Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 3, p 131-140
DOI 10.2478/v10136-009-0016-6
Streptococcus pneumoniae: from molecular biology to host-pathogen interactions
Pedro Garcia, Miriam Moscoso, Violeta Rodriguez-Cerrato, Jose Yuste, Ernesto Garcia
Address: Ernesto Garcia, Centro de Investigaciones Biologicas, CSIC, Ramiro de Maeztu, 9, 28040 Madrid, Spain
e.garcia@cib.csic.es
Received 23rd February 2010.
Revised 8th April 2010.
Published online 6th May 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Choline and cell wall hydrolases
Pneumococcal biofilms
Host-pathogen interactions
Enzybiotics and phage therapy
Acknowledgements
References
SUMMARY
Streptococcus pneumoniae is the main cause of community acquired pneumonia and also produces meningitis, bacteremia, and otitis media, among others. Worldwide, these infections are the cause of substantial morbidity and mortality. Many different virulence factors have been described and most of them are surface-located macromolecules, namely, the capsular polysaccharide and various pneumococcal proteins. Cell wall hydrolases (CWHs) specifically cleave covalent bonds of the peptidoglycan and associated polymers: most CWHs are choline-binding proteins (CBPs) and are among the most well-known surface proteins. Pneumococcal CBPs have been investigated due to their role in pathogenesis and as candidate antigens for improved vaccines. Among the complex host-parasite interactions characteristic of pneumococcal disease, nasopharyngeal colonization is the first step. CBPs appear to play a central role in the development of the carrier state, possibly by affecting biofilm formation and development. Although the role of biofilms in the pathogenesis of some chronic human infections is currently widely accepted, the molecular bases underlying the formation of pneumococcal biofilms are being only recently being studied. Among therapeutic strategies to combat multidrug-resistant pneumococcal infections, the use of purified phage- or bacteria-encoded CWHs both in vitro and in animal models is under investigation.
KEY WORDS
Pneumococcus; cell wall hydrolases; choline; phage therapy; biofilm; enzybiotics
INTRODUCTION
Streptococcus pneumoniae, a Gram-positive
facultative anaerobe, is currently one of the most
important human pathogens and a worldwide leading
cause of bacterial pneumonia, meningitis, and sepsis.
The bacterium is carried asymptomatically in the
nasopharynx of healthy children and, less frequently,
of healthy adults, with colonization beginning shortly
after birth. In the industrialized countries, serious
pneumococcal disease occurs mainly in children
below the age of five, the elderly, and
immunocompromised patients. In developing
countries, these diseases are common in children
under five, including newborn infants; but the rates of
the disease in the elderly population are largely
unknown. Respiratory infections are responsible for
the death of 4 million persons each year, and S.
pneumoniae is the predominant species in these
infections. Although in developed countries a
substantial reduction in pneumonia mortality during
the 20th century has been observed, in the low-income
countries of Asia and Africa, pneumonia is still the
main cause of child death. In developing countries,
over one-quarter of children have an episode of
clinical pneumonia each year throughout the first
5 years of their lives (Scott et al. 2008). Recent
estimates in children aged 1-59 months reported
about 14.5 million episodes of serious pneumococcal
disease and deaths ranging from 700,000 to 1 million
every year worldwide (O'Brien et al. 2009). Of the
14.5 million pneumococcal cases, 95% were
attributable to pneumonia.
Even though S. pneumoniae mutants with
increased resistance to penicillin had been isolated in
vitro and in vivo from experimental animals in the
1940s, clinical pneumococcal isolates were uniformly
susceptible to low concentrations of this antibiotic
until the mid-sixties. Two decades were to pass
before reports of resistant isolates from humans were
described in the United States and Australia.
Gradually, the spread of penicillin and
multiple-antibiotic resistant pneumococcal strains
became worldwide. In the United States, about 44%
of all pneumococcal strains are now resistant to
-lactam antibiotics; in Spain, about 41% are
resistant. This situation has prompted the
development of new anti-infectives for the treatment
of pneumococcal infections, particularly those
produced by multi-drug resistant pneumococci
(Maestro and Sanz 2007).
Growing resistance of S. pneumoniae to
conventional antibiotics emphasizes the urgent need
for new antipneumococcal drugs and vaccines to
control pneumococcal disease. The pneumococcal
capsular polysaccharide (CPS) is immunogenic and
induces type-specific protective immunity (Lopez
2006). Although a 23-valent non-conjugated CPS
vaccine for use in adults and children aged 5 years
and a heptavalent protein-polysaccharide conjugate
vaccine (designed for pediatric use) are currently
available, they are far from satisfactory. Pronounced
herd immunity has resulted in a decrease in invasive
pneumococcal diseases in vaccinees and
non-vaccinees and reduced antibiotic resistance rates.
However, recent studies report that serotypes
eradicated by the vaccines are being replaced by
non-vaccine pneumococcal serotypes. This so-called
'serotype replacement' might soon threaten the
success of vaccine use (Dagan 2009).
Our group has been working during more than
30 years on the molecular biology of S. pneumoniae,
namely in surface proteins involved in virulence (e.g.,
cell wall hydrolases; CWHs), CPS biosynthesis and
regulation, and its bacteriophages. As most of the
results obtained up to 2004 have been reviewed
(Lopez and Garcia 2004, Lopez et al. 2004a, Lopez et
al. 2004b, Garcia et al. 2005, Hermoso et al. 2007), in
the present review we will update our current research
efforts aimed at further insight into the
structure-function relationships of pneumococcal
CWHs and their role in virulence. Furthermore, the
state-of-the-art of biofilm development and regulation
as well as the therapeutic value of bacterium- and
phage-encoded CWHs will be discussed in the
framework of host-S. pneumoniae interplay.
CHOLINE AND CELL WALL HYDROLASES
S. pneumoniae has a unique physiological trait among
prokaryotes: it exhibits an absolute nutritional
requirement for choline. This aminoalcohol
incorporates as phosphorylcholine (PC) in the cell
wall teichoic acid (TA) and membrane lipoteichoic
acid. Depending on the particular strain, each repeat
unit of TA contains one or two PC residues. We have
studied some aspects of this auxotrophy and
confirmed the key role of TacF protein (a TA repeat
unit transporter) in the choline-dependent phenotype.
Besides this, we have also demonstrated that at least
two tacF mutations are required to confer an
improved fitness to the choline-independent
pneumococcal strains when growing in medium
lacking any aminoalcohol (Gonzalez et al. 2008).
S. pneumoniae contains several CWHs, enzymes
that degrade specific linkages of the cell wall. PC acts
as an anchor for a special class of surface-located
proteins, referred to as choline-binding proteins
(CBPs), through non-covalent interactions. Most
pneumococcal CWHs are CBPs and harbor two
functional modules: one is responsible of the substrate
binding and the other determines the site of action.
S. pneumoniae produces 13 to 16 CBPs depending on
the particular strain. The choline-binding module
(CBM) is formed by several repeats (choline-binding
repeats; CBRs), with the consensus motif
GWXK-X4-5-WYY-phi-X3-5-GXMX2-3, where X is any
residue and phi is a hydrophobic residue
(http://pfam.sanger.ac.uk/family?PF01473). The
relevant characteristics of the pneumococcal CBPs
mentioned in this review are summarized in Table 1.
The first crystal structure of a CBM showed that the
COOH- (C-)terminal module of the LytA major
pneumococcal autolysin, an N-acetylmuramoyl-
L-alanine amidase (NAM-amidase), adopts a peculiar
beta-solenoid structure (Fernandez-Tornero et al. 2001).
Each CBR comprises a beta-hairpin followed by a loop and a coiled region (Fig. 1a).
Choline-binding sites, as described for other CBPs,
are located at the interface of two consecutive CBRs,
where three structurally conserved aromatic residues
form a cavity in which the choline quaternary
ammonium moiety is stabilized primarily by cation-pi
interactions.
Table 1. Characteristics of selected pneumococcal and phage cell wall hydrolases.
| Protein |
Gene |
Signal
peptide |
Number of
amino acid
residuesa |
Molecular
mass (kDa)b |
Catalytic
activity |
Number
of CBRsc | | S. pneumoniaed |
|
|
|
|
|
| | LytA |
spr1754 |
No |
318 |
36.6 |
NAM-amidase |
7 (C) | | LytB |
spr0867 |
Yes |
679 (702) |
79.3 (81.9) |
endo--N-
acetylglucosaminidase |
18 (N) | | LytC |
spr1431 |
Yes |
468 (490) |
55.2 (57.4) |
Lysozyme |
11 (N) | | Pce
(CbpE) |
spr0831 |
Yes |
602 (627) |
69.4 (72.1) |
Phosphorylcholine
esterase |
10 (C) | | CbpF
(CbpC) |
spr0337 |
Yes |
311 (338) |
36.3 (39.3) |
NDe |
5 (C) | | CbpD |
spr2006 |
Yes |
408 (448) |
46.0 (50.4) |
Peptidase, putative |
4 (C) | | Spr1274 |
spr1274 |
No |
129 |
14.6 |
NDe |
4 (C) | | Phage Cp-1 |
|
|
|
|
|
| | Cpl-1 |
cpl1 |
No |
339 |
39.2 |
Lysozyme |
6 (C) | | Phage Dp-1 |
|
|
|
|
|
| | Pal |
pal |
No |
296 |
34.4 |
NAM-amidase |
7 (C) |
a The number of residues of the unprocessed protein is indicated in parentheses.
b The molecular mass of the unprocessed protein is indicated in parentheses.
c The numbers correspond to consensus and non-consensus choline-binding repeats (CBRs). N and C indicate, respectively, whether the CBRs are located at the N- or C- terminal region of the protein.
d The data correspond to the genome of the common laboratory strain R6. Alternative protein designations are shown in parentheses.
e ND, not determined.
The crystal structure elucidation of CBPs is a
crucial step to gain insight in the structural and
functional knowledge of these proteins. Fig. 1 shows
the crystal structures of host and phage-encoded
CBPs that have been solved to date. Relevant aspects
of the four CBPs most recently crystallized are the
following:
a) Pce, a phosphorylcholinesterase, removes a limited
number of PC residues from the cell wall TAs. Also,
Pce is able to hydrolyze the platelet-activating factor
(PAF; 1-alkyl-2-acetoyl-hexadecyl-glycero-3-
phosphoryl choline), which suggests that this enzyme
has other functions during infection, such as
degrading host PC-containing compounds. The cell
surface localization of Pce could facilitate the
hydrolysis of PAF from the bloodstream and might
play a role in the mechanism of pneumococcal
adherence and invasiveness (see below). Pce has a
globular NH2- (N-) terminal module containing a
binuclear Zn2+ catalytic center, and an elongated CBM
joined by a short linker of 12 amino acids (Fig. 1c).
This structure has revealed that the removal of PC
residues is limited by the configuration of the active
site and, thus, only residues located at the end of the
TA chains are accessible to the catalytic center
(Hermoso et al. 2005).
b) CbpF is not a CWH but presents unique
features compared with other CBPs, as it is assembled
entirely by CBRs composed both of consensus and
non-consensus repeats distributed along its length,
which markedly alter its shape, charge distribution
and binding ability, and organizing the protein into
two well-defined modules (Fig. 1e). Sequence
divergence observed in CbpF is generated by
mutations of the consensus amino acid residues of the
repeat, by amino acid additions in the -hairpin turn
or the loop, or by a combination of both. Biochemical
studies have shown that CbpF selectively modulates
the autolytic function of the LytC lysozyme and the
divergent CBRs that build its N-terminal module
have a crucial role in this effect. It is worth noting
that analyses of known pneumococcal genomes show
the existence of several proteins that are likely to
display the same architecture as CbpF, which might
constitute a new subfamily within the CBPs (Molina
et al. 2009).
c) The structure of the autolysin LytC presents an
unusual hook-shaped conformation with a catalytic
module resembling a flattened ellipsoid folding into
an irregular (beta/alpha/)5beta3 barrel, and eleven CBRs in the
substrate recognition module (Fig. 1f). Structural and
biochemical data allow an explanation of the
coordinated role of LytC and CbpD (another CBP
with CWH activity) in fratricide, a
competence-programmed mechanism of predation of
noncompetent sister cells (Claverys and Havarstein
2007). LytC is only able to hydrolyze non-crosslinked
peptidoglycan chains, a property probably connected
to a new type of choline-binding site with a
characteristic GYMA motif at the end of the third
repeat. Docking experiments have indicated that the
presence of these GYMA sites on the CBM could
enhance the affinity for the PC and
N-acetylgalactosamine sugars of the TAs by
providing a strong multivalent recognition and
attachment to the cell wall. The configuration around
the active site imposed limitations on hydrolysis of
peptidoglycan, which means that only when specific
cuts are introduced in the peptide stems of
peptidoglycan in the target cells, do glycan chains
become fully predisposed to the LytC activity.
Therefore, prior cleavage of the peptide stems
performed by CbpD should facilitate hydrolysis of the
non-crosslinked peptidoglycan chains by LytC
(Perez-Dorado et al. 2010).
d) Spr1274 is a putative CBP of unknown
function (Fig. 1d). The crystal structure of its CBM
(residues 44-129) has been solved with three
molecules in the asymmetric unit, forming a
pseudo-trimer. Chains A and C are antiparallel and
interact with the two termini of chain B. No common
threefold axis can be found in the pseudo-trimer, but
two fold axes are present between chains A and B and
between chains A and C. The overall shape is like the
letter V, with an angle of ~60° between the two
superhelices (Zhang et al. 2009).
Taking advantage of the specific binding of the
aminoalcohol choline and its analogs to the CBPs, we
have tested a highly promising new approach for the
development of drugs to treat pneumococcal
infections. It is is based on dendrimers (tree-like
branched molecules) used as a scaffold for the
attachment of choline groups to the tip of the
branches. Thus, the choline ends can simultaneously
occupy multiple choline-binding sites of the CBPs.
Exogenously choline-charged dendrimers
competitively inhibit the binding of CBPs to the cell
wall, blocking cell separation and the characteristic
autolysis at the end of the stationary phase of growth,
inducing instead the formation of long chains or even
preventing growth. These effects were observed at
low micromolar concentrations of the
higher-generation dendrimers, which represents a
103-104-fold increase of apparent affinity compared
to monovalent choline (Hernandez-Rocamora et al.
2009). A further development of this dendrimer
approach is currently under study using several
choline analogs that display a higher binding affinity
to CBPs than choline. In fact, inhibition of
pneumococcal CBPs and cell growth by esters of
bicyclic amines has already been demonstrated
(Maestro et al. 2007).
The CBM of LytA is essential to guarantee full
enzymatic activity through dimerization. In this sense,
analysis of a collection of 21 mutated LytA
NAM-amidases indicated that Ile-315, located in the
last CBR, is a key amino acid residue in both
enzymatic activity and folding (Romero et al. 2007).
Moreover, it is worth to mention that selective
interaction of the CBM of LytA with choline or its
analogs (tertiary or quaternary amines) has allowed its
use as a tag to construct a variety of fusion proteins
that may be purified using DEAE-cellulose, which
acts as an affinity matrix for CBM-containing
proteins (Sanchez-Puelles et al. 1992, Moldes et al.
2004). In this sense, a commercial kit based in this
system is already available (C-LYTAG;
http://www.biomedal.com). In addition to
amine-containing solid supports, aqueous two-phase
solutions containing polyethylene glycol have also
been used for purification of proteins tagged with
CBMs (Maestro et al. 2008).
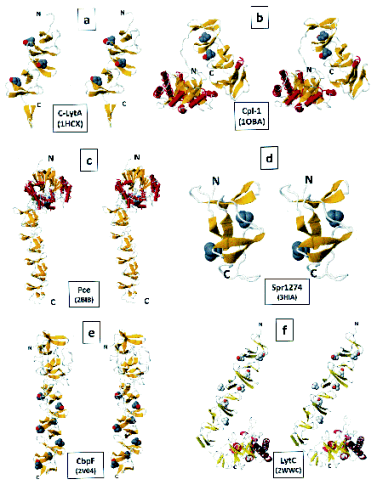
Fig. 1. Three-dimensional structures of several choline-binding proteins of S. pneumoniae. The stereo diagrams were
visualized with FISTGLANCE in JMOL (http://molvis.sdsc.edu/fgij/index.htm) or PyMOL (http://www.pymol.org/). For C-lytA
and Spr1274 only one monomer is shown for clarity. Rockets (or helices in panel f) and planks represent, respectively, alpha-helices
and beta-strands. N and C indicate the NH2- and the COOH- termini of the protein, respectively. PC molecules are depicted in CPK
mode. The PDB identification numbers are indicated in parentheses.
PNEUMOCOCCAL BIOFILMS
A biofilm is a sessile microbial community where
structured populations of microorganisms are adhered
to a surface or interface and embedded in an
extracellular matrix of polymeric substances. The
importance of biofilms in medical microbiology lies
in that more than 60% of human infections are related
to growth in biofilms and to the inherent tolerance of
these communities to antimicrobials and host immune
defense system. Biofilms have been associated with
several chronic infections (up to 80% of these)
including chronic otitis media. Formation of
pneumococcal biofilms on the mucosal epithelial cells
from children with recurrent or chronic middle ear
infections has been recently observed (Hoa et al.
2009). However, until five years ago no information
on pneumococcal biofilms at the structural or genetic
level was reported. Our research group has developed
the set-up of a suitable biofilm model for
S. pneumoniae in microtiter plates or glass-bottom
dishes to analyze the influence of several
environmental factors and to study its peculiar
structure (Moscoso et al. 2006, 2009).
Environmental changes analogous to those found
in the nasopharynx, such as nutrient content of the
medium, pH, and osmolarity, influence biofilm
formation in vitro. Nonencapsulated pneumococcal
strains generate a three-dimensional structure where
the adherent cells formed a mat of cells about 25 to
30 microm deep, as revealed by confocal laser scanning
microscopy (Moscoso et al. 2006). Also, using
low-temperature scanning electron microscopy, it was
found that pneumococcal cells growing in biofilms
are interconnected by small, thin filaments and
adopted regular shapes to form honeycomb-like
structures (Fig. 2).

Fig. 2. Low-temperature scanning electron micrograph
of a pneumococcal biofilm formed on the surface of a
glass coverslip (g). Arrows indicate filamentous material
linking pneumococci to each other and to the intercellular
matrix.
Extracellular DNA as well as extracytoplasmic
and/or surface-exposed proteins appear to be
important components of the biofilm matrix and are
required for pneumococcal biofilm formation and
maintenance (Moscoso et al. 2006). Extracellular
polysaccharides often determine the biofilm
architecture and provide the structural integrity for the
three-dimensional biofilm matrix. CPS, which is
absolutely required for pneumococcal virulence,
represents a partial physical barrier in the first steps
of biofilm formation since only reduced biofilm
formation was found when encapsulated, clinical
isolates or isogenic transformants were tested
(Moscoso et al. 2006). However, a minimum amount
of CPS is absolutely required for efficient
nasopharyngeal colonization in mice, although it
plays an essential protective role against phagocytosis
during invasive infection. It has been shown that
biofilm development may select nonencapsulated
phenotypic variants (Domenech et al. 2009). Several
different mutations were found among the type 3
capsular mutants that appeared in biofilms formed on
polystyrene plates. Most strains contained single
nucleotide polymorphisms in cap3A, the first gene of
the type 3 capsular operon (Moscoso and Garcia
2009) and encoding a UDP-glucose dehydrogenase;
one had a mutated -10 promoter hexamer
(CATAAT), and three had large deletions affecting
cap3A and, in one case, also cap3B. We have
proposed that non-encapsulated mutants of
pneumococcal type 3 strains are essentially involved
in the initial stages (the attachment stage) of biofilm
formation during colonization/pathogenesis
(Domenech et al. 2009).
The fact that pneumococcal biofilm is
CPS-independent indicates that one or more
extracellular polysaccharides other than CPS, are
involved in structuring of the biofilm matrix. Staining
of pneumococcal biofilms with various lectins has
provided evidence of the presence of an extracellular
polysaccharide (unpublished data).
Several genes have been reported to be important
for biofilm formation of pneumococci. Gene
expression profiles of pneumococci recovered from
the lungs and brains of infected mice have been
reported to be similar to those of pneumococci grown
in biofilms in vitro. Proteomic analysis confirmed that
the biofilm development process exhibited by
S. pneumoniae is correlated not only with differential
production of proteins but also with a dramatic
increase in the number of detectable proteins involved
in adhesion, resistance and virulence (Allegrucci et al.
2006). Mutations in genes encoding CBPs have been
shown to decrease the capacity of pneumococci to
form biofilms. Thus, CBPs such as the CWHs (e.g.,
the NAM-amidase LytA, the LytC lysozyme, or the
LytB glucosaminidase), the pneumococcal surface
protein A and the CbpA adhesin, were shown to
contribute to S. pneumoniae biofilm formation
(Moscoso et al. 2006). More recently, a large number
of transposon insertion strains were generated as an
approach to the identification of genes involved in
pneumococcal biofilm formation (Munoz-Elias et al.
2008). Such collection of mutants included strains
with genes encoding CBPs, adhesins, extracellular
proteases, efflux pumps, transporters, and
transcriptional regulators.
HOST-PATHOGEN INTERACTIONS
Pneumococcal disease is preceded by the colonization
of the nasopharynx, which is particularly common in
children (see above) and involves binding of the
bacterium to cell-surface carbohydrates. This process
is mediated by cell-wall-associated surface proteins
(Bogaert et al. 2004). Using CWH-deficient strains
and a human nasopharyngeal cell line we have found
that LytB and LytC play a key role in the colonization
of the upper respiratory tract and the establishment of
nasopharyngeal carriage. LytB or LytC deficient
strains displayed a reduced ability to colonize the
nasopharynx of infected mice when compared to the
wild-type strain. Our studies have demonstrated that
these CWHs are important surface-exposed proteins
involved in the adhesion and colonization of the
mucosal surfaces of the nasopharynx.
The transition from the carrier state to invasion
involves genotypic and phenotypic changes, many of
which lead to enhanced bacterial adherence to host
cells. Activation of endothelial or epithelial cells
results in up-regulation of the PAF receptor (PAFr) at
their surface, which in turn, promotes the recognition
of PAFr by PC, thus facilitating invasion by
S. pneumoniae throughout this receptor (Cundell et al.
1995). Choline is a key component of the bacterial
envelope of S. pneumoniae (see above) and it is
involved in the adhesion to nasopharyngeal epithelial
cells and the establishment of pneumococcal disease
(Kharat and Tomasz 2006). The hydrolysis of PC and
PAF by the Pce enzyme suggests that S. pneumoniae
has developed complex virulence mechanisms that
are involved in the processing of PC and host cell
adhesion as previously proposed (Hermoso et al.
2005). To establish the infection, a microorganism
must first overcome the host's innate immunity and,
in the case of pneumococcal infection, a key bacterial
element recognized by the innate immune system is
the cell wall. Cell wall fragments released in vivo
during bacterial autolysis mediated by pneumococcal
CWHs, contribute to inflammation and cell damage
(Lopez and Garcia 2004). Some of these components
interact with membrane-bound Toll like receptors
(TLRs), particularly TLR2, and induce a
proinflammatory response. Furthermore, it is
proposed that peptidoglycan degradation products
interact with Nod proteins that are intracellular
surveillance proteins that recognize subcomponents of
peptidoglycan (van der Poll and Opal 2008).
Therefore, CWHs might be involved in triggering
some of these physiological events.
Descriptions of virulence characteristics of
S. pneumoniae generally focus on its ability to escape
unharmed from the immune system and breach host
barriers leading to a widespread invasion. Acute
phase proteins such as the C-reactive protein (CRP)
or the serum amyloid P component (SAP) recognize
PC on the bacterial surface and activate
complement-mediated immunity, which is one of the
first defence lines against invading pathogens such as
S. pneumoniae, controlling bacterial replication in the
lungs and in the systemic circulation (Yuste et al.
2007). Limiting the content of PC by Pce might
impair the activation of the classical pathway
mediated by CRP or SAP, and therefore, Pce could
play an important role in pneumococcal pathogenesis.
To avoid complement immunity and phagocytosis,
S. pneumoniae has developed a wide arsenal of
bacterial virulence factors. Among them, PspA and
pneumolysin, which is released to the medium after
cell wall lysis mediated by LytA, contribute
synergistically to the innate immune diversion by
targeting complement immunity at different levels.
All these factors increase the ability of S. pneumoniae
to cause invasive disease (Yuste et al. 2005).
ENZYBIOTICS AND PHAGE THERAPY
The emergence and steady spread of
antibiotic-resistant S. pneumoniae infections have
determined the investigation of alternative treatment
strategies. A novel therapeutic approach is based on
the administration of purified recombinant CWHs
encoded either by bacteriophages (Lopez et al. 2004a,
Hermoso et al. 2007, Romero et al. 2009 a, b) or by
the bacterium itself (Rodriguez-Cerrato et al. 2007b)
to specifically kill bacterial species in which
enzyme-mediated lysis naturally occurs.
Bacteriophages have been used for years in the
prevention and/or treatment of bacterial infections in
some parts of the world (for a recent review, see
O'Flaherty et al. 2009). Several in vivo and in vitro
studies have showed the efficacy of phage therapy as
an alternative (or complement) to conventional
antibiotic therapy. Most bacteriophages encode a lytic
system containing a holin that causes membrane
damage, and at least one CWH (endolysin) that
destroy the bacterial cell wall and thus allow the
phage to disseminate its progeny.
Recently, the use of purified phage-encoded
endolysins as enzybiotics to control pathogenic
bacteria has been tested. Two purified pneumophage
lytic enzymes, the lysozyme Cpl-1 and the
NAM-amidase Pal, have been used with promising
efficacy in a murine sepsis model of pneumococcal
infection. This study demonstrated that Cpl-1, alone
or combined with Pal, afforded protection from
pneumococcal bacteremia and death (Lopez et al.
2004a). Therapy with Cpl-1 has also been tested in
other experimental models of S. pneumoniae
infections, including endocarditis, meningitis and
pneumonia, with promising results (O'Flaherty et al.
2009, Witzenrath et al. 2009). Although not tested yet
in vivo, a remarkable phage endolysin is the CHAP
domain-containing NAM-amidase Skl that is encoded
by a prophage from Streptococcus mitis SK137 strain
(Llull et al. 2006).
In an innovative approach in the field of
enzybiotic therapy, we have recently demonstrated
the antipneumococcal efficacy of recombinant LytA
and compared such effect with that of phage lysins
both in vitro and in a murine sepsis model
(Rodriguez-Cerrato et al. 2007a, b). The rationale for
the exogenous administration of purified LytA as
antibacterial agent was based on the role of LytA in
pneumococcal physiology, i.e., LytA causes bacterial
cell lysis during the late stationary phase of growth by
specifically breaking the amidase bonds between the
glycan strands and the peptide moieties of the
peptidoglycan.
Two serotype 3, penicillin-susceptible strains and
two penicillin- and cefotaxime-resistant (serotypes
19F and 19A, respectively) S. pneumoniae clinical
isolates were exposed to several combinations of
Cpl-1, Pal, and/or LytA, and antibiotics (cefotaxime
and moxifloxacin) using chequerboard and time-kill
assays (Rodriguez-Cerrato et al. 2007a). By the
chequerboard technique, although synergy between
LytA and Pal was not found for the four strains, the
combination of LytA and cefotaxime was synergistic
for one of the two cefotaxime-resistant strains
studied. The combined use of Cpl-1 and Pal was
synergistic for three of the four strains, as was Cpl-1
with antibiotics for two of the three strains studied.
In our study of mice infected with a
multidrug-resistant, meningeal pneumococcal strain,
intraperitoneal or intravenous therapy with purified
LytA and intraperitoneal therapy with high-dose
Cpl-1 achieved significant declines in bacterial counts
(ca. 3-5 log10 colony-forming units ml-1) in
peritoneum and blood, compared to those of
non-treated animals (Rodriguez-Cerrato et al. 2007b).
Further research using these and new lytic enzymes as
potential antipneumococcal agents is ongoing in our
laboratory.
ACKNOWLEDGEMENTS
We are indebted to J. A. Hermoso for his help in the
preparation of Fig. 1. This review was prepared
within the outlined actions of the Projects
SAF2006-00390 and SAF2009-10824. CIBER de
Enfermedades Respiratorias (CIBERES) is an
initiative of ISCIII. VR-C is supported by the
Subprograma Juan de la Cierva (JCI-2008-02690;
Ministerio de Ciencia e Innovacionn).
REFERENCES
Allegrucci M, Hu FZ, Shen K, Hayes J, Ehrlich GD, Post JC, Sauer K: Phenotypic characterization of Streptococcus pneumoniae biofilm development. J Bacteriol 188:2325-2335, 2006.
Bogaert D, de Groot R, Hermans PWM: Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 4:144-154, 2004.
Claverys J-P, Havarstein LS: Cannibalism and fratricide: mechanisms and raisons d'etre. Nat Rev Microbiol 5:219-229, 2007.
Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI: Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435-438, 1995.
Dagan R: Serotype replacement in perspective. Vaccine 27:C22-C24, 2009.
Domenech M, Garcia E, Moscoso M: Versatility of the capsular genes during biofilm formation by Streptococcus pneumoniae. Environ Microbiol 11:2542-2555, 2009.
Fernandez-Tornero C, Lopez R, Garcia E, Gimenez-Gallego G, Romero A: A novel solenoid fold in the cell wall anchoring domain of the pneumococcal virulence factor LytA. Nat Struct Biol 8:1020-1024, 2001.
Garcia P, Garcia JL, Lopez R, Garcia E: Pneumococcal phages. In Waldor MK, Friedman DLI, Adhya S (ed.): Phages: Their Role in Bacterial Pathogenesis and Biotechnology, ASM Press, Washington, D.C. 2005, pp. 335-361.
Gonzalez A, Llull D, Morales M, Garcia P, Garcia E: Mutations in the tacF gene of clinical strains and laboratory transformants of Streptococcus pneumoniae: impact on choline auxotrophy and growth rate. J Bacteriol 190:4129-4138, 2008.
Hermoso JA, Lagartera L, Gonzalez A, Stelter M, Garcia P, Martinez-Ripoll M, Garcia JL, Menendez M: Insights into pneumococcal pathogenesis from the crystal structure of the modular teichoic acid phosphorylcholine esterase Pce. Nat Struct Mol Biol 12:533-538, 2005.
Hermoso JA, Garcia JL, Garcia P: Taking aim on bacterial pathogens: from phage therapy to enzybiotics. Curr Opin Microbiol 10:461-472, 2007.
Hernandez-Rocamora VM, Maestro B, de Waal B, Morales M, Garcia P, Meijer EW, Merkx M, Sanz JM: Multivalent choline dendrimers as potent inhibitors of pneumococcal cell-wall hydrolysis. Angew Chem Int Ed Engl 48:948-951, 2009.
Hoa M, Tomovic S, Nistico L, Hall-Stoodley L, Stoodley P, Sachdeva L, Berk R, Coticchia JM: Identification of adenoid biofilms with middle ear pathogens in otitis-prone children utilizing SEM and FISH. Int J Pediatr Otorhinolaryngol 73:1242-1248, 2009.
Kharat AS, Tomasz A: Drastic reduction in the virulence of Streptococcus pneumoniae expressing type 2 capsular polysaccharide but lacking choline residues in the cell wall. Mol Microbiol 60:93-107, 2006.
Llull D, Lopez R, Garcia E: Skl, a novel choline-binding N-acteylmuramoyl-L-alanine amidase of Streptococcus mitis SK137 containing a CHAP domain. FEBS Lett 580:1959-1964, 2006.
Lopez R: Pneumococcus: the sugar-coated bacteria. Int Microbiol 9:179-190, 2006.
Lopez R, Garcia E: Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes, and bacteriophage. FEMS Microbiol Rev 28:553-580, 2004.
Lopez R, Garcia E, Garcia P: Enzymes for anti-infective therapy: phage lysins. Drug Discov Today Ther Strateg 1:469-474, 2004a.
Lopez R, Garcia E, Garcia P, Garcia JL: Cell wall hydrolases. In Tuomanen EI, Mitchell TJ, Morrison DA, Spratt BG (ed.): The Pneumococcus, ASM Press, Washington, D.C. 2004b, pp. 75-88.
Maestro B, Sanz JM: Novel approaches to fight Streptococcus pneumoniae. Recent Pat Antiinfect Drug Discov 2:188-196, 2007.
Maestro B, Gonzalez A, Garcia P, Sanz JM: Inhibition of pneumococcal choline-binding proteins and cell growth by esters of bicyclic amines. FEBS J 274:364-376, 2007.
Maestro B, Velasco I, Castillejo I, Arevalo-Rodriguez M, Cebolla A, Sanz JM: Affinity partitioning of proteins tagged with choline-binding modules in aqueous two-phase systems. J Chromatogr A 1208:189-196, 2008.
Moldes C, Garcia JL, Garcia P: Construction of a chimeric thermostable pyrophosphatase to facilitate its purification and immobilization by using the choline-binding tag. Appl Environ Microbiol 70:4642-4647, 2004.
Molina R, Gonzalez A, Stelter M, Perez-Dorado I, Kahn R, Morales M, Moscoso M, Campuzano S, Campillo NE, Mobashery S, Garcia JL, Garcia P, Hermoso JA: Crystal structure of CbpF, a bifunctional choline-binding protein and autolysis regulator from Streptococcus pneumoniae. EMBO Rep 10:246-251, 2009.
Moscoso M, Garcia E: Transcriptional regulation of the capsular polysaccharide biosynthesis locus of Streptococcus pneumoniae: a bioinformatic analysis. DNA Res 16:177-186, 2009.
Moscoso M, Garcia E, Lopez R: Biofilm formation by Streptococcus pneumoniae: role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. J Bacteriol 188:7785-7795, 2006.
Moscoso M, Garcia E, Lopez R: Pneumococcal biofilms. Int Microbiol 12:77-85, 2009.
Munoz-Elias EJ, Marcano J, Camilli A: Isolation of Streptococcus pneumoniae biofilm mutants and their characterization during nasopharyngeal colonization. Infect Immun 76:5049-5061, 2008.
O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, for the Hib and Pneumococcal Global Burden of Disease Study Team: Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893-902, 2009.
O'Flaherty S, Ross RP, Coffey A: Bacteriophage and their lysins for elimination of infectious bacteria. FEMS Microbiol Rev 33:801-819, 2009.
Perez-Dorado I, Gonzalez A, Morales M, Sanles R, Striker W, Vollmer W, Mobashery S, Garcia JL, Martinez-Ripoll M, Garcia P, Hermoso JA: Insights into pneumococcal fratricide from crystal structure of the modular killing factor LytC. Nat Struct Mol Biol 17:576-581, 2010.
Rodriguez-Cerrato V, Garcia P, del Prado G, Garcia E, Gracia M, Huelves L, Ponte C, Lopez R, Soriano F: In vitro interactions of LytA, the major pneumococcal autolysin, with two bacteriophage lytic enzymes (Cpl-1 and Pal), cefotaxime and moxifloxacin against antibiotic-susceptible and - resistant Streptococcus pneumoniae strains. J Antimicrob Chemother 60:1159-1162, 2007a.
Rodriguez-Cerrato V, Garcia P, Huelves L, Garcia E, del Prado G, Gracia M, Ponte C, Lopez R, Soriano F: Pneumococcal LytA autolysin, a potent therapeutic agent in experimental peritonitis-sepsis caused by highly beta-lactam-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother 51:3371-3373, 2007b.
Romero P, Lopez R, Garcia E: Key role of amino acid residues in the dimerization and catalytic activation of the autolysin LytA, an important virulence factor in Streptococcus pneumoniae. J Biol Chem 282:17729-17737, 2007.
Romero P, Garcia E, Mitchell TJ: Development of a prophage typing system and analysis of prophage carriage in Streptococcus pneumoniae. Appl Environ Microbiol 75:1643-1649, 2009a.
Romero P, Croucher NJ, Hiller L, Hu FZ, Ehrlich GD, Bentley SD, Garcia E, Mitchell TJ: Comparative genomic analysis of 10 Streptococcus pneumoniae temperate bacteriophages. J Bacteriol 191:4854-4862, 2009b.
Sanchez-Puelles JM, Sanz JM, Garcia JL, Garcia E: Immobilization and single-step purification of fusion proteins using DEAE-cellulose. Eur J Biochem 203:153-159, 1992.
Scott JAG, Brooks WA, Peiris JSM, Holtzman D, Mulhollan EK: Pneumonia research to reduce childhood mortality in the developing world. J Clin Invest 118:1291-1300, 2008.
van der Poll T, Opal SM: Host-pathogen interactions in sepsis. Lancet Infect Dis 8:32-43, 2008.
Witzenrath M, Schmeck B, Doehn JM, Tschernig T, Zahlten J, Loeffler JM, Zemlin M, Muller H, Gutbier B, Schutte H, Hippenstiel S, Fischetti VA, Suttorp N, Rosseau S: Systemic use of the endolysin Cpl-1 rescues mice with fatal pneumococcal pneumonia. Crit Care Med 37:642-649, 2009.
Yuste J, Botto M, Paton JC, Holden DW, Brown JS: Additive inhibition of complement deposition by pneumolysin and PspA facilitates Streptococcus pneumoniae septicemia. J Immunol 175:1813-1819, 2005.
Yuste J, Botto M, Bottoms SE, Brown JS: Serum amyloid P aids complement-mediated immunity to Streptococcus pneumoniae. PLoS Pathog 3:e120, 2007.
Zhang Z, Li W, Frolet C, Bao R, di Guilmi A-M, Vernet T, Chen Y: Structure of the choline-binding domain of Spr1274 in Streptococcus pneumoniae. Acta Crystallogr Section F Struct Biol Crystall Commun 65:757-761, 2009.
|
BACK
|



