Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 2, p 73-79
DOI 10.2478/v10136-009-0011-y
Circadian variations in biochemical markers of bone metabolism in horse of different age
Claudia Giannetto, Stefania Casella, Francesco Fazio, Vanessa Messina, Giuseppe Piccione
Address: Giuseppe Piccione, Dipartimento di Scienze Sperimentali e Biotecnologie Applicate, Facolta di Medicina Veterinaria, Universita degli Studi di Messina, Polo Universitario dell’Annunziata, 98168, Messina, Italy
giuseppe.piccione@unime.it
Received 26th November 2009.
Revised 16th December 2009.
Published online 23rd March 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
References
SUMMARY
The author studied in thirty Thoroughbred horses the influence of age and gender on daily rhythms of serum osteocalcin (OC). The animals were divided into two groups. Group A: six male and six female 2 years old; Group B: six male, six female and six geldings 6 years old. They were housed individually in box-like stalls under natural photoperiod and environmental conditions. Blood samples were collected every 3 hours over a 48 hour period. Statistical differences of serum OC concentration due to different gender were observed in both Groups A and B. Daily rhythms of serum OC concentration were observed only in Group B, with nocturnal acrophase. In females the acrophase was statistically postponed for 1 hour compared to male and gelding. Male and female showed a more robust daily rhythm than the geldings. The results showed that blood sampling for determination of serum OC should be strictly standardized with regard to the time of the day.
KEY WORDS
circadian rhythms; osteocalcin; age; gender; horses
INTRODUCTION
Bone turnover is characterized by two opposing, but
complementary, metabolic activities: the resorption of
old bone by osteoclastic cells and the deposition of
newly formed bone by osteoblasts. Age and rate of
growth at weaning may have an effect on the rate of
bone formation. The rate of bone turnover can be
assessed in vivo by measuring the serum, plasma or
urine concentrations of specific biochemical markers,
which, although mostly developed for studies of
human bone metabolism, have in some cases been
tested also in the horse (McIlwraith 2005).
The high incidence of orthopaedic diseases in
young and adult athletic horses raises questions
concerning the factors that contribute to these
problems, as well as a need to detect and monitor
them. Various techniques are described to
non-invasively evaluate and monitor equine bone
(Jeffcott et al. 1988, Lepage et al. 2001). Most of
them, however, are expensive, time consuming or
require specialized staff. Interestingly, osteocalcin
(OC) has been extensively used to assess bone
metabolism in this species. OC is a small abundant
non-collagenous calcium binding protein, indigenous
to the organic matrix of bone dentin and possibly
other mineralized tissues which circulate in the blood.
This protein is synthesised by osteoblasts,
incorporated in new bone matrix and released into the
circulation during bone resorption. As a result, its
serum level reflects bone turnover (Khosla and
Kleerekoper 2003). Several studies have
demonstrated that serum OC as a marker of bone
metabolism provides useful information in metabolic
bone diseases and in the management of their
treatment (Lepage et al. 1992). However, before
markers of bone turnover can be applied to study
orthopaedic diseases, sources of variation, such as age
differences and diurnal rhythms, must be described to
allow appropriate sample collection. Studies
conducted on 2 and 3 year old Thoroughbreds
indicated that bone biomarkers have a better
predictive value of bone diseases in older horses or
when measured serially in the same animals (Jackson
et al. 2009).
In Thoroughbred and Standardbred horses,
age-related changes in circulating levels of bone
markers have been observed, and changes reflect the
decrease in bone turnover with increasing skeletal
maturity (Lepage et al. 1990, Price et al. 1995). In
juvenile horses consistent age related patterns in
biomarker serum concentrations were found,
indicating a markedly higher metabolism before age
20 weeks. In these horses OC concentrations were not
affected by feeding level (Donabedian et al. 2008).
An age-related decrease in concentrations of bone
markers was also seen in Hanoverian foals during the
first 200 days of life. These changes were correlated
to the date of birth indicating that there are
differences in skeletal development between early-
and late-born foals (Vervuert et al. 2007). OC levels
were also observed to change in relation to the season
with higher concentrations during winter (Pastoret et
al. 2007).
In humans, it has been well-documented that
biochemical markers of bone metabolism show a
circadian rhythm (Nielsen et al. 1990, Hassager et al.
1992, Gertz et al. 1998). Circadian rhythmicity of
bone cell metabolic activity has been documented
also in rats and mice (Aardal and Laerum 1983,
Ohtsuka et al. 1998). In horses the results are not
clear: Black et al. (1999) investigated serum OC
diurnal variation in six Standardbred, one
Thoroughbred and one Quarter horse. They found a
rise in OC concentration during the night with the
lowest values during midday. This pattern was
similar to that reported by Lepage et al. (1991) in nine
adult Standardbreds, in which serum OC levels
reached their highest point during the night, but were
not significantly different during the day from 07:00
and 20:00. Jackson et al. (2003) found a circadian
rhythm of serum OC in six 2 years old Thoroughbred.
But in all these studies the procedures used in
circadian physiology for the analysis of full time
series were not applied (Refinetti 2006).
The purpose of this study was to determine
whether there is daily rhythm in serum OC
concentration in Thoroughbred horses and how this
may be affected by age and gender. It is of key
importance to establish the appropriate sampling time
for optimal use of biochemical markers to reflect bone
turnover change in clinical investigations of
metabolic disease and to value the effects of training
on skeletal adaptation.
MATERIALS AND METHODS
Animals
Our study was conducted on thirty Thoroughbred
horses regularly trained from the same horse training
centre. Before the start of the study, all subjects
underwent a heart examination, respiratory
auscultations, and routine haematology and plasma
biochemistry at rest. Only clinically healthy animals
were used. Animals were divided into two groups:
Group A consisted of twelve horses (six male and six
female), 2 years old and with a mean body weight of
380 ± 30 kg; Group B consisted of eighteen horses
(six male, six female and six geldings), 6 years old
and with a mean body weight of 560 ± 40 kg. They
were housed individually in box-like stalls (4.5 x
4.5 m) under natural photoperiod (sunrise at 06:30;
sunset at 18:30), at indoor temperature and humidity
(18-21 °C; 50-60 Rh%). Thermo-hygrometric
recordings were carried out inside the box throughout
the entire study by means of a data logger (Gemini,
Chichester, West Sussex, UK). The horses were fed
traditionally with hay and a mix of cereals (oats and
barley), three times a day (08:00, 12:00 and 19:00)
and received water ad libitum. General animal care
was carried out by professional staff not associated
with the research team. All housing and care
conformed to the standards recommended by the
Guide for the Care and Use of Laboratory Animals
and Directive 86/609 CEE.
Blood samples
Blood samples were collected at 3 hour intervals over
a 48 hour period (starting at 08:00 on day 1 and
finishing at 08:00 on day 3) via a jugular intravenous
catheter into Vacutainer tubes (Terumo Corporation,
Japan) without anticoagulant and stored at 4 °C for a
maximum of 30 min before centrifugation (3000 rpm
for 15 min) and freezing (-20 °C). Samples were
collected at 3 hour intervals in order not to
excessively disturb the animals. A dim red light
(<3lux, 15 W Safelight lamp filter 1A, Kodak Spa,
Milano, Italy) was used to sample horses during the
dark phase. Serum OC concentrations were quantified
by the use of an equine-specific RIA test (Diasorin
S.P.A., Saluggia, Italy). The validity of this kit for
horse OC was reported by Inoue et al. (2008) and
Donabédian et al. (2008). The serum OC
concentration was expressed as nanogram (ng) of OC
per millilitre (ml). The sensitivity of the RIA was
0.2 ng/ml.
Statistical analysis
All the results were expressed as mean ± SD. Data
were normally distributed (p<0.05,
Kolmogorov-Smirnov test). Two-way repeated
measure ANOVA was used to determine a statistical
significant effect of time of the day and age on the
serum OC concentration. An unpaired Student t-test
and an ANOVA were used to evaluate statistical
differences of serum OC concentration due to
different genders within Group A and B, respectively.
Data were evaluated at the significance level 2 = 0.05. The data was analyzed using
STATISTICA 7 software (StatSoft Inc., Tulsa, USA).
In addition, we applied a trigonometric statistical
model to the average values of each time series, so as
to describe the periodic phenomenon analytically, by
characterizing the main rhythmic parameters
according to the single cosinor procedure (Nelson et
al. 1979). Four rhythmic parameters were determined:
mean level, amplitude (the difference between the
peak, or trough, and the mean value of a wave),
acrophase (the time at which the peak of a rhythm
occurs), and robustness (strength of rhythmicity). For
each parameter, the mean level of each rhythm was
computed as the arithmetic mean of all values in the
data set (9 data points), the amplitude of a rhythm was
calculated as half the range of oscillation, which in its
turn was computed as the difference between peak
and trough. Rhythm robustness was computed as a
percentage of the maximal score attained by the
chi-square periodogram statistic for ideal data sets of
comparable size and 24 h periodicity (Refinetti 2004).
Robustness greater than 40% is above noise level and
indicates statistically significant rhythmicity.
RESULTS
The application of two-way repeated measure
ANOVA showed a statistical significant effect of time
of the day (F(7,524) = 2.94, statistically significant) and
of age (F(7,524) = 1050.26, statistically significant) on
the serum OC concentration. In Group A male and
female horses showed different trends of serum OC
concentration (Fig. 1); a higher serum OC
concentration in male (19.70 ± 0.36 ng/ml) than in
female was observed (18.37 ± 0.73 ng/ml) (t34 = 6.86,
statistically significant, unpaired Student t-test). In
Group B all horses showed the same trend of serum
OC concentration (Fig. 2); statistically significant
differences in serum OC concentration were observed
(F(2,53) = 45.31, statistically significant, one-way
ANOVA) between the genders (Figs 2-3). The higher
concentration was observed in males (13.96 ± 1.03
ng/ml) followed by geldings (12.30 ± 1.01 ng/ml) and
females (10.63 ± 1.10 ng/ml).
The application of the periodic model and the
statistical analysis of cosinor enabled us to define the
periodic parameters and their acrophases during the
48 h of monitoring. Group A did not show a daily
rhythm of serum OC concentration in both genders.
Group B showed a daily rhythm of serum OC
concentration in all genders. Statistical significant
differences of acrophases and robustness of rhythm
between the genders were observed. The mean level
for the four rhythmic parameters of serum OC levels
recorded during the 48 h of monitoring, with their
statistically differences, are reported in Fig. 3.
DISCUSSION
Our results showed a different bone metabolism in
horses of different ages and genders. A higher serum
OC concentration was observed in Group A than in
Group B. Serum OC is synthesized by osteoblasts and
reflects new bone formation. Therefore, its decrease
suggests a decrease in bone formation and resorption.
The immature growing skeleton of a 2 year old
Thoroughbred has neo-formative activity, and
maximal mineral content of the third metacarpal bone
is not reached until six years of age (Lawrence et al.
1994). More recent studies indicated a markedly
higher metabolism before 20 weeks of life
(Donabedian et al. 2008), the decrease in bone
metabolism persists during the first 200 days of life,
and seems to be related to the date of birth (Vervuert
et al. 2007).
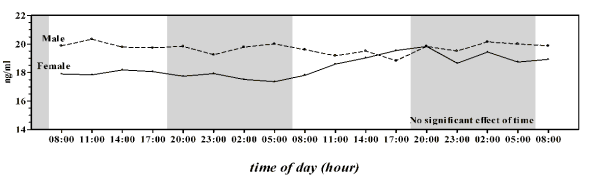
Fig. 1. Mean pattern of serum osteocalcin concentration in male and female Thoroughbred horses of Group A (2 year
olds). Each point represents the mean of six horses. The grey bar indicates the dark phase of the natural photoperiod.
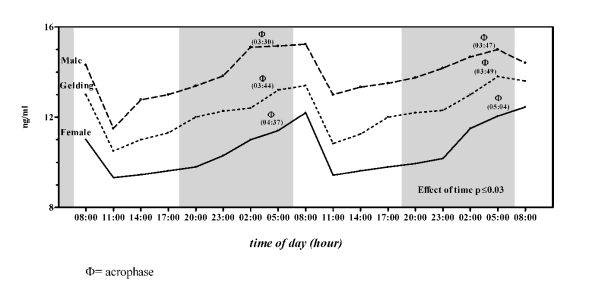
Fig. 2. Daily rhythms of serum osteocalcin concentration in male, female and gelding Thoroughbred horses of Group B
(6 year olds). Each point represents the mean of six horses. The grey bar indicates the dark phase of the natural photoperiod.
As previously reported by Lepage et al. (1991), in
Standardbred these data indicate a significant
slowdown in the rate of bone formation in adults
compared to foals. An age-related decrease of serum
OC had previously been described in female
Standardbred horses (Lepage et al. 1990) and in
humans (Kruse and Kracht 1986) in which the normal
range of serum OC is high in children and declines to adult levels with completion of puberty. In humans
the changes observed in serum OC at puberty showed
gender-related differences (Cole et al. 1985).
Influences of gender on serum OC concentrations
were also observed in Quarter horses (Fletcher et al.
2000) in contrast to the observations of Lepage et al.
(1992) which concluded that serum OC
concentrations of Standardbred horses less than
5 years of age were not affected by gender. The exact
hormonal mechanism that influence the serum OC
concentration in male and female horses during their
maturation period is not completely understood.
Fletcher et al. (2000) attributed gender differences to
the different levels of peripheral steroid hormone
concentration that act on the steroid receptors located
on the osteoblastic cells to a higher degree in females
than males and geldings.
The high rates of skeletal modelling and
remodelling during growth and the resulting greater
variability in bone marker concentrations were likely
to explain the lack of a circadian rhythm in young
Standardbred horses compared to adults (Black et al.
1999). In agreement with this, Fletcher et al. (2000)
also failed to observe any detectable change in serum
OC over a 24 hours sampling period in Quarter foals.
Hope et al. (1993) reported no significant changes in 24 hours serum OC concentrations in a study that
used animals covering a wide range of ages, whereas
Geor et al. (1995) observed no circadian rhythm in a
study of 3 to 5 years old Thoroughbreds. On the
contrary Jackson et al. (2003) reported in 2 years old
Thoroughbred a circadian rhythm of serum OC
concentration with an estimated peak time at 09:00.
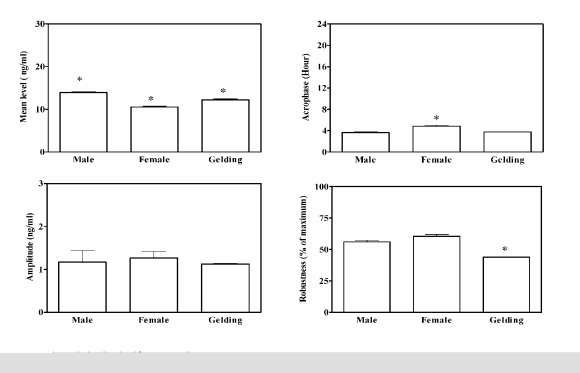
Fig. 3. Analysis of four rhythmic parameters of 48 h records of serum osteocalcin concentration in Group B (6 year olds). Each bar corresponds to the mean (±SD) of six horses of each gender in the 48 h of monitoring; * significant versus all.
Lepage et al. (1991) observed significant 24 hour
variation in serum OC concentrations in adult
Standardbred, and Black et al. (1999) also found
serum OC concentrations in adult geldings to exhibit
a significant circadian pattern. Studies in human and
animals have suggested that endogenous factors,
including hormones, may play an important role in
regulating daily rhythms in bone metabolism (Nielsen
et al. 1990, Ostrowska et al. 2002). It was noted by
Lepage et al. (1991) that circadian changes in serum
OC appeared to be related to photoperiod. They
suggest that physical activity does not explain the
differences between light and dark periods because
the activity of the horses was restricted to movements
in the box stall during the experiment. Also they
excluded an influence of meals on serum OC
concentration because after food it was not possible
to detect variations. In humans, it has been shown that
part of the circadian variation of serum OC may be
influenced by food intake (Schlemmer and Hassager,
1999, Bjarnason et al. 2002). On the basis of our
knowledge, in horses, the pattern of diurnal variation
was found to be affected by a number of endogenous
and exogenous factors, such as age, season, feeding
times and fasting, change in light intensity and
environmental temperature (Piccione et al. 2005,
2008a, Fazio et al. 2006, Bertolucci et al. 2008). To
find the exact zeitgeber it is necessary to test the
parameter under constant environmental conditions,
in which the circadian rhythms free-run with periods
slightly different from 24 hours, and to subject the
animals to different light/dark, temperature and
feeding schedules (Piccione et al. 2008b, Bertolucci
et al. 2009). Since circulating levels of serum OC
reflect bone turnover it has been proposed that
variations in the circulating pool may be linked to an
intrinsic rhythmic activity occurring in bone
(Gunderberg et al. 1985).
In summary, bone metabolism is different in
horses of different ages. It have a higher rate in 2 year
old than 6 year old Thoroughbreds. The different rate
of bone turnover influences also the circadian rhythm
of serum OC concentration, that is evident only in
mature Thoroughbreds. Within the same age-group
the influence of gender on serum OC concentration
has been observed. These differences were probably
due to the reproductive hormones, but no exact data
are present in the literature about their mechanism.
In conclusion, our results are indicative of the
existence of daily rhythms in osteoblastic activity.
Their activity peaks during the night and decreases in
the morning both in males and females, as well as in
geldings. The results, however, support the
interaction between sex hormones and bone
metabolism. Single samples from individuals are of
little value for monitoring marker activity; a series of
measurements should be taken over a period of time,
or samples should be collected at precise times for
results to be meaningful. Blood sampling for
determination of serum OC should be strictly
standardized with regard to the time of the day and
the time since last medication or intervention with
factors that may affect osteoblastic activity. Lack of
daily rhythm in serum OC of young horses suggests
that regulating the time of sampling for serum OC
determinations in metabolic studies may not be
necessary in skeletally immature horses.
REFERENCES
Aardal NP, Laerum OD: Circadian variation in mouse bone marrow. Exp Hematol 11:792-801, 1983.
Bertolucci C, Giannetto C, Fazio F, Piccione G: Seasonal variations in daily rhyhtms of activity in athletic horses. Animal 2:1055-1060, 2008.
Bertolucci C, Giudice E, Fazio F, Piccione G: Circadian intraocular pressure rhythms in athletic horses under different lighting regime. Chronobiol Int 26:348-358, 2009.
Bjarnason NH, Henriksen EE, Alexandersen P, Christgau S, Henriksen DB, Christiansen C: Mechanism of circadian variation in bone resorption. Bone 30:307-313, 2002.
Black A, Schoknecht PA, Ralston SL, Shapses SA: Diurnal variation and age differences in the biochemical markers of bone turnover in horses. J Anim Sci 77:75-83, 1999.
Cole DEC, Carpenter TO, Gundberg CM: Serum osteocalcin levels in children with metabolic bone disease. J Pediatr 106:770-776, 1985.
Donabedian M, Van Weeren PR, Perona G, Fluerance G, Robert C, Leger S, Bergero D, Lapage O, Martin-Rosset W: Early changes in biomarkers of skeletal metabolism and their association to the occurrence of osteochondrosis (OC) in the horse. Equine Vet J 40:1-7, 2008.
Fazio F, Assenza A, Crisafulli G, Piccione G, Caola G: The influence of exercise on daily rhythm of serum homocysteine in horses. J Physiol Sci 55:455-458, 2006.
Fletcher KL, Topliff DR, Cooper SR, Freeman DW, Geisert RD: Influence of age and sex on serum osteocalcin concentrations in horses at weaning and during physical conditioning. J Equine Vet Sci 20:124-126, 2000.
Geor R, Hope L, Lauper S, Piela S, Klassen J, King V, Murphy M: Effect of glucocorticoids on serum osteocalcin concentration in horses. Am J Vet Res 56:1201-1205, 1995.
Gertz BJ, Clemens DJ, Holland SD, Yuan W, Greenspan S: Application of a new serum assay for type-1 collagen cross-linked N-telopeptide: Assessment of diurnal changes in bone turnover with and without Alendronate treatment. Calcif Tissue Int 63:102-106, 1998.
Gunderberg CM, Markowitz ME, Mizruchi M, Rosen JF: Osteocalcin in human serum: a circadian rhythm. J Clin Endocrinol Metab 60:736-736, 1985.
Hassager C, Resteli J, Resteli L, Jensen SB, Christiansen C: Diurnal variation in serum markers of type I collagen synthesis and degradation in healthy premenopausal women. J Bone Miner Res 7:1307-1311, 1992.
Hope E, Johnston SD, Hegstad RL, Geor RJ, Murphy MJ: Effects of sample collection and handling on concentration of osteocalcin in equine serum. Am J Vet Res 54:1017-1020, 1993.
Inoue Y, Matsui A, Asai Y, Aoki F, Yoshimoto K, Matsui T, Yano H: Response of biochemical markers of bone metabolism to exercise intensity in Thoroughbred horses. J Equine Sci 19:83-89, 2008.
Jackson BF, Blumsohn A, Goodship AE, Wilson AM, Price JS: Circadian variation in biochemical markers of bone cell activity and insulin-like growth factor-I in two year old horses. J Anim Sci 81:2804-2810, 2003.
Jackson BF, Dyson PK, Lonnel C, Verheyen KLP, Pfeiffer DU, Pice JS: Bone biomarkers and risk of fracture in two- and three-year-old Thoroughbreds. Equine Vet J 41:410-413, 2009.
Jeffcott LB, Buckingham SH, McCarthy RN, Cleeland JC, Scotti E, McCartney RN: Non-invasive measurement of bone: a review of clinical and research applications in the horse. Equine Vet J 6:0S71-79, 1988.
Khosla S, Kleerekoper M: Biochemical markers of bone turnover. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 5th edition. Favus MJ (ed), American Society for Bone and Mineral Research, Washington, pp 166-172, 2003.
Kruse K, Kracht U: Evaluation of serum osteocalcin as an index of altered bone metabolism. Eur J Pediatr 145:27-33, 1986.
Lawrence LA, Ott EA, Miller GJ, Poulos PW, Piotrowski G, Asquith RL: The mechanical properties of equine third metacarpals as affected by age. J Anim Sci 72:2671-2623, 1994.
Lepage OM, Marcoux M, Tremblay A: Serum osteocalcin of bone Gla-protein, a biochemical marker for bone metabolism in horses: Differences in serum levels with age. Can J Vet Res 54:223-226, 1990.
Lepage OM, Des Coteaux L, Marcoux M, Tremblay A: Circadian rhythms of osteocalcin in equine serum. Correlation with alkaline phosphatase, calcium, phosphate and total protein levels. Can J Vet Res 55:5-10, 1991.
Lepage OM, Marcoux M, Tremblay A, Dumas G: Sex does not influence serum osteocalcin levels in Standardbred horses of different ages. Can J Vet Res 56:379-381, 1992.
Lepage OM, Carstanjen B, Uebelhart D: Non-invasive assessment of equine bone: an update. Vet J 161:10-22, 2001.
McIlwraith CW: Use of synovial fluid and serum biomarkers in equine bone and joint disease: a review. Equine Vet J 37:473-482, 2005.
Nelson W, Tong U, Lee J, Halberg F: Methods for cosinor rhythmometry. Chronobiologia 6:305-323, 1979.
Nielsen HK, Brixen K, Mosekilde L: Diurnal rhythm in serum activity of wheat-germ lectin-precipitable alkaline phosphatase: temporal relationships with the diurnal rhythm of serum osteocalcin. Scand J Clin Lab Invest 50:851-856, 1990.
Ohtsuka M, Saeki S, Igarashi K, Shinoda H: Circadian rhythms in the incorporation and secretion of 3H-Proline by odontoblasts in relation to uncremental lines in rat dentine. J Dental Res 77:1889-1895, 1998.
Ostrowska Z, Kos-Kudla B, Marek B, Kajdaniuk D, Ciesielska-Kopacz N: The relationship between the daily profile of chosen biochemical markers of bone metabolism and melatonin and other hormone secretion in rats under physiological conditions. Neuro Endocrinol Lett 23:417-425, 2002.
Pastoret V, Carstanjen B, Lejeune JP, Farnir F, Remy B, Reginster JY, Serteyn D, Gabriel A: Evaluation of serum osteocalcin and CTX_I in ardenner horses with special reference to Juvenile interphalangeal joint disease. J Vet Met A Physiol Pathol Clin Med 54:458-463, 2007.
Piccione G, Fazio F, Giudice E, Grasso F, Morgante M: Nycthemeral change of some haematological parameters in horses. J. Appl. Biomed. 3:123-128, 2005.
Piccione G, Giannetto C, Fazio F, Giudice E: Daily rhythm of tear production in normal horse. Vet Ophthalmol 1(Suppl. 1):57-60, 2008a.
Piccione G, Fazio F, Caola G, Refinetti R: Daily rhythmicity of glycemia in four species of domestic animals under various feeding regimes. J Physiol Sci 58:271-275, 2008b.
Price JS, Jackson B, Eastell R, Goodship AE, Blumsohn A, Wright I, Stoneham S, Lanyon LE, Russell RG: Age related changes in biochemical markers of bone metabolism in horses. Equine Vet J 27:201-207, 1995.
Refinetti R: Non-stationary time series and the robustness of circadian rhythms. J Theor Biol 227:571-581, 2004.
Refinetti R: Circadian Physiology. 2nd ed. Taylor & Francis Group, Boca Raton, 2006.
Schlemmer A, Hassager C: Acute fasting diminishes the circadian rhythm of biochemical markers of bone resorption. Eur J Endocrinol 140:332-337, 1999.
Vervuert I, Winkelsett S, Christmann L, Bruns E, Hoppen HO, Distl O, Hertsch B, Coenen M: Evaluation of the influences of exercise, birth date, and osteochondrosis on plasma bone marker concentrations in Hanoverian Warmblood foals. Am J Vet Res 68:1319-1323, 2007.
|
BACK
|




