Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 3, p 169-171
DOI 10.2478/v10136-009-0020-x
Involvement of the TGF-beta superfamily signalling pathway in hereditary haemorrhagic telangiectasia
Carmelo Bernabeu, Francisco Javier Blanco, Carmen Langa, Eva Maria Garrido-Martin, Luisa Maria Botella
Address: Carmelo Bernabeu, Centro de Investigaciones Biologicas, Ramiro de Maeztu, 9, Madrid 28040, Spain
bernabeu.c@cib.csic.es
Received 23rd February 2010.
Revised 29th April 2010.
Published online 13th May 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Hereditary haemorrhagic telangiectasia: clinical manifestations and genes involved
Regulated expression of HHT genes
Structure of endoglin and ALK1
Function of proteins encoded by HHT genes. The TGF-beta pathway
HHT therapeutic approaches. Molecular mechanisms of action
References
SUMMARY
Hereditary haemorrhagic telangiectasia (HHT) is a vascular hereditary autosomic dominant disease associated with epistaxis, telangiectases, gastrointestinal haemorrhages and arteriovenous malformations in lung, liver and brain. It affects 1-2 in 10,000 people. There are at least three different genes mutated in HHT, ENG, ACVRL1 and MADH4 that encode endoglin, activin receptor-like kinase (ALK1) and Smad4 proteins, respectively. These proteins are involved in the transforming growth factor (TGF)-beta superfamily signalling pathway of vascular endothelial cells. Mutations in ENG (HHT1) and ACVRL1 (HHT2) account for more than 90% of all HHT mutations. In this article, we review the underlying molecular and cellular bases and the therapeutic approaches that have been addressed in our laboratory in recent years.
KEY WORDS
transforming growth factor; endothelial cells; hereditary haemorrhagic telangiectasia; endoglin; ALK1; Smad; anti-fibrinolytic agents; estrogens
HEREDITARY HAEMORRHAGIC TELANGIECTASIA: CLINICAL MANIFESTATIONS AND GENES INVOLVED
Hereditary haemorrhagic telangiectasia (HHT) or
Rendu-Osler-Weber syndrome is a vascular
hereditary autosomic dominant disease associated
with epistaxis, telangiectases, gastrointestinal
haemorrhages and arteriovenous malformations in
lung, liver and brain. Prevalence is around 1-2 in
10,000 according to recent reviews (Abdalla and
Letarte 2006, Govani and Shovlin 2009). The disease
is included in the Online Mendelian Inheritance in
Man (OMIM; #187300; #600376; #175050). The
diagnosis is based on clinical criteria, known as the
Curaçao criteria (Shovlin et al. 2000). A person is
considered as an HHT patient if she/he has, at least 3
out of the following 4 criteria: (i) spontaneous and
recurrent epistaxis; (ii) multiple telangiectases at
characteristic locations (lips, oral cavity, fingers,
nose); (iii) visceral lesions (gastrointestinal
telangiectases, pulmonary, hepatic, cerebral or spinal
arteriovenous malformations-AVMs); or (iv) a first
degree relative with HHT. The penetrance of the
disease increases with age and at 45 years, is about
90% (Plauchu et al. 1989). Since HHT patients may
have lung and brain arteriovenous malformations
before the onset of epistaxis and telangiectasia, the
establishment of an early molecular diagnosis is
necessary. These malformations may give rise to
complications, such as brain ictus, brain infarction,
brain abscesses, massive haemoptysis and paralysis.
International guidelines for the diagnosis and
management of HHT have been recently reported
(Faughnan et al. 2010).
Two loci are involved by mutation in more than
90% of HHT cases. The first gene identified was
ENDOGLIN (ENG) that maps to chromosome 9
(Fernandez-Ruiz et al. 1993, McAllister et al. 1994),
representing between 39-59% of the total HHT
population. Next, ACVRL1 (activin receptor like
kinase 1, also known as ALK1) that maps to
chromosome 12 was described (Johnson et al. 1996)
as being involved in 25-57% of HHT cases.
Mutations in ENG and ACVRL1 give rise to HHT1
and HHT2 types, respectively. Up to date more than
700 different mutations have been described in ENG
and ALK1. In around 2% of the total HHT population,
the origin of the disease is a mutation in the MADH4
gene leading to the combined syndrome of Juvenile
Polyposis (JP) and HHT (JPHT), although an
overlapping spectra of MADH4 mutations in JP and
JPHT has been found (Gallione et al. 2010). Two
additional loci were described on chromosome 5 and
chromosome 7, whose genes are still unidentified
(Govani and Shovlin 2009). Haploinsufficiency is
accepted as the cause of HHT1 and HHT2
pathogenicity (Abdalla et al. 2006).
REGULATED EXPRESSION OF HHT GENES
Endoglin and ALK1 are expressed in endothelial cells
(ECs), which are the primary cell target in HHT.
Endoglin is expressed at low levels in resting ECs,
but at high levels in endothelial proliferating cells at
sites of active angiogenesis and during embryogenesis
(Bernabeu et al. 2009). Other cell types that express
endoglin at their surface are macrophages, erythroid
precursors in bone marrow, syncytiotrophoblasts and
several cell types closely related to the cardiovascular
system such as smooth muscle cells of atherosclerotic
plaques and cardiac fibroblasts (Bernabeu et al.
2007). The human ENG promoter does not contain
TATA or CAAT transcription initiation boxes but has
GC-rich regions and consensus sites for Sp1, Ets,
AP-2, NFB, GATA and Smad binding elements
(SBE) (Rius et al., 1998). The basal activity of ENG
transcription involves the proximal Sp1 motifs and an
Ets site at -68 (Rius et al. 1998, Botella et al. 2001).
Whereas the restricted expression to endothelium
requires the presence of enhancers that bind Ets
family members, a negative regulation involves the
presence of repressors that recruit Pu.1 and GATA-2
to inhibit ENG expression in blood stem/progenitors
(Pimanda et al. 2008).
Upregulated expression of endoglin was found in
inflamed or infected tissues, healing wounds,
psoriatic skin, synovial arthritis, upon vascular injury
and in tumoural vessels (Bernabeu et al. 2007,
Fonsatti et al. 2010). There is a variety of stimuli
responsible for the increased endoglin expression in
activated vessels, including hypoxia, vascular injury
and related cytokines. Indeed, endoglin expression is
upregulated after ischemia in the heart, kidney and
hind-limbs, as well as upon arterial injury (Botella et
al. 2002, Bernabeu et al 2007). Under hypoxic
conditions, the hypoxia inducible factor-1 (HIF-1)
complex binds a functional consensus hypoxia
responsive element (HRE) in the ENG gene promoter
(Sánchez-Elsner et al. 2002). TGF-beta signalling, via
Smad transcription factors, also potently stimulates
endoglin expression (Rius et al. 1998, Botella et al.
2001). Whereas hypoxia alone moderately stimulates
endoglin transcription, the addition of TGF-beta1 under
hypoxic conditions results in a transcriptional
cooperation between both signalling pathways,
leading to a marked stimulation of endoglin
expression. This synergic stimulation involves the
formation of a transcriptional multicomplex
containing Smad3/Smad4, Sp1, and HIF-1
(Sanchez-Elsner et al. 2002). Upon vascular injury, a
transcriptional activation of endoglin mediated by the
cooperative interaction between Sp1 and KLF6
transcription factors has been reported (Botella et al.
2002). Endoglin expression is also upregulated by
synthetic agonists of the liver X receptor alpha, which
binds to an LXR response element on the ENG
promoter, suggesting the in vivo involvement of
oxysterols, known as potent LXR activators
(Henry-Berger et al. 2008). By contrast, tumour
necrosis factor-alpha (TNF-alpha) decreases endoglin
protein levels in ECs (Bernabeu et al. 2007).
ALK1 expression has been reported not only in
highly vascularized tissues including lung, placenta,
and heart, but also at specific sites of
epithelial-mesenchymal interactions, and in other cell
types such as monocytes, microglia, skin fibroblasts,
stellate hepatic cells, chondrocytes, neural crest stem
cells and more recently myoblasts (Bernabéu et al.
2007, Velasco et al. 2008). Nonetheless, most studies
to date suggest that its major roles are related to the
endothelial specific expression pattern. ALK1 is
involved in angiogenesis and a regulatory region of
ACVRL1 gene is sufficient for endothelial expression
in arteries feeding ischemic tissues (Li et al. 2009).
The characterization of the ACVRL1 promoter and the
study of its transcriptional regulation remain largely
unknown. As in the case of ENG, the ACVRL1
proximal promoter does not contain TATA or CAAT
boxes, but has multiple GC-rich regions that recruit
Sp1 to regulate basal transcription. ALK1 presents
several transcriptional start sites and Sp1 is a key
factor involved in its transcriptional regulation.
Moreover, the methylation status of CpG islands
markedly modulates the activity of the ALK1
promoter region.
STRUCTURE OF ENDOGLIN AND ALK1
Both endoglin and ALK1 are type I membrane
proteins. Endoglin is expressed as a 180-kDa
disulfide-linked homodimer (Gougos and Letarte
1990). It contains a large extracellular domain of 561
amino acids, highly glycosylated mainly in asparagine
residues. Structurally, endoglin belongs to the Zona
Pellucida (ZP) family of proteins that share a ZP
domain of ~260 amino acid residues at their
extracellular region (Jovine et al. 2005, Llorca et al.
2007). The three-dimensional structure of the
extracellular domain of endoglin at 25Å resolution,
using single-particle electron microscopy has been
elucidated for the first time in our group (EMDB
Entry: EMD-1559) (Llorca et al. 2007). Endoglin is
arranged as a dome made of antiparallel orientated
monomers enclosing a cavity at one end. Each subunit
comprises three well-defined regions, two of them
corresponding to the ZP domain. The third region
does not show any significant homology to other
protein family/domain and thereby has been named
the "orphan" domain. A transmembrane region,
spanning 25 hydrophobic residues, acts as a linker
between the ectodomain and the cytosolic region.
Two different alternatively spliced isoforms, the
predominant long (L)-endoglin and the minor short
(S)-endoglin, are expressed in human and mouse
tissues (Gougos and Letarte 1990, Bellon et al. 2003,
Perez-Gomez et al. 2005). In humans, S-endoglin and
L-endoglin proteins vary from each other in their
cytoplasmic tails that contain 14 and 47 amino acids,
respectively, with a sequence of only 7 residues being
specific for S-endoglin. Both endoglin isoforms are
constitutively phosphorylated and can be targeted by
serine/threonine kinases, including the TGF-beta type I
(ALK1, ALK2 and ALK5) and II receptors
(Guerrero-Esteo et al. 2002, Bernabeu et al. 2007).
L-endoglin cytoplasmic domain contains a consensus
PDZ binding motif (SerSerMetAla) at the carboxyl
terminus that mediates endoglin interaction with
several PDZ domain-containing proteins and endoglin
phosphorylation of distal threonine residues
(Bernabeu et al. 2007, 2009).
ALK1 is a transmembrane protein of
approximately 55 kDa with an N-glycosylated
ectodomain of 97 amino acids carrying a cysteine-
rich small sequence which probably confers the
appropriate structural conformation to capture the
ligand. The ALK1 cytoplasmic region of 362 amino
acids contains (i) a GS domain, a conserved 30 amino
acids glycine/serine-rich sequence involved in the
regulation of the receptor activation and (ii) a
serine/threonine kinase domain. Phosphorylation of
serine/threonine residues of ALK1 in the GS domain
by the type II receptor (TbetaRII) leads to a
conformational change in ALK1 that allows
phosphorylation of the downstream signalling
molecules Smad1, Smad5 or Smad8 (Gordon and
Blobe 2008, Goumans et al. 2009). Although there
are no data about the three dimensional structure of
ALK1, it is possible to build a theoretical model of its
cytosolic domain using homology modelling
techniques based on the crystal structure of the type
I receptor ALK5 (PDB: 1IAS) (Fontalba et al. 2008).
The ALK1 structure of the cytosolic domain contains
the L45 loop, a small region that interacts with
Smads, which confers the signalling specificity
among different type I receptors. In addition, the
cytosolic region of ALK1 contains a consensus motif
between residues 399-406 for the interaction with the
scaffolding domain of caveolin-1, a major protein
component of caveolae (Santibanez et al. 2008). As
shown in Fig. 1, most of ALK1 mutations in HHT2
patients involve the cytoplasmic domain, at variance
with HHT1 where endoglin mutations map to the
extracellular domain (Fontalba et al. 2008).
FUNCTION OF PROTEINS ENCODED BY HHT GENES. THE TGF-BETA PATHWAY
The three identified genes mutated in HHT (ACVRL1,
ENG and MADH4) encode for proteins involved in
the TGF-beta signalling pathway (Gordon and Blobe
2008, Goumans et al. 2009, Govani and Shovlin
2009). Thus, ALK1 is a type I serine/threonine kinase
receptor, endoglin is an auxiliary co-receptor without
catalytic activity and Smad4 is a transcription factor
that mediates the TGF-beta signalling downstream of the
type I receptors (Fig. 2). Endoglin forms a protein
complex with the TGF-beta types I and II receptors and
the ligand. Several members of the TGF-beta
superfamily, including TGF-beta1, TGF-beta3, activin-A,
BMP-2, BMP-7 and BMP-9 are able to bind endoglin
and/or ALK1. This binding triggers the Smad-dependent downstream signalling
(Guerrero-Esteo et al. 2002, Blanco et al. 2005,
Gordon and Blobe 2008, David et al. 2009, Goumans
et al. 2009). In ECs, endoglin modulates ligand
binding and signalling by association with ALK1 and
ALK5 (Guerrero-Esteo et al. 2002, Blanco et al.
2005, Santibanez et al. 2007, Velasco et al. 2008).
Thus, endoglin inhibits the
TGF-beta/ALK5/Smad3-mediated cellular responses
such as the increased expression of the plasminogen
activator inhibitor 1 (PAI-1). By contrast, endoglin
promotes the ALK5/Smad2-mediated upregulation of
endothelial nitric oxide synthase (eNOS) as well as
the TGF-beta1/ALK1-mediated increase of Id1.
Interestingly, endoglin inhibits the BMP-9/ALK1
signalling in ECs. Overall, endoglin appears to be a
critical modulator of the balance between ALK1 and
ALK5 signalling. This balance plays a crucial role
during vascular remodelling and angiogenesis,
although the underlying molecular mechanisms
remain to be elucidated (Bernabeu et al. 2007, Lebrin
and Mummery 2008, David et al. 2009, Goumans et
al. 2009).
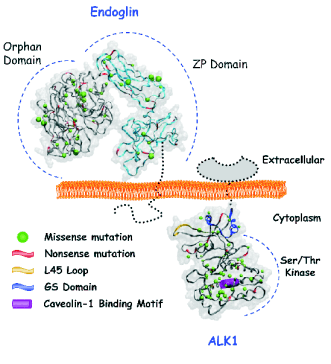
Fig. 1. Endoglin and ALK1 three dimensional structure and HHT mutations. The missense and nonsense mutations described
for the extracellular part of endoglin and the intracellular part of ALK1 are shown as green spheres and red segments,
respectively. The volume of the green spheres is exclusively related to the size of the mutated residue side chain. The L45 loop,
the GS domain and the caveolin-1 binding motif of ALK1 are also indicated.
Different studies support the view that endoglin
and ALK1 participate in a common signalling
pathway that is critical for EC responses to TGF-beta
family members (Bernabeu et al. 2007, 2009, Lebrin
and Mummery 2008). This conclusion agrees with the
fact that pathogenic mutations in ENG or ACVRL1
genes result in HHT and that ALK1 and endoglin null
mice have similar vascular phenotypes (Abdalla and
Letarte 2006). Recently, it has been shown that
S-endoglin is up-regulated during senescence of ECs
and exerts an antagonistic role to that described above
for L-endoglin. S-endoglin is able to interact with
both endothelial type I receptors, but showing much
more affinity for ALK5 than for ALK1.
Consequently, S-endoglin inhibits cellular
proliferation and promotes the expression of the
ALK5 target gene PAI-1, whereas the ALK1 target
Id1 is repressed (Blanco et al. 2008, Velasco et al.
2008).
Endoglin is involved in the control of vascular
tone. In fact, endoglin deficient mice (Eng+/-) show
decreased levels of eNOS and elevated expression of
cyclooxigenase-2 (COX-2), both of them key enzymes in the control of the vasodilator responses
(Jerkic et al. 2006, Santibanez et al. 2007). It is
noteworthy that transgenic mice expressing human
S-endoglin (S-ENG+) show a similar phenotype as
Eng+/- mice, in agreement with the opposing effects of
L-endoglin versus S-endoglin (Blanco et al. 2008).
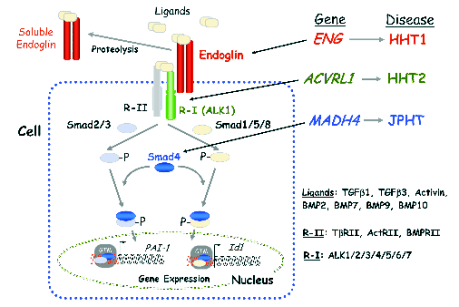
Fig. 2. HHT and the TGF-beta signalling pathway. Members of the TGF-beta family, which includes TGF-betas, activins and BMPs,
bind to specific type I (R-I) and type II (R-II) cell surface receptors that exhibit serine/threonine kinase activity. Endoglin is an
auxiliary receptor that associates with ligand, R-I and R-II and modulates signaling via R-I and R-II. A soluble form of endoglin
can be generated by juxtamembrane proteolysis of the membrane bound receptor that can sequester ligands and thereby
modulating their binding to R-I/R-II. The combinatorial heterodimeric association between R-I and R-II determines the specificity
of the ligand signalling. Upon ligand binding, the R-II transphosphorylates R-I, which subsequently propagates the signal by
phosphorylating the receptor-regulated Smad (R-Smad; Smad1, 2, 3, 5, 8) family of proteins. Once phosphorylated, R-Smads
form heteromeric complexes with a cooperating homologue named Co-Smad (Smad4), and translocate into the nucleus where
they regulate the transcriptional activity of target genes (Gordon and Blobe 2008, Goumans et al. 2009). In ECs the R-I, ALK1
and ALK5 activate signalling pathways via Smad1, 5, 8 (ALK1) or Smad2, 3 (ALK5), respectively. Endoglin, ALK1 and Smad4
proteins are encoded by ENG, ACVRL1 and MADH4 genes, whose pathogenic mutations give rise to HHT1, HHT2 and JPHT,
respectively. ActR, activin receptor; BMP, bone morphogenetic protein; BMPR, BMP receptor; GTM, general transcription
machinery.
Endoglin is also implicated in the cytoskeletal
organization. The cytoplasmic tail of L-endoglin
interacts with members of the LIM domain-containing
family of proteins, including zyxin and ZRP-1
(zyxin-related protein-1) (Sanz-Rodriguez et al.
2004). Both proteins serve as docking sites for the
assembly of multimeric protein complexes involved
in regulating cytoskeleton, assembly and cell motility.
Accordingly, blood outgrowth ECs from HHT
patients show an abnormal shape compared to
controls, exhibiting poor organization of the actin
cytoskeleton due to disorganized actin fibers and
depolymerization (Fernandez-L et al. 2005). The
organization of the capillary network during
angiogenesis depends on the structure of ECs so that
in the vasculature of HHT patients a disorganized
cytoskeleton is prone to cell breaking with changes in
shear stress and blood pressure. This might lead to
vessel haemorrhages and eventual disappearance of
the capillary network, as occurs in HHT.
Endoglin is emerging as a modulator of the TGF-beta
response with important roles in cancer. It is highly
expressed in the tumour-associated vascular
endothelium with prognostic significance in selected
neoplasias and is a vascular target for antiangiogenic
cancer therapy (Bernabeu et al. 2009, Fonsatti et al.
2010). On the other hand, expression of endoglin in
the tumour cells appears to play an important role in
the progression of cancer, influencing cell
proliferation, motility, invasiveness and
tumorigenicity ( Perez-Gomez et al. 2005, Wong et al.
2008, Bernabeu et al. 2009). In addition, in vitro and
in vivo experiments in which endoglin expression is
modulated have provided evidence that it acts as a
tumour suppressor (Perez-Gomez et al. 2007).
Increased levels of soluble endoglin have been
detected in plasma, serum and urine from patients
with different pathologies, including pre-eclampsia
and cancer (Bernabéu et al. 2009). Circulating soluble
endoglin is a reliable marker of preeclampsia and is
associated with poor prognosis in cancer. Whereas it
has been postulated a pathogenic role for soluble
endoglin in preeclampsia due to its anti-angiogenic
activity, the role of soluble endoglin in tumour
progression remains to be established (Perez-Gomez
et al. 2007).
HHT THERAPEUTIC APPROACHES. MOLECULAR MECHANISMS OF ACTION
So far, there is no cure for HHT and there is a need to
find an effective drug for its treatment. The
pharmacological therapeutic strategies should be
ideally aimed at: (i) improving the coagulation
process or preventing the haemorrhagic condition; (ii)
increasing the amount of endoglin or ALK1 on the
EC surface, since the pathogenesis of the HHT
condition is due to haplo-insufficiency; and (iii)
decreasing angiogenesis, because an excess of
abnormal angiogenesis has been reported in the HHT
condition. There are five different types of
pharmacological drugs used for the treatment of HHT
bleeding (Table 1). In this review, we will focus on
the clinical and molecular data regarding
antifibrinolytic drugs and raloxifene, therapeutic
agents used in our research group.
The basis for the efficiency of antifibrinolytic
agents, epsilon aminocaproic and tranexamic acid
(TA) relies on the inhibition of the fibrinolytic
activity, by binding the active center of plasmin in the
tissues that leads to clot stabilization. TA is a
derivative of lysine (4-aminomethyl
cyclohexanecarboxylic acid), which binds reversibly
to plasminogen, avoiding fibrin degradation by
plasmin (Manucci 1998). TA is indicated in severe
bleedings with hyperfibrinolysis as it is the case of
HHT, showing hyperfibrinolysis secondary to
intravascular coagulation. Previous studies have
described the use of TA for the treatment of HHT
patients with an improvement in epistaxis and the
associated anaemia (Sabba et al. 2001).
Since 2003, more than 250 patients from more
than 100 different families have been screened by the
HHT unit in Sierrallana Hospital (Cantabria, Spain).
A pilot series of oral TA treatment was conducted
with a total of 14 patients which had severe epistaxis
interfering with their quality of life. In all these cases
side-effect risks of thrombosis were absent. All
patients showed a decrease in the intensity and
frequency of nose bleeds after the first week of
treatment. None of them have presented adverse
side-effects until now (Fernandez-L et al. 2007).
However, there is a contraindication for TA in those
patients prone to suffer thrombosis. In patients with
high levels of coagulation factors, therapies that avoid
bleeding may lead to deep venous thromboembolism,
therefore alternative therapeutic sources to counteract
HHT epistaxis are needed. Hormonal therapy, using
estradiol/norethindrone for epistaxis and
gastrointestinal management of HHT, has shown a
variable degree of efficacy depending on the patient.
A case based report with long-term cessation of
epistaxis using tamoxifen in a postmenopausal
woman was described (Zacharski et al. 2001). Based
on these reports, the efficacy of raloxifene, another
estrogen receptor modulator (SERM) as tamoxifen,
was assessed in 19 postmenopausal HHT women
(Albinana et al. 2010). Raloxifene, a second
generation SERM, exhibits an improved clinical
profile versus that of tamoxifen and is currently used
for the treatment and prevention of post-menopausal
osteoporosis. These HHT women patients diagnosed
with osteoporosis with ages ranging from 47 to 74
years, had no contraindication for the hormonal
therapy, and were good candidates for a hormonal
substitutive therapy. After the treatment, all of them
showed an improvement of the HHT symptoms
concerning epistaxis. The effects on epistaxis were
evaluated after 6 months based on the
Sadick-designed scale. This scale evaluates the
amount and frequency of nose bleeds. In the nineteen
patients treated with raloxifene, a decrease in the
frequency and the quantity of epistaxis was observed
in all patients with at least one grade in the Sadick
scale: average of 2.36 versus 1.31 after treatment and
2.26 versus 1.42 after treatment, respectively
(Albinana et al. 2010).
Using in vitro cellular experiments, we have also
addressed the possible molecular mechanism of
action of TA and raloxifene. Both, TA and raloxifene
upregulate endoglin and ALK1 protein and mRNA
levels as well as their gene promoter activities,
suggesting a positive effect on gene transcription. In
this regard, we were able to show by chromatin
immunoprecipitation experiments that estrogen
receptors are involved in the raloxifene-induced
transcription. In addition, tube formation in matrigel
and wound healing experiments indicate that TA and raloxifene promote functions dependent on the
TGF-beta/ALK1/endoglin pathway. The mechanism by
which these drugs are able to stimulate this TGF-beta
pathway remains to be elucidated (Fernandez-L et al.
2007, Albinana et al. 2010). In summary, these
experiments support the hypothesis that TA and
raloxifene are counteracting, at least partially,
endoglin or ALK1 haploinsufficiency in HHT
patients.
Table 1. Different types of pharmacological drugs used to treat bleeding in HHT Centers.
| Therapeutic strategy |
Observations | | Antifibrinolytics |
-aminocaproic acid (AC), tranexamic acid (TA).
Not appropriate when patients have pro-thrombotic clinical history, or high
level of coagulation factors V and VIII. | | Hormonal estrogen therapy |
Estrogen/progesterone, ethinyl, estradiol/norethindrone, danazol,
phyto-estrogens, SERM (Selective Estrogen Receptor Modulator) like
tamoxifene and raloxifene. | | Anti-angiogenic drugs (anti-VEGF) |
Thalidomide, bevacizumab (avastin).
Currently used in trials. Only for severe cases. | | Immunosuppressant agents |
Sirolimus, tacrolimus, IFNgamma.
Only case reports available on kidney/liver HHT transplanted patients. Not
enough information up to the moment. | | Anti-inflammatory/antioxidant
compounds |
N-acetylcysteine |
REFERENCES
Abdalla SA, Letarte M: Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease. J Med Genet 43:97-110, 2006.
Albinana V, Bernabeu-Herrero ME, Zarrabeitia R, Bernabeu C, Botella LM: Estrogen therapy for hereditary haemorrhagic telangiectasia (HHT): Effects of raloxifene, on Endoglin and ALK1 expression in endothelial cells. Thromb Haemost 103:525-534, 2010.
Bellon T, Corbi A, Lastres P, Cales C, Cebrian M, Vera S, Cheifetz S, Massague J, Letarte M, Bernabeu C: Identification and expression of two forms of the human transforming growth factor-beta-binding protein endoglin with distinct cytoplasmic regions. Eur J Immunol 23:2340-2345, 1993.
Bernabeu C, Conley BA, Vary CP: Novel biochemical pathways of endoglin in vascular cell physiology. J Cell Biochem 102:1375-1388, 2007.
Bernabeu C, Lopez-Novoa JM, Quintanilla M: An emerging role of TGF-beta co-receptors in cancer. Biochem Biophys Acta 1792:954-973, 2009.
Blanco FJ, Santibanez JF, Guerrero-Esteo M, Langa C, Vary CP, Bernabeu C: Interaction and functional interplay between endoglin and ALK-1, two components of the endothelial transforming growth factor-beta receptor complex. J Cell Physiol 204:574-584, 2005.
Blanco FJ, Grande MT, Langa C, Oujo B, Velasco S, Rodriguez-Barbero A, Perez-Gomez E, Quintanilla M, Lopez-Novoa JM, Bernabeu C: S-endoglin expression is induced in senescent endothelial cells and contributes to vascular pathology. Circ Res 103:1383-1392, 2008.
Botella LM, Sanchez-Elsner T, Rius C, Corbi A, Bernabeu C: Identification of a critical Sp1 site within the endoglin promoter and its involvement in the transforming growth factor-beta stimulation. J Biol Chem 276:34486-3494, 2001.
Botella LM, Sanchez-Elsner T, Sanz-Rodriguez F, Kojima S, Shimada J, Guerrero-Esteo M, Cooreman MP, Ratziu V, Langa C, Vary CP, Ramirez JR, Friedman S et al.: Transcriptional activation of endoglin and transforming growth factor-beta signaling components by cooperative interaction between Sp1 and KLF6: their potential role in the response to vascular injury. Blood 100:4001-4010, 2002.
David L, Feige JJ, Bailly S: Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev 20:203-212, 2009.
Faughnan ME, Palda VA, Garcia-Tsao G, Geisthoff UW, McDonald J, Proctor DD, Spears J, Brown DH, Buscarini E, Chesnutt MS, Cottin V, Ganguly A, et al.: International Guidelines for the Diagnosis and Management of Hereditary Hemorrhagic Telangiectasia. J Med Genet published online June 23, 2009 doi:10.1136/jmg.2009.069013
Fernandez-L A, Sanz-Rodriguez F, Zarrabeitia R, Perez-Molino A, Hebbel RP, Nguyen J, Bernabeu C, Botella LM: Blood outgrowth endothelial cells from hereditary haemorrhagic telangiectasia patients reveal abnormalities compatible with vascular lesions. Cardiovasc Res 68:235-248, 2005.
Fernandez-L A, Garrido-Martin EM, Sanz-Rodriguez F, Ramirez JR, Morales-Angulo C, Zarrabeitia R, Perez-Molino A, Bernabeu C, Botella LM: Therapeutic action of tranexamic acid in hereditary haemorrhagic telangiectasia (HHT): regulation of ALK-1/endoglin pathway in endothelial cells. Thromb Haemost 97:254-262, 2007.
Fernandez-Ruiz E, St-Jacques S, Bellon T, Letarte M, Bernabeu C: Assignment of the human endoglin gene (END) to 9q34>qter. Cytogenet Cell Genet 64:204-207, 1993.
Fonsatti E, Nicolay HJ, Altomonte M, Covre A, Maio M: Targeting cancer vasculature via endoglin/cd105: a novel antibody-based diagnostic and therapeutic strategy in solid tumors. Cardiovasc Res 86:12-19, 2010.
Fontalba A, Fernandez-L A, Garcia-Alegria E, Albinana V, Garrido-Martin EM, Blanco FJ, Zarrabeitia R, Perez-Molino A, Bernabeu-Herrero ME, Ojeda ML, Fernandez-Luna JL, Bernabeu C et al.: Mutation study of Spanish patients with hereditary hemorrhagic telangiectasia. BMC Med Genet 9:75, 2008.
Gallione C, Aylsworth AS, Beis J, Berk T, Bernhardt B, Clark RD, Clericuzio C, Danesino C, Drautz J, Fahl J, Fan Z, Faughnan ME et al.: Overlapping spectra of SMAD4 mutations in juvenile polyposis (JP) and JP-HHT syndrome. Am J Med Genet A. 152A:333-339, 2010.
Gordon KJ, Blobe GC: Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta 1782:197-228, 2008.
Gougos A, Letarte M: Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem 265:8361-8364, 1990.
Goumans MJ, Liu Z, ten Dijke P: TGF-beta signaling in vascular biology and dysfunction. Cell Res 19:116-127, 2009.
Govani FS, Shovlin CL: Hereditary haemorrhagic telangiectasia: a clinical and scientific review. Eur J Hum Genet 17:860-871, 2009.
Guerrero-Esteo M, Sanchez-Elsner T, Letamendia A, Bernabeu C: Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor-beta receptors I and II. J Biol Chem 277:29197-29209, 2002.
Henry-Berger J, Mouzat K, Baron S, Bernabeu C, Marceau G, Saru JP, Sapin V, Lobaccaro JM, Caira F: Endoglin (CD105) expression is regulated by the liver X receptor alpha (NR1H3) in human trophoblast cell line JAR. Biol Reprod 78:968-975, 2008.
Jerkic M, Rivas-Elena JV, Santibanez JF, Prieto M, Rodriguez-Barbero A, Perez-Barriocanal F, Pericacho M, Arevalo M, Vary CP, Letarte M, Bernabeu C, Lopez-Novoa JM: Endoglin regulates cyclooxygenase-2 expression and activity. Circ Res 99:248-256, 2006.
Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, Guttmacher AE, Jackson CE et al.: Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet 13:189-195, 1996.
Jovine L, Darie CC, Litscher ES, Wassarman PM: Zona pellucida domain proteins. Annu Rev Biochem 74:83-114, 2005.
Lebrin F, Mummery CL: Endoglin-mediated vascular remodeling: mechanisms underlying hereditary hemorrhagic telangiectasia. Trends Cardiovasc Med 18:25-32, 2008.
Li X, Yonenaga Y, Seki T: Shortened ALK1 regulatory fragment maintains a specific activity in arteries feeding ischemic tissues. Gene Ther 16:1034-1041, 2009.
Llorca O, Trujillo A, Blanco FJ, Bernabeu C: Structural model of human endoglin, a transmembrane receptor responsible for hereditary hemorrhagic telangiectasia. J Mol Biol 365:694-705, 2007.
Mannucci PM: Hemostatic drugs. N Engl J Med 339:245-253, 1998.
McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, McCormick MK, Pericak-Vance MA et al.: Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 8:345-351, 1994.
Perez-Gomez E, Eleno N, Lopez-Novoa JM, Ramirez JR, Velasco B, Letarte M, Bernabeu C, Quintanilla M: Characterization of murine S-endoglin isoform and its effects on tumor development. Oncogene 24:4450-4461, 2005.
Perez-Gomez E, Villa-Morales M, Santos J, Fernandez-Piqueras J, Gamallo C, Dotor J, Bernabeu C, Quintanilla M: A role for endoglin as a suppressor of malignancy during mouse skin carcinogenesis. Cancer Res 67:10268-10277, 2007.
Pimanda JE, Chan WY, Wilson NK, Smith AM, Kinston S, Knezevic K, Janes ME, Landry JR, Kolb-Kokocinski A, Frampton J, Tannahill D, Ottersbach K et al.: Endoglin expression in blood and endothelium is differentially regulated by modular assembly of the Ets/Gata hemangioblast code. Blood 112:4512-4522, 2008.
Plauchu H, de Chadarevian JP, Bideau A, Robert JM: Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet 32:291-297, 1989.
Rius C, Smith JD, Almendro N, Langa C, Botella LM, Marchuk DA, Vary CP, Bernabeu C: Cloning of the promoter region of human endoglin, the target gene for hereditary hemorrhagic telangiectasia type 1. Blood 92:4677-4690, 1998.
Sabba C, Gallitelli M, Palasciano G: Efficacy of unusually high doses of tranexamic acid for the treatment of epistaxis in hereditary hemorrhagic telangiectasia. N Engl J Med 345:926, 2001.
Sanchez-Elsner T, Botella LM, Velasco B, Langa C, Bernabeu C: Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-beta pathways. J Biol Chem 277:43799-43808, 2002.
Santibanez JF, Letamendia A, Perez-Barriocanal F, Silvestri C, Saura M, Vary CP, Lopez-Novoa JM, Attisano L, Bernabeu C: Endoglin increases eNOS expression by modulating Smad2 protein levels and Smad2-dependent TGF-beta signaling. J Cell Physiol 210:456-468, 2007.
Santibanez JF, Blanco FJ, Garrido-Martin EM, Sanz-Rodriguez F, del Pozo MA, Bernabeu C: Caveolin-1 interacts and cooperates with the transforming growth factor-beta type I receptor ALK1 in endothelial caveolae. Cardiovasc Res 77:791-799, 2008.
Sanz-Rodriguez F, Guerrero-Esteo M, Botella LM, Banville D, Vary CP, Bernabeu C: Endoglin regulates cytoskeletal organization through binding to ZRP-1, a member of the Lim family of proteins. J Biol Chem 279:32858-32868, 2004.
Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H: Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 91:66-67, 2000.
Velasco S, Alvarez-Munoz P, Pericacho M, Dijke PT, Bernabeu C, Lopez-Novoa JM, Rodriguez-Barbero A: L- and S-endoglin differentially modulate TGFbeta1 signaling mediated by ALK1 and ALK5 in L6E9 myoblasts. J Cell Sci 121:913-919, 2008.
Wong VC, Chan PL, Bernabeu C, Law S, Wang LD, Li JL, Tsao SW, Srivastava G, Lung ML: Identification of an invasion and tumor-suppressing gene, Endoglin (ENG), silenced by both epigenetic inactivation and allelic loss in esophageal squamous cell carcinoma. Int J Cancer 123:2816-2823, 2008.
Zacharski LR, Dunbar SD, Newsom WA, Jr.: Hemostatic effects of tamoxifen in HHT. Thromb Haemost 85:371-372, 2001.
|
BACK
|



