Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 3, p 121-130
DOI 10.2478/v10136-009-0015-7
Cell cycle and Alzheimer's disease: studies in non-neuronal cells
Natividad de las Cuevas, Ursula Munoz, Fernando Bartolome, Noemi Esteras, Carolina Alquezar, Angeles Martin-Requero
Address: Angeles Martin-Requero, Centro de Investigaciones Biologicas (CSIC), Ramiro de Maeztu 9, 28040 Madrid, Spain
amrequero@cib.csic.es
Received 24th February 2010.
Revised 27th April 2010.
Published online 6th May 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Cell cycle and AD
Mitotic signals
Cellular and animal models
Cell cycle disturbances in non-neuronal cells
Proliferative activity of
immortalized lymphocytes from control and AD patients
Vulnerability of control and AD
lymphoblasts to serum deprivation
Signalling pathways and mediators involved in increased proliferation and survival of AD lymphocytes
Conclusions
Acknowledgements
References
SUMMARY
The most common cause of dementia in the elderly is Alzheimer disease (AD). In Europe, AD is a leading cause of death. The prevalence of this
disease in developed countries is increasing because of very significant shifts in life expectancy and demographic parameters. AD is characterized
by progressive cognitive impairment, resulting from dysfunction and degeneration of neurons in the limbic and cortical regions of the brain. Two
prominent abnormalities in the affected brain regions are extracellular deposits of beta-amyloid, and intracellular aggregates of tau protein in
neurofibrillary tangles. The role of these features in AD pathogenesis and progression is not yet completely elucidated. Research over the last
decade has revealed that the activation of cell cycle machinery in postmitotic neurons is one of the earliest events in neuronal degeneration in
AD. Here we summarize evidence to support the hypothesis that cell cycle alterations occur in cells other than neurons in AD sufferers.
Immortalized lymphocytes from AD patients have show an enhanced rate of proliferation associated with G1/S regulatory failure induced by
alterations in the cyclin/CDK/pRb/E2F pathway. In addition, these cells have a higher resistance to serum deprivation-induced apoptosis. These
neoplastic-like features, cell cycle dysfunction and impaired apoptosis can be considered systemic manifestations of AD disease.
KEY WORDS
Alzheimer's disease; lymphocytes; cell cycle; cell survival; p27; p21; calmodulin; PI3K/Akt; ERK1/2
INTRODUCTION
Alzheimer's disease (AD) is a progressive
neurodegenerative disorder affecting aged people; AD
prevalence is approximately 1% between 65 and
69 years and is higher than 50% in individuals above
95 years. The predominant clinical manifestation is
memory loss, but a number of other changes in brain
functioning, including impairments in language and
visual-spatial skills, and disorientation also
characterize this disease. With increasing life
expectancy across the world, dementia is a rapidly
growing socioeconomic and medical problem. The
hallmarks for AD are beta-amyloid plaques,
neurofibrillary tangles, and regionalized neuronal
loss. However our understanding of the role that these
features of AD play in the etiology of the disease
remains incomplete.
AD is extremely complex and genetically
heterogeneous. The majority of patients with the
so-called "sporadic" disease exhibit clinical signs
during the seventh decade, whereas individuals with
inherited AD (FAD) often become demented in mid
life. To date autosomal dominant FAD has been
linked to the presence of mutations in the genes APP,
PS1, and PS2, which are genes encoding the amyloid
precursor protein (APP), located in chromosome 21 or
the presenilins PS1 or PS2 in chromosomes 14 and 1
respectively. The late-onset or sporadic AD has been
associated with genetic factors modifying the risk of
suffering AD. Notably, the risk of apoE allele type is
a susceptibility locus with apoE4 showing a
dose-dependent contribution to AD (Strittmatter et al.
1993). Various methods of genetic analysis indicate
that additional genes predisposing to AD exist. A
complete list of susceptibility genes associated with
increased risk for AD can be found in
http://alzgene.org (Bertram et al. 2007).
CELL CYCLE AND AD
Despite progress in uncovering many of the factors
that contribute to the etiology of this disease, the
cause of neuronal death is largely unknown. One
promising theory to explain neurodegeneration in AD
is that neuronal loss could be associated with cell
cycle disturbances. This hypothesis receives support
from evidence that a variety of cyclins and
cyclin-dependent kinases (CDKs) are up-regulated in
the brain of AD patients (Nagy et al. 1998),
suggesting that elements that normally control cell
cycle progression in proliferating cells may modulate
neuronal death as well. Hippocampal and selected
cortical neuronal populations in AD exhibit
phenotypic changes characteristic of cells re-entering
the cell division cycle (Arendt 2003), and it has been
further demonstrated that a significant fraction of the
hippocampal pyramidal and basal forebrain neurons
have fully or partially replicated four independent loci
of three different chromosomes (Yang et al. 2001).
The successful duplication of DNA indicates that
some neurons had completed the S phase of cell cycle
(Mosch et al. 2007). These anomalies were not found
in unaffected regions of AD brains or in the
hippocampus of non-demented age-matched controls.
Recent work has shown that cell cycle-related events
are present many months before the appearance of
plaques or signs of inflammation in brain of murine
models of AD (Yang et al. 2006), as well as in brain
of patients with Mild Cognitive Impairment (MCI)
(Ueberham and Arendt 2005). MCI is a risk stage for
development of AD within the next 3-5 years.
Therefore, it appears that cell cycle dysfunction is
implicated in disease onset and early development
and it seems to confer selective vulnerability to
neurons (Webber et al. 2005). Importantly, most of
the AD pathological features, including beta-amyloid,
tau, presenilin mutations, oxidative stress, dystrophic
neuritis, DNA damage and aneuploid are somehow
related to cell cycle control, providing a link between
cell cycle disturbances and neurodegeneration (Zhu et
al. 2007, Zekanowski and Wojda 2009). A causal
relationship between cell cycle re-entry and
neurodegeneration has been recently reported in a
transgenic mouse model in which conditional and
neuron-specific expression of the proto-oncogene
c-Myc leads to cognitive deficit and
neurodegeneration (Lee et al. 2009a). It is now
assumed that adult neurons, rather than staying
permanently postmitotic, must constantly keep their
cell cycle in check (Herrup and Yang 2007). If
control of neuronal cell cycle fails, the consequence
is the entrance of neurons into an altered and
vulnerable state, often leading to death (Zhu et al.
2007). Based on these findings, some authors have
come to consider AD disease as an abortive
neoplastic disorder, that is, a disease of the cell cycle,
and thus, the knowledge of the intimate involvement
of cell-cycle checkpoints in molecular pathogenesis
of AD might be important for diagnostic purposes and
particularly in the search for treatment strategies (see
Lee et al. 2009b for a review).
MITOTIC SIGNALS
The search for factors responsible for the formation of
neurofibrillary tangles and amyloid deposits has
yielded several clues to the hypothesis that cell
cycle-related phenomena is implicated in the
accumulation of AD pathology. CDKs are involved in
the phosphorylation of tau (Weishaupt et al. 2003),
the main component of tangles as well as a key
protein for cytoskeleton organization that occurs
during neurite outgrowth and perhaps in aberrant
neuronal sprouting (Webber et al. 2005). Beta-amyloid
has been identified as mitogenic in vitro (Milward et
al. 1992). APP may induce the activation of cell cycle
proteins in neurons (Neve and McPie 2006).
Conversely, cell cycle proteins that normally control
the progression of cell cycle at the G1/S checkpoint
are present in tangle bearing neurons (McShea et al.
2007). The accumulation of potentially mitogenic
growth factors (EGF, bFGF) in diffuse amyloid
deposits could represent the trigger that initiates the
re-entry of neurons into the cell cycle (McShea et al.
1999). DNA damage induced by oxidative stress has
been associated with overexpression of the tumor
suppressor protein p53 and cell cycle reentry-induced
apoptosis in cultured neurons (Kruman et al. 2004).
p53 could activate cell cycle or apoptosis depending
on the success of the DNA repairing process
(Zekanowski and Wojda 2009). Other factors,
including many of the identified risk factors for
Alzheimer's disease, such as elevated plasma
homocysteine levels, ageing, menopause, low thyroid
levels, low level prolonged oxidative stress or head
injury, can either represent mitogenic signalling for
neurons or facilitate cell cycle re-entry in vulnerable
neuronal populations (reviewed by Arendt 2003).
CELLULAR AND ANIMAL MODELS
Limitations in the use of the postmortem brain for
examining molecular mechanisms underscore the
need to develop cell or animal models representative
of the pathogenesis that characterize AD. Thus, there
has been a strong impetus in the last decade to
develop a number of different transgenic mouse
models of AD that overexpress the human FAD genes
in the context of the mouse. This has proven to be a
valuable resource in the study of APP processing and
in the exploration and design of disease therapies
(Morrissette et al. 2009). The AD mice show
microglial activation, astrocytosis, and changes in
neuronal cytoskeleton proteins including tau. Many of
these model organisms have also been shown to have
significant memory deficits. Although most of these
mice don't show significant loss of neuronal bodies,
cell cycle-related events appear to occur also in the
mouse brain. Recently, it has been demonstrated in
four different plaque bearing mice, that neurons in the
most vulnerable areas have begun a true cell cycle
(Yang et al. 2006).
An alternative strategy to study the pathogenesis
of AD is the use of non-neuronal cells from patients.
Numerous observations indicate that, while the
predominant clinical expression arises from brain, AD
has systemic expression at the cellular and molecular
levels. Considerable precedent exists for studying AD
with peripheral tissues, including lymphocytes,
fibroblasts, and platelets (Etcheberrigaray and
Ibarreta 2001, Casoli et al. 2008). The use of
peripheral tissues complement studies of autopsy
samples and provide a useful tool to investigate
dynamic processes such as signal transduction
mechanisms, oxidative metabolism, etc.
CELL CYCLE DISTURBANCES IN NON-NEURONAL CELLS
Work from our laboratory and others have shown cell
cycle disturbances in peripheral cells, such as
lymphocytes from AD patients, suggesting that
dysfunction of the cell cycle is a more general
phenomenon affecting cells other than neurons.
Immortalized lymphocytes from AD patients showed
altered response to mitogenic stimulation relative to
control subjects (Urcelay et al. 2001, Nagy et al.
2002). Fibroblasts collected from AD patients also
show an aberrant cell cycle-dependent Ca2+ response
(Tatebayashi et al. 1995). We, and others have also
found failure of the G1/S transition checkpoint,
similar to that reported in AD brain, in lymphocytes
from AD subjects (Nagy et al. 2002, de las Cuevas et
al. 2003), and interestingly in MCI patients as well
(Nagy et al. 2002, Zhou and Jia 2010).
We also found that lymphocytes from AD patients
were more resistant to serum withdrawal-induced cell
death (de las Cuevas et al. 2005), suggesting that
control of cell fate depending on the presence or
absence of growth stimulatory signals is impaired in
peripheral cells from AD sufferers. These features
might represent an adaptative response for AD cells
that are exposed to chronic stress. It has been
considered that susceptible neurons in AD survive for
long time in a compromised way by delaying the
apoptotic process, a mechanism termed abortosis or
abortive apoptosis (Jellinger 2006).
PROLIFERATIVE ACTIVITY OF
IMMORTALIZED LYMPHOCYTES FROM CONTROL AND AD PATIENTS
To investigate the distinct cell cycle regulation in AD
at the systemic level, we performed a comparative
study on cell proliferation, cell cycle profiles, and
expression levels of key cell cycle regulatory proteins
in lymphoblasts derived from control and late-onset
AD subjects. These lymphoblastoid cell lines,
obtained by infecting peripheral blood mononuclear
cells with the Epstein Barr virus, retained the cellular
response of freshly obtained lymphocytes, to serum
addition or withdrawal (Bartolome et al. 2007, Munoz
et al. 2008a).
Lymphoblasts from AD patients exhibited a serum
dose-dependent enhanced rate of proliferation
compared with cells from normal age-matched
controls (Urcelay et al. 2001, de las Cuevas et al.
2003). AD lymphoblasts show cell cycle progress
modifications such as a decrease of cells in G1, and
an increased number of cells in S phase, together with
altered expression and phosphorylation of several
proteins involved in regulation of the G1/S transition
check point (de las Cuevas et al. 2003, 2005, Munoz
et al. 2005). AD lymphoblasts showed increased
phosphorylation of the retinoblastoma protein (pRb)
and other members of the family of pocket proteins
compared with cell lines derived from normal
age-matched controls (de las Cuevas et al. 2003,
Munoz et al. 2005). pRb is sequentially
phosphorylated by two sets of protein kinases, the
cyclinD/CDK4 and cyclin E/CDK2 complexes
(Mittnacht 1998). The activity of the latter, was found
to be enhanced in AD lymphoblasts (Munoz et al.
2008a). Furthermore, we demonstrated that the
increase in CyclinE/CDK2 activity was not due to
changes in the expression levels of either cyclin E or
CDK2, but rather, to the decreased levels of the CDK
inhibitor p27 found in AD lymphoblasts (de las
Cuevas et al. 2003, Munoz et al. 2005).
Once pRb-related proteins are phosphorylated, the
transcription factor E2F is released and activated
(Weinberg 1995). Accordingly, nuclear extracts from
AD lymphoblasts showed reduced E2F-DNA binding
activity as determined by EMSA analysis (de las
Cuevas et al. 2005). In contrast the activity of NF-kappaB
was found to be decreased in lymphocytes from AD
patients, and was not related to the serum-induced
enhanced proliferation, but associated instead with
decreased vulnerability of AD cells to serum
deprivation (de las Cuevas et al. 2005).
Two different reports have shown that freshly
obtained lymphocytes from AD patients are less
sensitive to G1/S transition blockers, thus suggesting
a failure of the G1/S checkpoint function (Nagy et al.
2002, Zhou and Jia 2010). These authors also found
these cell cycle alterations in MCI or mild-AD
patients.
Further work focused in delineating the molecular
mechanisms underlying the p27 down-regulation in
AD cells. It was found that this effect was due to
increased p27 degradation. A shorter half-life of the
p27 protein was detected in AD lymphoblasts as
compared with control cells (Munoz et al. 2008a).
p27 proteolysis is a three-step process that requires
phosphorylation at Thr187, recognition by the F-box
protein SKP2, ubiquitination, and degradation by the
26S proteasome. Increased phosphorylation of p27
protein at Thr187 in AD cells, rather than changes in
the 26S proteasome machinery, seems to account for
decreased p27 levels. An inverse relationship between
phospho-p27 and p27 content was found, while total
proteasome activity and accumulation of
ubiquitin-tagged proteins did not change significantly
(Munoz et al. 2008a, b).
Interestingly, the enhanced proliferative activity
and changes in cell cycle regulatory proteins, can be
modulated pharmacologically by treating AD cells
with the anti-inflammatory cyclopentenone
15-deoxy-prostaglanding J2 or simvastatin (Munoz et
al. 2005, 2008b, Sala et al. 2008). Therefore these
observations provide a plausible explanation for the
reported apparent benefits of these drugs preventing
or delaying the clinical features of AD (Stewart et al.
1997, Wolozin et al. 2000).
VULNERABILITY OF CONTROL AND AD LYMPHOBLASTS TO SERUM
DEPRIVATION
Lymphoblasts from AD subjects were found to be
more resistant to serum withdrawal (de las Cuevas et
al. 2005, Bartolome et al. 2007). In control cells, there
was a progressive appearance of cell death after 24 h
of serum starvation. However, little cell death, as
assessed by decreasing levels of MTT reduction was
observed in AD cells even after 96 h of serum
deprivation (de las Cuevas et al. 2005, Bartolome et
al. 2007). Selective impairment of the mechanisms
involved in cell death has been also reported in
fibroblasts from AD patients. The protective
mechanism of AD fibroblasts against H2O2 was
related to an impairment of cell cycle arrest and a
diminished induction of apoptosis (Uberti et al. 2002).
The lower sensitivity of AD lymphoblasts to
serum withdrawal was associated with changes in the
balance of pro- and anti-apoptotic proteins. Moreover
it was shown that the survival of AD cells was
accompanied by enhanced p21 content as compared
with that of control cells (Bartolomé et al. 2009b). A
number of recent studies pointed out that in addition
to being an inhibitor of cell proliferation, p21 may
protect cells from apoptosis (Gartel and
Radhakrishnan 2005). For example, it has been
reported that up-regulation of p21 blocked the
oxidative stress-induced death of human myeloma
U266 cells (Kim et al. 2001) and that inducible
expression of exogenous p21 render glioblastoma
cells resistant to chemotherapy drugs (Ruan et al.
1998). Thus the increase in p21 cellular content in
AD lymphoblasts may confer these cells a survival
advantage.
SIGNALLING PATHWAYS AND
MEDIATORS INVOLVED IN INCREASED PROLIFERATION AND SURVIVAL OF AD LYMPHOCYTES
Since dysregulation of calcium homeostasis is among
the major cellular alterations in AD (Thibault et al.
2007) we investigated whether alterations in the
major cellular Ca2+-binding protein, calmodulin
(CaM) were involved in the altered cellular response
of AD lymphoblasts. We found that two structurally
unrelated antagonists of CaM, like calmidazolium
(CMZ) and W-7, inhibited the proliferation of
lymphoblasts exclusively from AD patients (Urcelay
et al. 2001, de las Cuevas et al. 2003).
The CaM antagonists were also able to revert the
resistance of AD lymphoblasts to cell death induced
by serum deprivation (de las Cuevas et al. 2005,
Bartolome et al. 2007). Therefore, CaM seems to play
a pivotal role in transmitting proliferative/survival
signals from the plasma membrane to the nucleus.
Whether CaM contributes to cell proliferation or
apoptosis may depend on cellular CaM levels and/or
activity, as well as the presence of growth-stimulatory
signals.
The combination of CaM antagonist and specific
inhibitors of intracellular pathways potentially
implicated in the regulation of cell proliferation and
apoptosis, revealed the interaction of Ca2+/CaM with
PI3K/Akt and ERK1/2 pathways in the presence or in
the absence of serum, respectively. Table I shows
how, in contrast to the selective effect of CMZ,
preventing the enhanced stimulation of AD cells,
Ly294002, the inhibitor of PI3K/Akt, decreased
proliferation of both control and AD lymphoblasts.
SB202190, the inhibitor of p38, and PD98059,
inhibitor of ERK1/2 had no effect on cell
proliferation. However, treatment of control cells with
PD98059 prevented cell death induced by serum
starvation (Table I). This inhibitor had no effect in
AD cells, but blunted the effects of CMZ inducing
apoptosis in these cell lines. PI3K/Akt activity, as
assessed by increased Akt phosphorylation, was
found to be enhanced in AD cells following serum
stimulation, compared with the activity observed in
control cells (Munoz et al. 2008a, Bartolome et al.
2009a, b). In contrast a reduced sustained
phosphorylation of ERK1/2 was observed in AD cells
upon serum deprivation (Bartolome et al. 2007). Both
overactivation of PI3K/Akt and downregulation of
ERK1/2 pathways in AD cells were prevented by
CaM antagonists (Bartolome et al. 2007, Munoz et al.
2008a).
The Ca2+/CaM dependent overactivation of
PI3K/Akt appears to be the upstream event in the
serum-mediated enhanced proliferation of AD
lymphoblasts. The CaM antagonists and the inhibitor
of PI3K/Akt have similar effects in p27
phosphorylation at Thr187, and protein degradation
(Munoz et al. 2008a). It was reported that
overactivation of PI3K/Akt in AD cells may favor the
CyclinE/CDK2-mediated phosphorylation of p27 at
Thr187. In addition, PI3K/Akt phosphorylate other
p27 residues (Thr157, Thr159), which are important
in controlling the nucleo-cytoplasmic traffic of p27
(Liang et al. 2002). The exclusion of p27 from the
nucleus would then facilitate its degradation by the
proteasome (Munoz et al. 2008a,b) and thus relieve
cyclinE/CDK2 kinase activity from p27 inhibition. In
fact, it was observed that pRb hyperphosphorylation,
E2F, and cyclinE/CDK2 activities, as well as p27
content, were all affected by CaM antagonists (de las
Cuevas et al. 2003, 2005, Munoz et al. 2008a, b). On
the other hand, the Ca2+/CaM dependent down
regulation of ERK1/2 seems to be the key event in
protecting AD cells from serum deprivation-induced
cell death (Bartolomé et al. 2007). By decreasing the
activity of ERK1/2, it induces the overexpression of
p21 protein, which in turn blocks the serum
withdrawal-induced apoptosis (Bartolome et al.
2009b). Interestingly, it was found that the CaM
binding proteins CaMKII and calcineurin are not
involved in the serum-enhanced proliferation of AD
lymphoblasts (de las Cuevas et al. 2003, Munoz et al.
2008a). However CaMKII seems to play an important
role in controlling cell survival under serum
deprivation, since either CaM antagonists or the
CaMKII inhibitor KN-62 sensitize AD cells to death
triggered by the absence of growth stimulatory signals
(Bartolome et al. 2007). CaM antagonists had no
effect in control cells, suggesting a threshold for CaM
activation as the survival signal (de las Cuevas et al.
2005, Bartolome et al. 2007, Munoz et al. 2008a). In
fact, higher CaM content was found in lymphoblasts
from AD patients (Munoz et al. 2008b).
As reported for other cell types (Perez-Garcia et
al. 2004), we were able to observe that CaM binds to
the p85 subunit of PI3K (Munoz et al. 2008b).
Therefore, CaM could contribute to PI3K
overactivation in AD cells through this mechanism, as
association of CaM with the SH2 domain in p85 leads
to PI3K activation (Perez-Garcia et al. 2004).
The mechanism(s) by which CaM downregulates
the ERK1/2 pathway in AD lymphoblasts is not yet
known, thought a mechanism of association of
CaMKII with Ras-Raf-1/MEK/ERK1/2 seems likely.
This issue is currently under investigation in our
laboratory.
In summary, our work revealed a functional
relationship between Ca2+/CaM and PI3K/Akt or
ERKs in serum-induced signalling in immortalized
lymphocytes, controlling cell fate (proliferation/death
or survival) depending on growth factor availability.
The proposed scenario is represented schematically in
Fig. 1.
Table I. Influence of signalling pretubation on cell proliferation/cell death.
| Treatment |
Control |
AD | |
Inhibition of cell proliferation (%) | | +10% FBS |
- |
- | | 20 microM Ly294002 |
35 ± 3 |
41 ± 5 | | 20 microM PD98059 |
2 ± 4 |
8 ± 5 | | 20 microM SB202190 |
6 ± 4 |
5 ± 3 | | 1 microM CMZ |
4 ± 2 |
30 ± 3* | | 1 microM CMZ + 20 microM Ly294002 |
33 ± 4 |
36 ± 5 | |
Cell survival (%) | | -10% FBS |
69 ± 1 |
92 ± 5‡ | | 20 microM Ly294002 |
71 ± 4 |
87 ± 2 | | 20 microM PD98059 |
96 ± 2‡ |
92 ± 9 | | 10 microM SB202190 |
66 ± 6 |
87 ± 6 | | 1 microM CMZ |
60 ± 3 |
61 ± 6 | | 1 microM CMZ + 20 microM PD98059 |
93 ± 6‡ |
87 ± 6 |
* Lymphoblasts from control and AD individuals were seeded at an initial density of 1 × 106/ml on day 0, in the presence
or in the
absence of FBS, and treated with the indicated concentrations of drugs for 3 days. Cell survival was expressed as % of cells at
day 0. Values shown are the mean 6 SEM for 4-6 observations.
p<0.05 significantly different from control cells; ‡ p<0.05 significantly from untreated AD cells.
Finally, it is worth mentioning that a deregulation
of both PI3K/Akt and ERK1/2 signalling pathways
has been reported in AD brains. Increased phospho-Akt (Ser473) has been detected in AD
temporal cortex, accompanied by increased levels of
phosphorylation of Akt substrates such as GSK3,
mTOR, tau and lower levels of p27 (Griffin et al.
2005). Increased phosphorylated p27 (Thr 187) has
been also found in AD brains. Importantly,
Thr187-p27 shows a considerable overlap with
tau-positive neurofibrillary pathology, including
neurofibrillary tangles and dystrophic neuritis (Ogawa
et al. 2003). On the other hand, ERK1/2 activation
has been shown in degenerative neurons in close
association with neurofibrillary tangles, suggesting
the implication of this pathway in AD pathogenesis
(Knowles et al. 1999).
Thus, our results obtained in peripheral cells from
AD patients, demonstrated that dysfunction of
PI3K/Akt, and ERK1/2 signalling also occur outside
the CNS, supporting the hypothesis that AD has
systemic expression at cellular and molecular levels.
Therefore, peripheral cells from patients may be a
potential useful surrogate for diagnosis, prognosis and
therapeutic monitoring of AD.
While most of the studies on AD lymphocytes had
been focused on detecting disease-specific changes
that may serve as biomarkers, the clinical
consequences, if any, of the enhanced proliferative
response of lymphocytes in AD patients, remain to be
established, as well as the role that they may play in
the chronic inflammation associated with this disease.
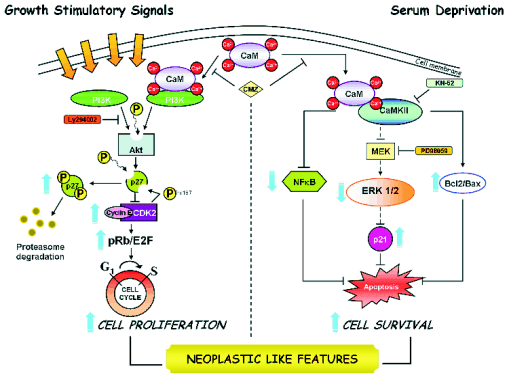
Fig. 1. Depiction of the proposed signalling pathways altered in AD lymphoblasts. In AD cells, increased levels of CaM
synergise with serum stimulation and promote overactivation of PI3K/Akt leading to enhanced p27 degradation, and activation
of cyclin/CDK/pRb/E2F, therefore favouring, the progression of cells through the cell cycle. In the absence of serum, the
Ca2+/CaM-binding protein, CaMKII, decreases the serum-deprivation induced NF-kappaB and ERK1/2 activation in comparison with
control cells, and increases the cellular content of p21, which then seems to protect AD lymphoblasts from apoptosis induced
by the absence of trophic support.
CONCLUSIONS
Cell cycle disturbances are evident in non-neuronal
cells from AD patients. While the precise origins of
cell cycle alterations are not fully understood, a
complex interaction of Ca2+/CaM with the PI3K/Akt and ERK1/2 pathways seems to be the master
regulator of cell survival, controlling cell proliferation
or preventing apoptosis depending on growth
conditions. Two cell cycle regulatory proteins, the
CDK inhibitors, p27 and p21, are ultimately
responsible for the enhanced proliferation and
increased resistance to cell death, respectively.
Whereas downregulation of p27 in a
Ca2+/CaM-dependent manner induces the enhanced
proliferative response of immortalized lymphocytes
from AD patients, upregulation of p21 seems to help
AD cells to escape from serum deprivation-induced
apoptosis. The distinct cell cycle and apoptosis
control in lymphoblastoid cells from AD patients
offer a noninvasive tool for investigating the
pathogenesis of AD and suggest a number of
molecular targets for potential AD therapies.
ACKNOWLEDGEMENTS
We are grateful to former members of the laboratory
involved in cell lines generation and ionic
homeostasis studies. We would like to thank to all
patients, their families and clinicians involved in this
study. Work in the authors's laboratory has been
supported from grants from the Spanish Fondo de
Investigaciones Sanitarias (FIS 01/1194 and
PI040312), the Spanish Ministry of Science and
Innovation (SAF 2003-01458 and SAF 2007-62405),
and the Fundacion E. Rodriguez Pascual.
REFERENCES
Arendt T: Synaptic plasticity and cell cycle activation in neurons are alternative effector pathways: the 'Dr. Jekyll and Mr. Hyde concept' of
Alzheimer's disease or the yin and yang of neuroplasticity. Prog Neurobiol 71:83-248, 2003.
Bartolome F, de las Cuevas N, Munoz U, Bermejo F, Martin-Requero A: Impaired apoptosis in lymphoblasts from Alzheimer's disease patients:
cross-talk of Ca2+/calmodulin and ERK1/2 signalling pathways. Cell Mol Life Sci 64:1437-1448, 2007.
Bartolome F, Munoz U, Esteras N, Esteban J, Bermejo-Pareja F, Martin-Requero A: Distinct regulation of cell cycle and survival in lymphocytes from
patients with Alzheimer's disease and amyotrophic lateral sclerosis. Int J Clin Exp Pathol 2:390-398, 2009a.
Bartolome F, Munoz U, Esteras N, Alquezar C, Collado A, Bermejo-Pareja F, Martin-Requero A: HMG-CoA reductase inhibitor simvastatin overcomes the
resistance to serum withdrawal-induced apoptosis of lymphocytes from Alzheimer's disease patients. XXXII SEBMM meeting, Oviedo Spain 2009b.
Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE: Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene
database. Nat Genet 39:17-23, 2007.
Casoli T, Di Stefano G, Giorgetti B, Balietti M, Recchioni R, Moroni F, Marcheselli F, Bernardini G, Fattoretti P, Bertoni-Freddari C: Platelet as
a physiological model to investigate apoptotic mechanisms in Alzheimer beta-amyloid peptide production. Mech Ageing Dev 129:154-162, 2008.
de las Cuevas N, Urcelay E, Hermida OG, Saiz-Diaz RA, Bermejo F, Ayuso MS, Martín-Requero A: Ca2+/calmodulin-dependent modulation of
cell cycle elements pRb and p27kip1 involved in the enhanced proliferation of lymphoblasts from patients with Alzheimer dementia. Neurobiol Dis
13:254-263, 2003.
de las Cuevas N, Muoz U, Hermida OG, Martin-Requero A: Altered transcriptional regulators in response to serum in immortalized lymphocytes from
Alzheimer's disease patients. Neurobiol Aging 26:615-624, 2005.
Etcheberrigaray R, Ibarreta D: Ionic channels and second messenger alterations in Alzheimer's disease. Relevance of studies in nonneuronal cells.
Rev Neurol 33:740-749, 2001.
Gartel AL, Radhakrishnan SK: Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res 65:3980-3985, 2005.
Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O'Connor R, O'Neill C: Activation of Akt/PKB, increased phosphorylation of Akt
substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer's disease pathology. J Neurochem 93:105-117, 2005.
Jellinger KA: Challenges in neuronal apoptosis. Curr Alzheimer Res 3:377-391, 2006.
Herrup K, Yang Y: Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci 8:368-378, 2007.
Kim DK, Cho ES, Lee SJ, Um HD: Constitutive hyperexpression of p21(WAF1) in human U266n myeloma cells blocks the lethal signalling induced by
oxidative stress but not by Fas. Biochem Biophys Res Commun 289:34-38, 2001.
Knowles RB, Chin J, Ruff CT, Hyman BT: Demonstration by fluorescence resonance energy transfer of a close association between activated MAP kinase
and neurofibrillary tangles: implications for MAP kinase activation in Alzheimer disease. J Neuropathol Exp Neurol 58:1090-1098. 1999.
Kruman II, Wersto RP, Cardozo-Pelaez F, Smilenov L, Chan SL, Chrest FJ, Emokpae R Jr., Gorospe M, Mattson MP: Cell cycle activation linked to
neuronal cell death initiated by DNA damage. Neuron 41:549-561, 2004.
Lee HG, Casadesus G, Nunomura A, Zhu X, Castellani RJ, Richardson SL, Perry G, Felsher DW, Petersen RB, Smith MA: The neuronal expression of MYC
causes a neurodegenerative phenotype in a novel transgenic mouse. Am J Pathol 174:891-897, 2009a.
Lee HG, Casadesus G, Zhu X, Castellani RJ, McShea A, Perry G, Petersen RB, Bajic V, Smith MA: Cell cycle re-entry mediated neurodegeneration and
its treatment role in the pathogenesis of Alzheimer's disease. Neurochem Int 54:84-88, 2009b.
Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, Franssen E, Slingerland JM:
PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med 8:1153-1160, 2002.
McShea A, Zelasko DA, Gerst JL, Smith MA: Signal transduction abnormalities in Alzheimer's disease: evidence of a pathogenic stimuli. Brain Res
815:27-242, 1999.
McShea A, Lee HG, Petersen RB, Casadesus G, Vincent I, Linford NJ, Funk JO, Shapiro RA, Smith MA: Neuronal cell cycle re-entry mediates Alzheimer
disease-type changes. Biochim Biophys Acta 1772:467-472, 2007.
Milward EA, Papadopoulos R, Fuller SJ, Moir RD, Small D, Beyreuther K, Masters CL: The amyloid protein precursor of Alzheimer's disease is a
mediator of the effects of nerve growth factor on neurite outgrowth. Neuron 9:29-37, 1992.
Mittnacht S: Control of pRB phosphorylation. Curr Opin Genet Dev 8:21-27, 1998.
Morrissette DA, Parachikova A, Green KN, LaFerla FM: Relevance of transgenic mouse models to human Alzheimer disease. J Biol Chem 284:6033-6037,
2009.
Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T: Aneuploidy and DNA replication in the normal human brain and Alzheimer's disease. J
Neurosci 27:6859-6867, 2007.
Munoz U, de las Cuevas N, Bartolome F, Hermida OG, Bermejo F, Martin-Requero A: The cyclopentenone 15-deoxy-delta(12,14)-prostaglandin
J2 inhibits G1/S transition and retinoblastoma protein phosphorylation in immortalized lymphocytes from Alzheimer's disease patients.
Exp Neurol 195:508-517, 2005.
Munoz U, Bartolome F, Bermejo F, Martin-Requero A: Enhanced proteasome-dependent degradation of the CDK inhibitor p27(kip1) in immortalized
lymphocytes from Alzheimer's dementia patients. Neurobiol Aging 29:1474-1484, 2008a.
Munoz U, Bartolome F, Esteras N, Bermejo-Pareja F, Martin-Requero A: On the mechanism of inhibition of p27 degradation by
15-deoxy-Delta12,14-prostaglandin J2 in lymphoblasts of Alzheimer's disease patients. Cell Mol Life Sci 65:3507-3519, 2008b.
Nagy Z, Esiri MM, Smith AD: The cell division cycle and the pathophysiology of Alzheimer's disease. Neuroscience 87:731-739, 1998.
Nagy Z, Combrinck M, Budge M, McShane R: Cell cycle kinesis in lymphocytes in the diagnosis of Alzheimer's disease, Neurosci Lett 317:81-84,
2002.
Neve RL, McPhie DL: The cell cycle as a therapeutic target for Alzheimer's disease. Pharmacol Ther 111:99-113, 2006.
Ogawa O, Lee HG, Zhu X, Raina A, Harris PL, Castellani RJ, Perry G, Smith MA: Increased p27, an essential component of cell cycle control, in
Alzheimer's disease. Aging Cell 2:105-110, 2003.
Perez-Garcia MJ, Cena V, de Pablo Y, Llovera M, Comella JX, Soler RM: Glial cell line-derived neurotrophic factor increases intracellular calcium
concentration. Role of calcium/calmodulin in the activation of the phosphatidylinositol 3-kinase pathway. J Biol Chem 279:6132-6142, 2004.
Ruan S, Okcu MF, Ren JP, Chiao P, Andreeff M, Levin V, Zhang W: Overexpressed WAF1/Cip1 renders glioblastoma cells resistant to chemotherapy agents
1,3-bis(2-chloroethyl)-1-nitrosourea and cisplatin. Cancer Res 58:1538-1543, 1998.
Sala SG, Munoz U, Bartolome F, Bermejo F, Martin-Requero A: HMG-CoA reductase inhibitor simvastatin inhibits cell cycle progression at the G1/S
checkpoint in immortalized lymphocytes from Alzheimer's disease patients independently of cholesterol-lowering effects. J Pharmacol Exp Ther
324:352-329, 2008.
Stewart WF, Kawas C, Corrada M, Metter EJ: Risk of Alzheimer's disease and duration of NSAID use. Neurology 48:626-632, 1997.
Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD: Binding of human
apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci
USA 90:8098-8192, 1993.
Tatebayashi Y, Takeda M, Kashiwagi Y, Okochi M, Kurumadani T, Sekiyama A, Kanayama G, Hariguchiand S, Nishimura T: Cell-cycle-dependent abnormal
calcium response in fibroblasts from patients with familial Alzheimer's disease, Dementia 6:9-16, 1995.
Thibault O, Gant JC, Landfield PW: Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging Cell
6:307-317, 2007.
Uberti D, Carsana T, Bernardi E, Rodella L, Grigolato P, Lanni C, Racchi M, Govoni S, Memo M: Selective impairment of p53-mediated cell death in
fibroblasts from sporadic Alzheimer's disease patients. J Cell Sci 115:3131-3138, 2002.
Ueberham U, Arendt T: The expression of cell cycle proteins in neurons and its relevance for Alzheimer's disease. Curr Drug Targets CNS Neurol
Disord 4:293-306, 2005.
Urcelay E, Ibarreta D, Parrilla R, Ayuso MS, Martin-Requero A: Enhanced proliferation of lymphoblasts from patients with Alzheimer dementia
associated with calmodulin-dependent activation of the Na+/H+ exchanger. Neurobiol Dis 8:289-298, 2001.
Weinberg RA: The retinoblastoma protein and cell cycle control. Cell 81:323-330, 1995.
Webber KM, Raina AK, Marlatt MW, Zhu X, Prat MI, Morelli L, Casadesus G, Perry G, Smith MA: The cell cycle in Alzheimer disease: a unique target
for neuropharmacology. Mech Ageing Dev 126:1019-1025, 2005.
Weishaupt JH, Neusch C, Bahr M: Cyclin-dependent kinase 5 (CDK5) and neuronal cell death. Cell Tissue Res 312:1-8, 2003.
Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G: Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl
coenzyme A reductase inhibitors. Arch Neurol 57:1439-1443, 2000.
Yang Y, Geldmacher DS, Herrup K: DNA replication precedes neuronal cell death in Alzheimer's disease. J Neurosci 21:2661-2668, 2001.
Yang Y, Varve NH, Lamb BT, Herrup K: Ectopic cell cycle events link human Alzheimer's disease and amyloid precursor protein transgenic mouse
models. J Neurosci 26:775-784, 2006.
Zekanowski C, Wojda U: Aneuploidy, chromosomal missegregation, and cell cycle reentry in Alzheimer's disease. Acta Neurobiol Exp (Wars) 69:232-253,
2009.
Zhou X, Jia J: P53-mediated G1/S checkpoint dysfunction in lymphocytes from Alzheimer's disease patients. Neurosci Lett 468:320-325, 2010.
Zhu X, Lee HG, Perry G, Smith MA: Alzheimer disease, the two-hit hypothesis: an update. Biochim Biophys Acta 1772:494-502, 2007.
|
BACK
|


