) is
induced by infectious challenges (Kluver et al. 2006).
The production of HBD-2 and 3 is influenced by
different types of pathogens. HBD-2 and 3 represent
human defensins shown to be produced following
stimulation of the epithelial cells with
microorganisms (Schibli et al. 2002). HBD-2 exhibits
a potent antimicrobial activity against gram-negative
bacteria and Candida, and only bacteriostatic activity
against Staphylococcus aureus (Claeys et al. 2003).
Po-Hsu and Shee-Yiu (2004) note that HBD-2 has a
bactericidal effect against a number of gram-negative
and gram-positive bacteria including S. aureus. HBD-3 shows bactericidal activity against gram-negative as
well as gram-positive bacteria, including S. aureus
(Claeys et al. 2003); this is in contrast to the findings
of Harder et al. (1997) who describe a strong
bactericidal effect on gram-negative bacteria, a high
antimycotic potency but only a weak bacteriostatic
effect on gram-positive S. aureus in oral tissues. It
was found that the highest concentrations of S. aureus
are found immediately distal to the anterior hairy
epidermis in a moist squamous epithelium devoid of
hair, cilia and microvilli. This localization may be due
to the lack of ciliary clearance of nasal fluid from this
area, so its resistance to colonization depends largely
on the intrinsic antimicrobial properties of the nasal
fluid (Cole et al. 2001). A very interesting finding is
the detection of different levels of HBD-2 and 3 in the
inflamed nasal mucosa with and without S. aureus.
HBD-2 and 3 were negligibly induced in nasal
epithelial cells exposed to the nasal carrier strain of
S. aureus in comparison to the non-carrier strain. It
suggests that carrier strains of S. aureus retain a
competitive advantage over non-carrier strains by
delaying the host innate response to epithelial
colonization and infection (Quinn and Cole 2007).
HBD-1 was expressed in all nasal tissue samples,
at levels that did not differ significantly and HBD-2
was found in the nasal polyps but not in healthy nasal
mucosa (Lee et al. 2002). Some other authors wrote
that HBD-2 was detected only in several samples of
the healthy nasal mucosa. It was predominantly
localized in the surface epithelial cells with the
strongest positivity in the basal layer (Po-Hsu and
Sheen-Yie 2004). Claeys et al. (2003) found no
up-regulation for HBD-2 and 3 in paranasal mucosa
in patients with chronic sinusitis or nasal polyposis
compared with healthy nasal mucosa.
There are differing opinions as to the presence of
beta-defensins in tonsillar tissue. HBD-2, 3 have been
demonstrated both in the surface epithelium and in
the epithelium of the tonsillar crypts. The expression
of HBD-2, 3 was confirmed in tonsillar tissue with no
significant difference between idiopathic hypertrophic
tonsillar disease and recurrent tonsillitis. The deep
crypts and the excessive microorganical load and
bacterial diversity in tonsils could explain the more
pronounced expression of inducible antimicrobial
peptides in this organ (Claeys et al. 2003). Some
others found that significantly increased levels of
HBD-2 were detected only in chronic inflamed tonsils
(Weise et al. 2002). Ball et al. investigated very
interesting results: the surface epithelium of tonsils
from recurrent acute tonsillitis patients showed
reduced amounts of antimicrobial peptides HBD-1
and 3, compared to healthy controls. It may increase
these patients' susceptibility to infection (Ball et al.
2007).
The aim of this study was to demonstrate the
occurrence of human beta-defensin subfamilies 1, 2 and
3 in healthy human nasal mucosa and human palatine
tonsils and during chronic inflammation (nasal
polyposis and chronic tonsillitis). Findings in patients
with nasal polyposis were divided into two groups -
the first group of patients with a positive colonization
of Staphylococcus aureus in the nasal mucosa and the
second one with negative results of cultivation.
Another aim of this study was to compare the
occurrence of human beta-defensins in these two
different localizations.
MATERIAL AND METHODS
Staphylococcus aureus in the nasal mucosa (n = 10),
the second one with the negative cultivation from
nasal mucosa (n = 40). The samples of tonsils were
collected during surgery from patients suffering from
chronic tonsillitis (n = 11) or obstructive sleep apnea
syndrome - these were clinically healthy tonsils (n = 8). The microbial cultivation from patients with
chronic tonsillitis was made before tonsillectomy.
Various types of pathogens were found in the
cultivations from the tonsils. For light microscopy the
samples were fixed in 4% paraformaldehyde (PFA) in
a phosphate buffer solution (PBS) (pH 7.4) for
embedding to paraffin or snap frozen in liquid
nitrogen and kept frozen at -80 °C. Paraffin and
cryostate sections were used for the detection of
defensins (HBD-1, 2, 3) via the three-step
immunoperoxidase methods. The detection on
cryostat sections was carried out on 4% PFA in
PBS-fixed frozen sections. The paraffin sections were
deparaffined using xylen and 96% ethanol. The 2%
fetal bovine serum (Biosera, UK) in PBS was used for
30 minutes to block non-specific binding of
immunoglobulins. The next step of processing was
incubation with a primary antibody
[Anti-Rabbit-HBD-1 (Alpha-Diagnostic, Inc., USA)
1:400, Anti-Rabbit-HBD-2 (Peptide Institute, Inc.,
Japan) 1:200, Anti-Rabbit-HBD-3 (Orbigen, USA)
1:100]. This was carried out for 60 minutes at room
temperature. After washing in PBS, the sections were
incubated with biotinylated Goat-Anti-Rabbit IgG in
PBS (Sigma-Aldrich, USA) 1:200 as the secondary
antibody for another 30 minutes at room temperature.
After rinsing in PBS for another 5 minutes - repeated
three times - the sections were incubated with
Vectastain ABC Elite kit peroxidase (VECTOR lab.,
USA) and diluted in PBS for ABC buffer (1:50) for
30 minutes at room temperature. The preparations,
gently rinsed in PBS for five minutes were exposed to
Diaminobenzidine (DAB) peroxidase substrate
solution (DAKO Cytomation, Denmark) until the
brown staining appeared. The nuclei were
counterstained with hematoxylin for 5 seconds.
Antigen retrieval was performed after
deparaffination in TRIS buffer base (pH 9.5) in a
microwave oven (1 minute 560W and 5 minutes
240W). Possible activity of endogenous peroxidase
was blocked by 70% methanol and 1% hydrogen
peroxide for 10 minutes. The above mentioned
procedures were performed on cryostate sections with
exception of the antigen retrieval.
RESULTS
Clinically healthy nasal mucosa from inferior turbinate
The production of HBD-1 was higher in this
localization than in polyps and tonsils. The reaction
product was found in the glandular ducts, in the
serous cells of the serous demilunes but not in the
superficial epithelium. HBD-2, 3 were found in very
large amounts especially in the glandular serous cells
and in the excretory parts. Precipitate was
accumulated in the whole cytoplasm. HBD-3 was
detected in high amounts in the whole cytoplasm of
superficial epithelial cells as well as in the epithelial
lining of the glandular ducts.
Nasal polyps with occurrence of Staphylococcus aureus
The production of all three human beta-defensins (HBD-1, 2, 3) was very low; they were detected only in
several localizations of superficial pseudostratified
columnar epithelium and in several glandular duct
cells. The distribution of precipitate in the cytoplasm
had a granular pattern.
Nasal polyps without occurrence of Staphylococcus aureus
HBD-1 was detected irregularly in some serous
glandular cells, cells of excretory ducts and in
individual cells of the surface epithelium in very low
amounts. HBD-2, 3 were found in large amounts
especially in the serous and excretory parts of the
seromucous glands and in the superficial epithelial
cell cytoplasm. HBD-2 was present diffusely in the
apical part of the cytoplasm of the epithelial cells and
on the surface of this epithelium, HBD-3 had a
granular pattern in the whole cytoplasm of the
superficial epithelium and glandular ducts (Fig. 1).
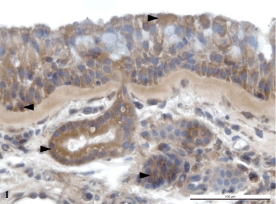
Fig. 1. The nasal polyp with negative cultivation. The
presence of HBD-3 in the superficial pseudostratified
columnar epithelium, in the excretory duct of a seromucous
gland and in the cytoplasm of serous cells.
Immunoperoxidase reaction (DAB). Counterstained by
hematoxylin.
Clinically healthy tonsils
A quite low but regular amount of HBD-1 was found
especially in the stratified squamous epithelium
covering. Very low levels of HBD-2 were proven in
the healthy tonsillar epithelium especially in the
stratum spinosum. HBD-3 was present in the
superficial epithelium in higher amounts than HBD-2.
It was found especially in the endothelial cell
cytoplasm of small veins (Fig. 2).
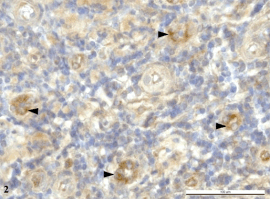
Fig. 2. The lymphoid tissue with small veins in clinically
healthy tonsil. The detection of HBD-3 in the endothelial
cell cytoplasm of small veins. Immunoperoxidase reaction
(DAB). Counterstained by hematoxylin.
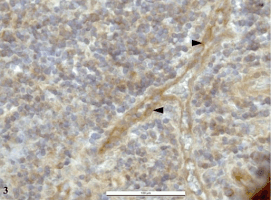
Fig. 3. A small branching vein in the lymphoid tissue of
chronically inflamed tonsil. HBD-3 is localized in the
cytoplasm of endothelial cells. Immunoperoxidase reaction
(DAB). Counterstained by hematoxylin.
Chronically inflamed tonsils
HBD-1 was detected in the stratum spinosum layer of
the epithelium of crypts. HBD-2 was present in quite
high levels in the superficial epithelium and
occasionally in the marginal zone of lymphatic
follicles. The HBD-3 was proven in the highest level
in the superficial epithelium of crypts in the marginal
zone of lymphatic follicles and a very expressive
positivity was found in the endothelial cell cytoplasm
of small veins (Fig. 3).
DISCUSSION
It is thought that HBD-1 gene is expressed
constitutively and that the production of a peptide is
not up-regulated by pathogens (Dunsche et al. 2001,
Po-Hsu and Sheen-Yie 2004, Kluver et al. 2006). In
the literature some authors have found that the levels
of HBD-1 do not differ significantly in various tissue
samples (Lee et al. 2002); another text asserts that
HBD-1 is reduced in the tonsils of patients with
recurrent acute tonsillitis (Ball et al. 2007). Our
results show that HBD-1 was detected in larger
amounts in healthy nasal mucosa than in the healthy
tonsils. Chronically inflamed samples did not produce
significant differences between nasal and tonsillar
localization. Only in nasal mucosa with positive
S. aureus was the presence of HBD-1 very low.
HBD-2 was found only in several samples of nasal
mucosa (Po-Hsu and Sheen-Yie 2004), in polyps it
was detected regularly (Lee et al. 2002). In tonsillar
tissue the production of HBD-2 does not differ in
healthy and inflamed samples (Claeys et al. 2003). In
other publications, authors report significantly higher
production of HBD-2 in inflamed tonsillar tissue in
comparison to healthy controls (Weise et al. 2002).
The expression of HBD-2 is induced by different
types of pathogens (Schibli et al. 2002, Kluver et al.
2006). We focused our work on the production of
HBD-2 during chronic inflammation in nasal and
tonsillar mucosa.
From our results we can say that HBD-2 was
found in all tissues examined but in variable amounts.
It was proven in very high levels in healthy nasal
mucosa as well as in nasal and tonsillar inflamed
samples with the exception of polyps with positive S.
aureus. From literature we know that HBD-2 is able
to act against S. aureus (Weise et al. 2002, Claeys et
al. 2003) but our results have proven only very low
production of this defensin in case of colonization of
nasal mucosa by S. aureus. In the healthy tonsils the
production of this defensin was not proven.
According to the literature, HBD-3 similar to
HBD-2 was detected in healthy nasal mucosa as well
as in nasal polyps. An up-regulation was not found in
these two types of samples. HBD-3 was demonstrated
in both healthy and chronically inflamed tonsils
(Claeys et al. 2003).
We investigated whether HBD-3 was present in
healthy nasal as well as tonsillar tissues in larger
amounts than HBD-2. In healthy nasal mucosa this
defensin was found in very high levels. The
production in chronically inflamed nasal and tonsillar
tissues was quite high with the exception of polyps
with positive S. aureus. Very interesting was the
localization of HBD-3 in the endothelial cell
cytoplasm of small veins especially in tonsils.
HBD-1 is involved in innate immunity; our results
show that HBD-1 is present more in healthy nasal
mucosa than in healthy tonsils. A possible reason is
that in the innate immunity of nasal mucosa the lining
fluid is more involved with the antimicrobial
substances in comparison to tonsils where the most
effective mechanism against pathogens are the cells
of the immune system (lymphocytes, leucocytes etc.).
Even when HBD-2 and 3 are included in the group of
antimicrobial peptides up-regulated by pathogens, we
found these defensins in very high amounts also in
healthy nasal mucosa. These results show its possible
contribution to innate immunity. Our results have
proven up-regulation of HBD-2 and 3 after the
invasion of pathogens into the nasal as well as
tonsillar region. The localization of HBD-3 in the
endothelial cell cytoplasm of small veins in tonsils is
very interesting. It shows the possible transport of
HBD-3 from the tonsillar region to the circulatory
system or in the opposite direction. This result is very
important because it shows that the possible effect of
HBD-3 is not only in the place of origin but that it can
be transported to any other region.
The colonization of the nasal mucosa with
S. aureus is a very important matter. The effect of
HBD-2 and 3 against S. aureus is known. Some
authors refer the bacteriostatic (Claeys et al. 2003) or
bactericidal (Po-Hsu and Sheen-Yie 2004) effect of
these defensins to S. aureus. HBD-2, 3 are very
important components of the defense against
pathogens. But our results showed that in case of
mucosal colonization with S. aureus, the cells
expressed very reduced ability to produce HBD-1, 2,
3 so the organism's immune defense is weakened.
These results can be also explained by the
principally different role the lymphatic compartment
has in the tonsillar region which surely take place in
the processes of antigen determination during
alimentary passages in comparison to nasal mucosa
which is exposed to very high amounts of airborne
pathogens during every inspiration. The most
effective manner in which the nasal mucosa can fight
against this infection is by means of the antimicrobial
peptides in the superficial lining fluid.
In summary, it can be concluded that HBD-1, 2, 3
are synthesized more intensively in healthy nasal
mucosa in comparison in healthy palatine tonsil.
There was no confirmation of any significant
difference in the production of HBD-1, 2, 3 in the
nasal polyps without the presence of S. aureus and in
the chronically inflamed tonsils. Very low - nearly
none - incidence of HBD-1, 2, 3 was detected in the
nasal polyps with positive S. aureus. HBD-3 was
found in the endothelial cell cytoplasm of the small
veins in both healthy and chronically inflamed tonsils.
ACKNOWLEDGEMENT
The authors would like to express their gratitude to
Mrs. Blazkeova, Mrs. Kratinova, Mrs. Kolarova and
Miss Kosova of the Inst. of Histology and
Embryology for their technical assistance in
processing the material and realizing the
immunohistochemical procedures.
This project was supported by Grant project No.
9077 NR, IGA Ministry of Health, CR.
REFERENCES
Ball SL, Siou GP, Wilson JA, Howard A, Hirst BH, Hall J: Expression and immunolocalisation of antimicrobial peptides within human palatine tonsils. J Laryngol Otol 121:973-978, 2007.
Claeys S, de Belder T, Holtappels G, Gevaert P, Verhasselt B, van Cauwenberge P, Bachert C: Human beta-defensins and toll-like receptors in the upper airway. Allergy 58:748-753, 2003.
Cole AM, Tahk S, Oren A, Yoshioka D, Kim YH, Park A, Ganz T: Determinants of Staphylococcus aureus nasal carriage. Clin Diag Immunol 8:1064-1069, 2001.
Dunsche A, Acil Y, Siebert R, Harder J, Schroder JM, Jepsen S: Expression profile of human defensins and antimicrobial proteins in oral tissues. J Oral Pathol Med 30:154-158, 2001.
Harder J, Bartels J, Christophers E, Schroeder JM: A peptide antibiotic from human skin. Nature 387:861, 1997.
Kluver E, Adermann K, Schulz A: Synthesis and structure-activity relationship of beta-defensins, multi-functional peptides of the immune system. J Pept Sci 12:243-257, 2006.
Lee SH, Kim JE, Lim HH, Lee HM, Choi JO: Antimicrobial defensin peptides of the human nasal mucosa. Ann Otol Rhinol Laryngol 111:135-141, 2002.
Po-Hsu Ch, Sheen-Yie F: Expression of human beta-defensin 2 in human nasal mucosa. Eur Arch Otorhinolaryngol 216:238-241, 2004.
Quinn GA, Cole AM: Suppression of innate immunity by nasal carriage strain of Staphylococcus aureus increases its colonization on nasal epithelium. Immunology 122:80-89, 2007.
Schibli DJ, Hunter HN, Aeyev V, Starner TD, Wiencek JM, Mccray PB: The solution structures of human beta-defensin 3 lead to better understanding of the potent bactericidal activity of HBD-3 against Staphylococcus aureus. J Biol Chem 277:8279-8289, 2002.
Weise JB, Meyer JE, Helmer H, Wittrock H, Maune S: A newly discovered function of palatine tonsils in immune defence: the expression of defensins. Otolaryngol Pol 56:409-413, 2002.
|
BACK
|




