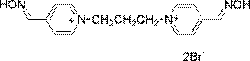Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 2, p 87-92
DOI 10.2478/v10136-009-0008-6
The effect of trimedoxime on acetylcholinesterase and on the cholinergic system of the rat bladder
Ondrej Soukup, Ondrej Holas, Jiri Binder, Kumar Killy, Gunnar Tobin, Daniel Jun, Josef Fusek, Kamil Kuca
Address: Kamil Kuca, Centre of Advanced Studies, Department of Toxicology, Faculty of Military Health Science, University of Defense,
Trebesska 1575, 500 01 Hradec Kralove, Czech Republic
kucakam@pmfhk.cz
Received 20th August 2009.
Published online 13th January 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
References
SUMMARY
Trimedoxime is a bisquaternary oxime that is widely used in the treatment of organophosphorous poisoning caused by tabun and paraoxon. We tested its affinity to acetylcholinesterase (AChE), its mechanism of interaction and effect on the cholinergic system of the rat bladder. The half maximal inhibitory concentration (IC50) of trimedoxime to recombinant AChE was found to be 82.0 mM ± 30.1 mM. This represents a weak inhibition. Its interaction with AChE seems to be very similar to obidoxime - one aromatic nucleus interacts with the peripheral anionic site and the other with the residues TYR337 and TYR341 inside the cavity. Also the oxime moiety is moving towards the catalytic triade ready for the reactivation of the inhibited AChE. In the organ bath experiment no significant effect of trimedoxime was observed on the contraction of the detrusor caused by the muscarinic agonist metacholine.
KEY WORDS
acetylcholinesterase; trimedoxime; antidote; muscarinic receptors; reactivation
INTRODUCTION
Organophosphorous compounds are an extremely
toxic group of substances. Members of this group are
nerve agents (sarin, soman, cyclosarin) and pesticides
(paraoxon, chlorpyrifos, parathion). Some of them are
used in agriculture (chlorpyrifos, paraoxon etc.), in
human and veterinary medicine as drugs, and as
military weapons (tabun, sarin, soman, cyclosarin),
most notably as nerve agents. The mechanism of OP
poisoning involves phosphorylation of the serine
hydroxyl group in the active site of the
acetylcholinesterase (AChE) (Marrs 1993). The
poisoning manifests as a cholinergic syndrome, and
the clinical effects of excessive stimulation of the
cholinoreceptors depend on their localization and the
type of receptor. Muscarinic, nicotinic and central
symptoms are typical of organophosphate poisoning
(Bajgar 2004). There are two main groups of
treatment for the poisoning: (1) anticholinergics such
as atropine are used as functional antidotes since they
are able to antagonize the effects of excessive ACh by
a blockade of mainly muscarinic receptors, and (2)
reactivators of AChE - oximes - are able to reactivate
inhibited AChE (Kassa 2002). Anticholinergics and
reactivators are usually administered together because
of their synergistic effect. There are a number of
compounds used in the treatment of organophosphate
poisoning - pralidoxime, trimedoxime, methoxime,
obidoxime and HI-6 (Kassa et al. 2009, Kuca et al.
2009). However, the reactivators differ in their
efficacy against individual nerve agents. No universal
antidote has yet been developed (Kuca et al. 2007,
Zdarova Karasova et al. 2009) and other mechanisms
not related to the reactivation are being investigated.
Trimedoxime (Fig. 1) is a bisquaternary oxime
that is widely used in the treatment of
organophosphorous poisoning caused by tabun
(Kassa 2002, Kassa et al. 2006). In vitro and in vivo
testing have shown its effect as significantly greater
than the others in the case of tabun poisonings (Cabal
et al. 2004, Kassa et al. 2005). Moreover it has very
good reactivation potency in vitro on paraoxon
inhibited AChE (Jun et al. 2008). Trimedoxime (and
obidoxime), although relatively toxic, are the most
potent in inducing reactivation of AChE inhibited by
the majority of organophosphorous pesticides. Since
there are currently no reports of controlled clinical
trials on the use of trimedoxime in human
organophosphate pesticide poisoning, obidoxime is
prefered in the treatment of pesticide poisoning
(Antonijevic and Stojiljkovic 2006).
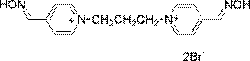
Fig. 1. Structure of trimedoxime bromide.
The high therapeutic efficacy of oximes, may in
addition to its reactivating potency, be also due to
other antidotal mechanisms based probably on direct
anticholinergic actions (Hamilton and Lundy 1989).
It has been discovered that oximes (HI-6) block the
nicotinic receptor and thus contribute to a recovery of
the diaphragm muscle from soman poisoning
(Tattersall 1993). Moreover examination of the
cholinolytic effect of trimedoxime has shown that a
blockade of open end-plate ionic channels underlies
the reactivating effect (Giniatullin et al. 1988).
The aim of this study was to investigate the
binding of trimedoxime to the AChE using docking
simulation, its inhibitory effect on AChE using
Ellman's method and also its influence on the whole
tissue containing muscarinic receptors. A contraction
study on rat bladder has been chosen for this purpose.
MATERIALS AND METHODS
Chemicals
Trimedoxime: TMB-4, 1,3-bis(4-hydroxyimino-methylpyridinium)propane dibromide previously
synthesized at the Department of Toxicology, Faculty
of Military Health Sciences, University of Defence,
Hradec Kralove, Czech Republic. Commercial
recombinant AChE (Sigma-Aldridge, Czech
Republic), acetylthiocholine,
5,5'-dithiobis(2-nitrobenzoic) acid (all
Sigma-Aldridge, Czech Republic), methacholine:
2-acetyloxypropyl-trimethyl-azanium
(Sigma-Aldridge, Czech Republic).
The assessment of AChE IC50
The activity of AChE was assessed by the standard
spectrophotometric Ellman's method with
acetylthiocholine iodide as substrates and use of
5,5'-dithiobis(2-nitrobenzoic) acid as chromogen
(Ellman et al. 1961); modified in wavelength
(412 nm). The spectrophotometer Helios Alpha
(Thermo Scientific, Czech Republic) was used for
determination of absorbance. The inhibitor (K112)
was added 10 min prior to measurement. The results
were calculated using the Origin 6.1 software
(OriginLab Corporation, Northampton, USA).
Docking studies
Docking studies were performed using the
AUTODOCK 3.0.5 (Morris et al. 1998) to deduce
conformation and orientation of trimedoxime in
AChE. The initial model of AChE (Mus musculus,
mAChE) for docking studies was built based on the
X-ray crystal structure of the obidoxime - AChE
complex which was obtained from the Protein Data
Bank (PDB entry 2GYW) (Ekstrom et al. 2006). The
original ligand was removed while water molecules
present in the PDB file were maintained in their
position. Residues with missing side chain atoms
were rebuilt using the Swiss-PdbViewer 4.0.
Hydrogen atoms were added to all amino acid
residues. The search and scoring grid for Autodock
was centered in the cavity. The grid size was set to
90 x 90 x 90 points with a spacing of 0.3 A. The
Lamarckian genetic search algorithm was performed
200 times by using a population size of 500, an upper
limit for the number of energy evaluations of
5 000 000 and the maximum number of generations
were set to 300 000. The parameters for mutation and
crossover were kept at their default settings of 0.02
and 0.80, respectively. The local-energy-minimization
algorithm was limited to 100 steps for 6% of the
population. To explore the conformational space of
ligands, the overall translation steps was set to 0.2 Å,
and the overall rotation and torsion rotation step were
set to 5 in the docking studies. RMS tolerance during
the clustering was set to 1.5 A to reduce the number
of clusters. All other values were used at default
settings. VMD software (NIH Center for Research
Resources, University of Illinois, USA) was used for
structure drawing.
Contraction study
The procedure has been described previously (Giglio
et al. 2007). In the current study, Sprague-Dawley
rats (250-300 g) were used. The rats were killed by
carbon dioxide and the bladders were dissected out
and bladder strips (6 x 2 mm) were prepared. The
detrusor strips were then mounted by a thread
between two steel rods where one was fixed and the
other adjustable and connected to an isometric force
transducer (Linton) in 25 ml organ baths. The organ
baths contained Krebs solution with the following
composition (in mM): NaCl 118, KCl 4.6, CaCl2 1.25,
KH2PO4 1.15, MgSO4 1.15, NaHCO3 25 and glucose
5.5 (all substances from Sigma Chemicals Co., St.
Louis, MO, USA), gassed with 5% CO2 in O2 and
kept at 37 °C by a thermo-regulated water circuit and
at pH 7.4. The detrusor strips were thereafter
pre-stretched and let at rest for 30 min to produce a
stable tension of around 5 mN. Data were recorded
using an MP100WSW data acquisition system and
Acquire software (Biopac, Goleta, USA).
Methacholine and trimedoxime were administered to
the organ baths at a volume of 200 microl and the
concentrations mentioned in the text refer to the final
ones in the organ bath chambers. The methacholine
concentration was increased in a sequential manner
from 10-6 M to 10-4 M. A subsequent dose of
methacholine was added immediately when the
maximal contractile response to the previous
administration could be observed. Trimedoxime was
added to the bath 30 min prior to the administration of
the agonist. Student's t-test (two populations) has
been applied.
RESULTS
IC50 for recombinant AChE was found 82.0 mM ±
30.1 mM (Fig. 2).
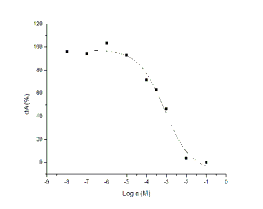
Fig. 2. Inhibitory potency of trimedoxime on AChE.
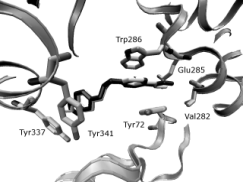
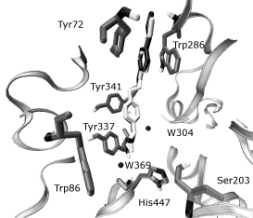
Figs 3, 4. Comparison of the obidoxime gained from the
crystal structure (white) with the results of docking
(black). The enzyme (PDB entry 2GYW) is represented by
the grey ribbon, selected residues by tubes, water molecules
are shown as black spheres.
We selected the crystal structure of the complex
Obidoxime-AChE for the docking study since
obidoxime and trimedoxime (TMB-4) are analogical
compounds. A similar interaction was expected. A
redocking procedure was used in order to verify that
the docking parameters specified in the input file for docking method are correct and able to recover a
known complex's structure and interactions (Figs 3,
4). Docking studies show that the peripheral
pyridinium ring is sandwiched via cation-
interactions with the side chains of Tyr124 and
Trp286. The oxime moiety is able to form H-bond to
the carbonyl oxygen of Val282, to the main-chain
nitrogen of Trp286 or of Glu285 according to actual
conformation. The central chain of trimedoxime is
accommodated in the active-site gorge. The
pyridinium ring that carries the attacking oxime forms
cation- interactions with the aromatic surface that is
created by the side chains of Tyr337 and Tyr341.The
oxime oxygen of trimedoxime can form the H-bond
to the water molecule W369 or to the W304 (Figs 5,
6).
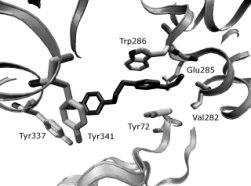
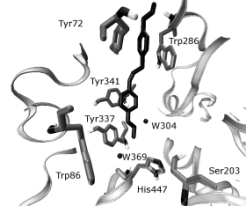
Figs 5, 6. Selected geometry of trimedoxime in the
AChE obtained by AutoDock simulation. The interaction
between the Peripheral anionic site (PAS; TRP286, ASP74,
TYR124, TYR72) of mAChE and trimedoxime (A), and the
corresponding interaction with the catalytic site (SER203
and TRP86) shown in (B). The enzyme is represented by
grey ribbon, selected residues by tubes, water molecules are
shown as black spheres.
Contractions of the detrusor showed a
concentration-dependent increase with the largest
response to methacholine 10-3 M. However,
trimedoxime does not cause any significant changes -
according to Student's t-test - during the
methacholine-evoked contractions (Fig. 7).
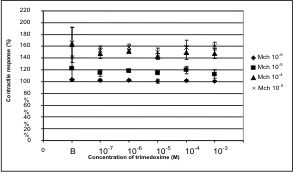
Fig. 7. Effect of trimedoxime on methacholine-evoked
contraction. B stands for basal response - in the absence
of trimedoxime.
DISCUSSION
Trimedoxime is a potent reactivator of AChE which
also seems to be a weak reversible inhibitor of AChE.
Compared to classic AChE inhibitors like donepezil
or tacrine (Ogura et al. 2000) it is a much weaker
inhibitor and compared to the reactivator HI-6,
trimedoxime is also weaker (Soukup et al. 2008). This
might be an advantage when comes to
organophosphorous poisoning, where a blockade of
AChE by organophosphates occurs.
Docking studies confirm the hypothesis that the
interaction with AChE is similar to obidoxime. One
aromatic nucleus interacts with the PAS and the other
with the residues TYR337 and TYR341 inside the
cavity. The oxime moiety is headed for the catalytic
triade ready for reactivation of the inhibited AChE.
However, obidoxime has been reported to be of
higher affinity to AChE than trimedoxime.
Methacholine is a potent muscarinic agonist with
also a slight effect on nicotinic receptors. It causes the
contraction of the smooth muscles via IP3 and Ca2+
(Collins and Crankshaw 1986). In the rat bladder
cholinergic system M2 and M3 subtypes are
predominant. Even though the M2 subtypes
outnumber the M3 subtype, it is mainly the M3
subtype that plays a key role in the contraction of the
bladder. The M2 subtype seems only to enhance the
contractile response to the M3 receptor activation
(Abrams et al. 2006 ).
In the tissue bath experiment, the effect of
trimedoxime on the whole cholinergic system (AChE
and muscarinic receptors) should have been evident.
However, comparison of the contractile responses in
the absence and in the presence of trimedoxime,
found no significant effect. Interestingly, after use of
a similar reactivator - HI-6 - in the same in vitro
experiment it was found that, at low concentrations an
inhibitory effect on AChE was observed, and at
higher concentrations an inhibitory effect on
muscarinic receptors predominated (Soukup et al.
2008). This might be due to a low inhibitory potency
on AChE or due to a balanced effect on AChE and on
muscarinic receptors.
ACKNOWLEDGEMENT
This work was supported by the grant of Ministry of
Defense (Czech Republic) No. FVZ0000604.
REFERENCES
Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, Kay G, Laties A, Nathanson NM, Pasricha PJ, Wein AJ: Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol 148:565-578, 2006.
Antonijevic B, Stojiljkovic MP: Unequal efficacy of pyridinium oximes in acute organophosphate poisoning. Clin Med Res 5:71-82, 2006.
Bajgar J: Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem 38:151-216, 2004.
Cabal J, Kuca K, Kassa J: Specification of the structure of oximes able to reactivate tabun-inhibited acetylcholinesterase. Basic Clin Pharmacol Toxicol 95:81-86, 2004.
Collins SM, Crankshaw DJ: Dissociation of contraction and muscarinic receptor binding to isolated smooth muscle cells. Am J Physiol 251:G546-552, 1986.
Ekstrom F, Pang YP, Boman M, Artursson E, Akfur C, Borjegren S: Crystal structures of acetylcholinesterase in complex with HI-6, Ortho-7 and obidoxime: Structural basis for differences in the ability to reactivate tabun conjugates. Biochem Pharmacol 72:597-607, 2006.
Ellman GL, Courtney KD, Andres V, Featherstone RM: A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88-95, 1961.
Giglio D, Aronsson P, Eriksson L, Tobin G: In vitro characterization of parasympathetic and sympathetic responses in cyclophosphamide-induced cystitis in the rat. Basic Clin Pharmacol Toxicol 100:96-108, 2007.
Giniatullin RA, Shabunova IA, Nikolskii EN, Bukharaeva EA: Reactivating and cholinolytic action of trimedoxime bromide at the neuromuscular-junction of warm-blooded animals. Neurophysiology 20:256-260, 1988.
Hamilton MG, Lundy PM: HI-6 therapy of soman and tabun poisoning in primates and rodents. Arch Toxicol 63:144-149, 1989.
Jun D, Musilova L, Kuca K, Kassa J, Bajgar J: Potency of several oximes to reactivate human acetylcholinesterase and butyrylcholinesterase inhibited by paraoxon in vitro. Chem Biol Interact 175:421-424, 2008.
Kassa J: Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J Toxicol Clin Toxicol 40:803-816, 2002.
Kassa J, Kuca K, Cabal J: Comparison of the efficacy of currently available oximes against tabun in rats. Biologia (Bratisl) 60:77-79, 2005.
Kassa J, Kuca K, Cabal J, Paar M: A comparison of the efficacy of new asymmetric bispyridinium oximes (K027, K048) with currently available oximes against tabun by in vivo methods. J Toxicol Environ Health A 69:1875-1882, 2006.
Kassa J, Zdarova Karasova J, Tesarova S: Evaluation of the neuroprotective efficacy of individual oxime (HI-6) and oxime mixtures (HI-6 + trimedoxime, HI-6 + K203) in tabun-poisoned rats. J Appl Biomed 7:189-199, 2009.
Kuca K, Jun D, Bajgar J: Currently used cholinesterase reactivators against nerve agent intoxication: Comparison of their effectivity in vitro. Drug Chem Toxicol 30:31-40, 2007.
Kuca K, Musilek K, Jun D, Pohanka M, Zdarova Karasova J, Novotny L, Musilova L: Could oxime HI-6 really be considered as "broad-spectrum" antidote? J Appl Biomed 7:143-149, 2009.
Marrs TC: Organophosphate poisoning. Pharmacol Ther 58:51-66, 1993.
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ: Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639-1662, 1998.
Ogura H, Kosasa T, Kuriya Y, Yamanishi Y: Comparison of inhibitory activities of donepezil and other cholinesterase inhibitors on acetylcholinesterase and butyrylcholinesterase in vitro. Methods Find Exp Clin Pharmacol 22:609-613, 2000.
Soukup O, Pohanka M, Tobin G, Jun D, Fusek J, Musilek K, Marek J, Kassa J, Kuca K: The effect of HI-6 on cholinesterases and on the cholinergic system of the rat bladder. Neuro Endocrinol Lett 29:759-762, 2008.
Tattersall JE: Ion channel blockade by oximes and recovery of diaphragm muscle from soman poisoning in vitro. Br J Pharmacol 108:1006-1015, 1993.
Zdarova Karasova J, Bajgar J, Novotny L, Kuca K: Is a high dose of Huperzine A really suitable for pretreatment against high doses of soman? J Appl Biomed 7:93-99, 2009.
|
BACK
|