Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 3, p 159-167
DOI 10.2478/v10136-009-0019-3
Membrane-active peptides as anti-infectious agents
Luis Rivas, Juan Roman Luque-Ortega, Maria Fernandez-Reyes, David Andreu
Address: Luis Rivas, Centro de Investigaciones Biologicas - CSIC, Ramiro de Maeztu 9, E-28040 Madrid, Spain
luis.rivas@cib.csic.es
Received 15th March 2010.
Revised 23rd April 2010.
Published online 12th May 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Antimicrobial peptides from eukaryotic sources
Pharmacological exploitation of AMPs
Cell-penetrating peptides as anti-infectious
agents?
Acknowledgements
References
SUMMARY
The lipid components of pathogen cell membranes have been considered as a poor pharmacological target, due to their universal distribution and
apparent homogeneity throughout living organisms. Among the rare exceptions to this view one could mention polyene antibiotics such as
amphotericin, or peptide antibiotics such as the polymyxins and the gramicidins. In the last two decades, however, the above notion has been
challenged by two main lines of discovery; first, natural antimicrobial peptides (AMPs) that kill pathogens by interaction with phospholipids and
membrane permeabilization, and secondly, cell-penetrating peptides (CPPs), capable of introducing into cells a variety of cargoes in the absence of
specific receptors, again by interaction at some point with membrane phospholipids. For both AMPs and CPPs, the pharmacological proof-of-concept
has been successfully demonstrated, and promising applications as nanobiotechnological tools have been envisaged though not hitherto materialized
in clinical settings. In this review we briefly examine the pros and cons of these two classes of therapeutic agents, as well as strategies aimed
at rationalizing and expanding their potentiality.
KEY WORDS
membrane; cell-penetrating peptide; antimicrobial peptide; antibiotic resistance; infectious disease
Abbreviations
AMP, antimicrobial peptide
CPP, cell-penetrating peptide
DC, dendritic cell
HD, human beta defensin
HNP, human neutrophil peptide (human alpha-defensin)
IM, inner membrane
OM, outer membrane
PG, phosphatidylglycerol
PL, phospholipid
PM, plasma membrane
INTRODUCTION
The plasma membrane (PM), made up of
phospholipids (PLs) and other PL-embedded
components, constitutes the ultimate barrier isolating
the intracellular milieu from the external
environment, and as such, the arena where the
pathogen, on one side, and the host immune defences
and antimicrobial drugs on the other side, draw their
battle lines. Aside from a minority of free-diffusing
molecules, drug entry is controlled by transporters
and pores imposing rather stringent structural
requirements for internalization. Though protective of
cell integrity, strict PM crossing requirements become
an Achilles' heel for drugs, particularly
antimicrobials, as even minor mutations in proteins
involved in PM passage may partially or fully block
drug uptake and subsequent activity (Hopkins et al.
2005).
A somewhat different antimicrobial paradigm,
relying on the lethal hit of an antimicrobial peptide
(AMP) on the PLs of the pathogen PM (Zhang and
Falla 2009), is currently under scrutiny at pharma
companies. Membrane-active AMPs of bacterial
origin, such as gramicidins and polymyxins,
previously ignored due to their toxicity, are
undergoing a reappraisal as the repository of classical
antibiotics without reported resistance problems is
increasingly exhausted (Mogi and Kita 2009);
daptomycin, another membrane-targeting peptide
active against Gram-positive infections, has recently
received clinical approval. This review will focus on
various aspects of PM-antimicrobial interactions, with
an emphasis on eukaryotic-derived AMPs and their
man-made surrogates, as well as on the more recently
discovered cell-penetrating peptides (CPPs).
ANTIMICROBIAL PEPTIDES FROM EUKARYOTIC SOURCES
Description and natural occurrence
Eukaryotic AMPs play a major role as a first chemical
barrier against invading pathogens. As key
components of innate immunity, AMPs are
characterized by (i) activity on a broad variety of
pathogens; (ii) constitutive expression or fast
induction after contact with the pathogen; (iii)
absence of immunological memory. These features
are in contrast with acquired immune responses based
on exquisite, highly specific antigen recognition but
penalized by a fairly long onset, during which time
host defence largely depends on the innate response
of which AMPs are an essential part. Such immediacy
in the face of infection is one reason why AMPs may
have been preserved through evolution and are now
pervasively found throughout eukaryotes, from
unicellular amoebae to such simple pluricellular
organisms as Hydra, and on to higher organisms
including primates and man, in a display of universal
efficacy associated to a rather tight gene economy.
For a given eukaryotic organism, an
armamentarium based on a variety of AMPs is
typical; e.g. for Drosophila, there are 20 inducible
AMPs grouped in 7 families (Lemaitre and Hoffmann
2007). Partial target specificity can be found within a
given antimicrobial peptidome, e.g., the much higher
antifungal than antibacterial activities observed for
drosomycin in Drosophila (Lemaitre and Hoffmann
2007) or histatin in humans.
As expected from their invasion-preventing role,
AMPs are most abundant in anatomical locations
where first contact with pathogens is likely: skin,
mucosal tissues, biological fluids and professional
phagocytes (Metz-Boutigue et al. 2009). Some have
rather precise locations, e.g., dermicidin in sweat
glands or histatins in higher primate saliva, while
others are more generally distributed, e.g., human
LL-37. In addition, the AMP repertoire of a given
organism can be further increased through
proteolysis, e.g., by trimming of a pre-existing AMP,
as in histatin (Sun et al. 2009) and defensin isoforms,
or by protease-mediated unmasking or enhancement
of the antimicrobial activity of a large precursor, such
as lactoferricin (from lactoferrin), haemocidins (from
hemoglobin), or buforin (from histones). Also,
microbicidal activities have been postulated for
proteins or peptides classified otherwise, e.g.,
hepcidin, a Fe2+ transporter, RNAses or various
chemokines (Zasloff 2009), with the caveat of
whether their physiological concentration is high
enough for pathogen killing.
Natural AMPs epitomize biodiversity. The almost
boundless variety of natural AMP leads, the
increasing number of available genomes, as well as
the development of peptidomics and of algorithms for
AMP identification, have raised exponentially the
number of putative or real AMPs. Exhaustive
compilations of AMP sequences appear in free-access
databases, either for peptides in general
(http://pepbank.mgh.harvard.edu/) or solely for
AMPs, such as AMSD (http://www.bbcm.units.it/~tossi/amsdb.html),
APD (http://aps.unmc.edu/AP/main.php),
CAMP (http://www.bicnirrh.res.in/antimicrobial/),
RAPD for recombinant AMPs (http://faculty.ist.unomaha.edu/chen/rapd/index.php),
PhytAMP for plant AMPs (http://phytamp.pfba-lab-tun.org/main.php),
or specific for certain AMPs such as defensins
(http://defensins.bii.a-star.edu.sg/) or shrimp
peneidins (http://penbase.immunaqua.com/).
The structure-based AMP classification originally
proposed by Boman remains largely in effect, with
the inclusion of some new groups (Table 1). In
general, AMP structural plasticity is inversely related to the number of disulfide bonds; thus, most alpha-helical
peptides are unstructured in aqueous media, and only
become structured upon contact with PLs or
membrane-mimicking solvents such as trifluoethanol.
Table 1. Classification of antimicrobial peptides by structural criteria.
| Peptide group |
Representative
peptide |
Sequence |
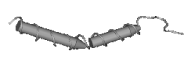
Structure | | Linear helical
peptides without Cys |
LL-37
(Homo sapiens) |
LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES |
 | | Linear peptides
without Cys but
enriched in a given
amino acid except
Arg or Lys
|
Indolicidin
(Bos taurus)
|
ILPWKWPWWPWRR-NH2*
|
 | | Peptides with a single
internal disulfide
bond
|
Lactoferricin
(Bos taurus)
|
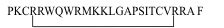 |
 | | Peptides with two
or more internal
disulfide bonds
|
HbetaD-1
(Homo sapiens)
|
 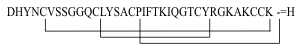 |
 | | Cyclic peptides
|
theta defensin
(Maccacus rhesus)
|
|
 |
* Amidation of the C-terminal group.
Although most AMPs have been described in
monomeric form, a few examples of covalent
heterodimeric AMPs linked by a disulfide bond are
known, such as the amphibian distinctin, or halocydin
from the tunicate Halocynthia auratum. Hetero- and
homodimer formation has also been reported for
murine intestinal cryptdins, leading to diversified
antimicrobial performance with respect to the original
monomeric repertoire (Hornef et al. 2004).
Only one cyclic AMP, rhesus theta-defensin, has been
so far isolated in vertebrates, as the result of
head-to-tail ligation of two nonapeptides from
truncated defensins. Retrocylin, its
pseudogene-encoded human ortholog, appears to be
unexpressed but its chemically synthesized version
showed anti-HIV and lectin activities (Selsted 2004).
A high cationic character and some type of
amphipathic higher structure appear to be the only
common structural motifs shared by the vast majority
of AMPs hitherto described. A few exceptional cases
of anionic AMPs originating from prozymogen
processing are known (Brogden et al. 1998).
Mechanism of action
Ever since the initial discovery of AMPs, disruption
of cellular membranes in the targeted organism has
been consistently observed (Lehrer et al. 1989) and
reproduced on artificial membranes (Christensen et al.
1988). Furthermore, AMP enantiomers were
equipotent with their allL counterparts (Wade et al.
1990), ruling out chirality as a requirement for
activity. Altogether, the PM permeabilization caused
by AMP interaction with PLs is now unanimously
regarded as an essential hallmark of AMP lethal
action.
Several models have been postulated to account
for AMP permeabilization of the PM, mostly deduced
from model membrane studies. A brief description of
the most prevailing models is outlined below. The
reader is referred to recent and specialized reviews
about this subject (e.g., Melo et al. 2009). The
following events can be arbitrarily defined as
sequential steps of the mechanism:
1. The AMP accumulates massively at the PM outer
leaflet, with its axis parallel to the membrane plane.
To minimize the free-energy of the system, the polar
surface of the (amphipathic) AMP remains exposed to
the external medium, and in interaction with PL polar
head groups, while the hydrophobic surface contacts
the fatty acid aliphatic chains of PLs.
2. Next, massive peptide insertion into the PM leads
to an expansion of the outer leaflet and to mechanical
stress by imbalance with the internal leaflet. Once a
threshold value for this stress is reached, the system
reacts by either "micellization" of the membrane by
formation of supramolecular aggregates ("carpet-like"
model), or by re-orienting a fraction of the
membrane-bound AMP in a transversal mode, leading
to the formation of mixed PL-AMP pores
("worm-hole", toroidal, or two-state model), and
promoting PL interchange between leaflets and
membrane curvature.
3. In a final step, the mixed pore disintegrates,
sending peptide randomly to both sides of the
membrane. If the average lifetime of the pore is long
enough, this will itself suffice for an irreversible loss
of the pathogen's internal homeostasis. Alternatively,
a brief enough pore will give rise to a transitory,
usually reversible disruption, which appears to be the
typical course for those few AMPs known to act not
primarily by PM disruption but upon intracellular
targets.
4. As an alternative to step 2 above, the "barrel stave"
model postulates an exclusively peptide-based pore
formed at much lower AMP-PL stoichiometry than
the carpet model, and driven by AMP self-affinity
stronger than AMP-PL affinity.
5. Finally, formation of HII, non-lamellar inverted
hexagonal PL phases, defining an inverted micelle
inside the membrane, has also been invoked as a
mechanism of action for membranes made up of PLs
promoting negative curvature, with the polycationic
AMP acting as nucleating core for the micelle
(Powers et al. 2005). This model accounts for AMP
translocation without membrane permeabilization. In
this and the above models, AMP aggregation in
solution prior to membrane insertion would result in
local AMP concentrations capable of inducing
permeabilization at much lower concentrations than
the isolated monomer, along with decreased
membrane specificity.
Target specificity
The mainstay of AMP specificity for bacteria and
fungi over higher eukaryotes is that the former show
a higher percentage of anionic PLs that favour
electrostatic interaction with polycationic AMPs. In
addition, in higher eukaryotes the anionic PLs are
confined to the cytosolic leaflet of the membrane,
initially inaccessible to the AMP. This recognized
fact, however, cannot by itself explain the marked
variations in antibiotic activity observed for different
AMPs. Among other factors having a modulating role
on activity, several refer to the PL composition of the
membrane, including (i) type and shape of the anionic
PLs. Thus, for PLs with a bulky polar head (e.g., PS),
AMP insertion induces a positive (convex) curvature
of the PM that favours formation of toroidal pores.
For anionic PLs with an area of polar head region
smaller than that of the hydrophobic tail, resulting in
an inverted cone shape (e.g., PG), thus promoting a
negative curvature a higher peptide stoichiometry is
required for pore formation (Matsuzaki 2009); (ii)
length and degree of unsaturation of the acyl chain,
not only as a key factor for membrane fluidity, but
also influencing AMP insertion ability. As a rule of
thumb, the longer the chain, the lower the
perturbation caused by the AMP; in addition,
membranes with a high percentage of unsaturated
fatty acids, and thus of higher fluidity, are leakier
than more saturated ones (Matsuzaki 2009); (iii)
sterols cause membrane rigidification and may thus
impair AMP insertion. Prokaryotes, with the
exception of Mollicutes (e.g., mycoplasmas), lack
sterol in their PM, which explains the superior
permeabilization by AMPs in comparison with
eukaryote, sterol-rich PMs. The presence of
ergosterol in fungi, yeasts and some parasitic
Protozoa, is less protective against AMPs than the
cholesterol of higher eukaryotes.
Other non-lipidic factors also affecting the
outcome of an AMP-microbe encounter include (i)
external barriers such as the outer membrane (OM) of
Gram-negative bacteria, with anionic
lipopolysaccharide (LPS) as the major component of
its external leaflet. In order to reach its PM target, an
AMP traverses the OM by a mechanism called
"self-promoting uptake" (Rosenfeld and Shai 2006)
whereby it interacts first with anionic (lipid A) sites
of LPS, displacing Mg2+ ions that crosslink LPS
molecules, thereby disrupting the OM and allowing
access of other AMP molecules to the periplasm and
interaction with the PM. A further consequence of
AMP-LPS binding is neutralization of endotoxic
activity, preventing the shock caused by LPS release
after massive killing of bacteria by other antibiotics
(Jerala and Porro 2004); (ii) in Gram-positives, the
peptidoglycan layer is also a serious hurdle for AMP
translocation. Again, AMP interaction with (anionic)
teichoic and lipoteichoic acid units abrogates their
endotoxic character, avoiding an exalted
inflammatory response; (iii) other anionic
exopolysaccharides such as alginate or poly-gamma-DL-glutamic acid, produced by fungal and bacterial
biofilms, compete for AMP binding with anionic PLs
of the PM, reducing AMP efficacy (Otto 2006).
Encapsulated forms of fungi and bacteria are also
more resistant to AMPs (Rodriguez-Hernandez et al.
2006); (iv) the potential across the PM, negative at
the cytoplasmic side, promotes AMP insertion; hence,
AMP activity increases in polarized cells (Matsuzaki
2009). Finally (v) proteases specifically secreted by
each type of pathogen degrade AMP molecules to
various extents, thus contributing to the heterogeneity
of AMP activity observed for different pathogens
(Peschel 2002).
Intracellular targets
An increasing number of reports show that
permeation of pathogen membranes cannot
exclusively account for the lethal action of some
AMPs, and thus point to intracellular targets (Otvos
2005). In mechanistic terms, either the toroidal pore
or the inverted micelle models can satisfactorily
explain AMP translocation through a PL bilayer. For
AMPs acting in this way, the fact that the all-D enantiomer is inactive - in contrast to AMPs acting
purely through a PM-disruption mechanism - is
usually taken as solid evidence in this direction. In
most cases, the nature of the intracellular targets
remains unknown. For those few that have been
identified, targets are evolutionarily conserved
housekeeping systems essential for viability, such as
the DnaK chaperone for proline-rich AMPs
pyrrochorycin and A3-APO, or DNA for the
amphibian buforin (Otvos 2005). Mitochondria is the
target for histatin 5, an AMP from human saliva
(Luque-Ortega et al. 2008).
Alternative activities
Increasing evidence of AMP activities other than
pathogen killing and often differing in important
aspects from microbicidal action has been
accumulating over the last decades. Most though not
all of such activities can be said to follow a canonical
pattern of peptide-receptor recognition, usually at
concentrations one log below microbicidal ones, or in
environments where antimicrobial activity is in some
way blunted. These activities, in addition, tend to be
strictly specific for a given peptide, in contrast with
the broad antibiotic specificity of many AMPs. The
structural constraints of these additional activities do
not necessarily overlap with those of antimicrobial
action (Wu et al. 2003); for instance, chiral
discrimination tends to exist, in contrast with
membrane permeabilization, where the all-D enantiomer is usually equipotent with the natural
version.
Most of these additional features are beneficial
and synergistic vis-a-vis tissue repair and pathogen
elimination. Thus, AMPs are known to promote
proliferation of skin and mucosal cells, either directly
or through mobilization of growth factors bound to
the anionic sites of the glycocalyx (Otte et al. 2009).
For other AMPs such as LL-37 or PR-39, angiogenic
activity has been reported (Schittek et al. 2008). Also,
AMP cooperation with the antigen-specific immune
response has been described as taking place through
various mechanisms, including (i) induction of
vascular permeability by mast cell degranulation; (ii)
stimulation of chemokyne and chemotactic-like
activities on different types of immune cells; (iii)
maturation of antigen-processing cells; (iv) activation
of professional phagocytes, or (v) modulation of
cytokine maturation and release (Niyonsaba et al.
2009, Steinstraesser et al. 2009, Yang et al. 2009).
PHARMACOLOGICAL EXPLOITATION OF AMPs
AMPs have drawn the attention of the pharmaceutical
industry by (i) the potential of their unique killing
mechanism, different from the highly specific but
resistance-prone mechanisms of other antimicrobials;
(ii) their broad spectra of activity on a wide variety of
pathogens; (iii) their extremely low - though not nil
(Otto 2009) - levels of resistance induction; (iv) their
fast killing rates. On the downside of an AMP-based
therapy are high manufacturing costs (about 10x
those of typical antibiotics), poor bioavailability due
to protease degradation and sequestration by serum
components, and low tissue penetration.
Despite these caveats, AMPs remain an attractive
option for exogenous antibiotic chemotherapy. The
low levels of postranslational modification found in
animal or plant AMPs as compared with fungal and
bacterial counterparts make their chemical synthesis
and structural manipulation both feasible and
attractive. The goals of such modifications tend to be,
first, improved activity and, second, size reduction in
search of minimally active sequences that can
diminish immunogenicity and synthesis costs. Several
strategies along these lines are outlined in Table 2.
AMPs have also been proposed as candidate drugs
against novel or multirresistant pathogens, or reagents
against biological warfare (Dawson and Liu 2008),
given their ability to exploit common traits in
different pathogens, and their scarce cross-resistance
with other antibiotics.
Other potential uses for AMPs include infection
imaging, by means of AMP-99mTc conjugates which
allow discrimination between infectious and sterile
inflammation processes by scintigraphic methods
(Lupetti et al. 2003), and coating of medical devices
(bone and dental cements, surgical sutures) to prevent
biofilm or any other formation of infectious foci.
Encapsulation in biodegradable vehicles would allow
AMP sustained delivery for treating or preventing
infections difficult to reach by systemic antibiotic
administration, e.g., osteomyelitis. AMP inclusion in
chewing gum to prevent caries formation has also
been proposed.
The fact that AMPs are gene-encoded opens the
possibility of expression either in the original or in an
alien organism; expression can be transitory, e.g.,
encoded on adenoviral vectors, or permanent, e.g., in
transgenic plants or cattle. For instance, artificial skin
engineered to express AMPs can be grafted on
severely burned patients (Carretero et al. 2004), or
applied to stimulate re-epithelisation of chronic
wounds. Also, pups fed with lactoferrin-expressing
transgenic milk show improved survival and lower
infection rates, and protegrin 1-expressing transgenic
mice double their survival rate when infected with
Actinobacillus suis. Also highly promising is the
transgenic expression of AMPs in plants to prevent
phytopathogen infection (Marcos et al. 2008).
CELL-PENETRATING PEPTIDES AS ANTI-INFECTIOUS AGENTS?
The main plus of CPPs is their ability to transport
across the PM of cells a broad range of cargoes
including small drugs, metal ions, peptides and
proteins, nucleic acids or quantum dots (Torchilin
2008). The repertoire of CPPs has been constantly
growing, from the early Antennapedia and Tat
sequences to oligoarginines, proline-rich motifs or
Pep-1, which can deliver non-covalently-bound
cargoes (Vives et al. 2008). CPPs share structural
traits with AMPs, such as cationic character and
amphipathicity (to a degree). Their ability to
translocate across membranes has parallels with the
accepted mechanisms of action of AMPs (see 2.2
above); indeed, AMPs with intracellular targets (see
2.4 above) behave de facto as CPPs. Nevertheless,
CPP internalization mechanisms, involving
macropinocytosis, endocytosis by clathrin or caveolae
and possibly other processes in a non-mutually
excluding manner, appear to be more diverse and
complex than those of conventional AMPs (Alves et
al. 2010).
Some potential applications of CPPs in
anti-infectious therapy have hitherto been envisaged,
including i) uptake of drugs bypassing the absence or
defective function of a dedicated receptor (Koczan et
al. 2002); ii) uptake of otherwise PM-impermeable
cargo molecules such as "pepducins", peptide
inhibitors interfering with signal transduction
pathways, successfully assayed on cancer cells
(Watkins et al. 2009); iii) drug delivery and
accumulation into otherwise inaccessible
compartments (Rao et al. 2009), or organelle-specific
targeting by CPPs fitted with distinctive import motifs (Santra et al. 2005); iv) real-time monitoring of
infections by delivery of nuclease-resistant
fluorescent tags targeting specific regions of a
pathogen's genome; v) RNA anti-pathogen therapy
by means of antisense phosphorodiamidate
morpholino oligomers (PMO) directed to the RNA of
pathogens such as Ebola virus or Salmonella (Mitev
et al. 2009), and vi) boosting the immune response,
by immunogen coupling to a CPP and improved
uptake by antigen-processing cells.
Table 2. Some strategies of AMP modification.
| Modification |
Example |
Comments | | Optimization at a given
residue/position |
Cryptdin 4 |
Role of conservative
R K substitution in
antimicrobial activity | | Replacement of specific residue(s) |
Bactenecin 2 |
Optimization of antimicrobial activity | | Non-natural amino acid surrogates |
Ampetoids |
Improvement of biological stability | | Domain swapping |
Magainin 2 |
Modification of antimicrobial
and haemolytic activity | | Delineation of minimal active
sequence |
Cecropin-melittin hybrids |
Size reduction from 26 to 11 amino acids | | Increasing cationic character |
Short artificial peptides |
Improvement of antifungal activity | | Dimerization by interchain disulfide |
Murine beta defensin |
Improvement of antimicrobial activity | | Sequence hybridation |
Cecropin A-melittin |
Improved antimicrobial activity,
lower toxicity | | Juxtaposition of sequences with
different mechanisms |
Dermaseptin-RNAIII inhibiting
peptide |
Synergic antimicrobial activity | | Heterodimerization |
Distinctin |
Improved membrane interaction
and protease stability | | Linearization (disulfide reduction) |
Plant defensin IB-Amp1 |
Improved antimicrobial activity | | Cyclization (head-to-tail disulfide) |
Histatin 3 |
3-log improvement of re-epithelization
activity | | Disulfide engineering |
Minimal defensin template |
Modification of antimicrobial and
chemotactic profiles | | Dimerization;
intra interchain disulfide formation |
Bactenecin 5 |
Improved antimicrobial activity even
at high ionic strength | | Retro-enantio version |
Cecropin-melittin hybrids |
Improved stability and antimicrobial
activity | | Diastereomer formation |
alpha-helical linear peptides |
Reduced toxicity and protease susceptibility | | Acylation |
Cecropin A-melittin hybrids |
Improved antimicrobial activity |
CONCLUSIONS AND OUTLOOK
Membrane active agents, particularly peptides, are
currently at the threshold of a renewed lease of life.
Formerly disregarded by their poor selectivity, they
are undergoing a positive reappraisal that includes old
antibiotics such as polymyxin or gramicidin, as well
as AMPs and CPPs. Despite their many promising
features, no AMP has yet reached the status of a
clinically approved drug, and some authorized
opinions would limit AMP use to topical or colutory
formulations, given the high cost and therapeutic
uncertainty (partly due to lack of adequate trials)
associated with systemic administration. The more
recent arrival of CPPs, and the possibility of using
them in proof-of-concept trials for an increasing
number of drugs, will however face strong
competition from other nanopharmacological
approaches. Even so, it seems reasonable to foresee a
continued expansion of the membrane-active peptide
field, an area where biophysics, biochemistry, cell
biology and pharmacology meet together.
ACKNOWLEDGEMENTS
Work supported by the European Union
(HEALTH-2007-223414, Leishdrug, to L.R. and
D.A.), the Fondo de Investigaciones Sanitarias
(PI061125, PS09/01928 and RETICS-FEDER RD
06/0021/0006 to L.R.), the regional governments of
Madrid (S-BIO-0260/2006 to L.R) and Catalonia
(2009 SGR 492 to D.A.), and the Spanish Ministry of
Science and Innovation (PET2006-0139 to L.R. and
D.A.).
REFERENCES
Alves ID, Jiao CY, Aubry S, Aussedat B, Burlina F, Chassaing G, Sagan S: Cell biology meets biophysics to unveil the different mechanisms of
penetratin internalization in cells. Biochim Biophys Acta 2010, doi:10.1016/j.bbamem.2010.02.009.
Brogden KA, Ackermann M, Huttner KM: Detection of anionic antimicrobial peptides in ovine bronchoalveolar lavage fluid and respiratory epithelium.
Infect Immun 66:5948-5954, 1998.
Carretero M, Del Rio M, Garcia M, Escamez MJ, Mirones I, Rivas L, Balague C, Jorcano JL, Larcher F: A cutaneous gene therapy approach to treat
infection through keratinocyte-targeted overexpression of antimicrobial peptides. FASEB J 18:1931-1933, 2004.
Christensen B, Fink J, Merrifield RB, Mauzerall D: Channel-forming properties of cecropins and related model compounds incorporated into planar
lipid membranes. Proc Nat Acad Sci USA 85:5072-5076, 1988.
Dawson RM, Liu CQ: Properties and applications of antimicrobial peptides in biodefense against biological warfare threat agents. Crit Rev Microbiol
34:89-107, 2008.
Hopkins KL, Davies RH, Threlfall EJ: Mechanisms of quinolone resistance in Escherichia coli and Salmonella: recent developments. Int
J Antimicrob Agents 25:358-373, 2005.
Hornef MW, Putsep K, Karlsson J, Refai E, Andersson M: Increased diversity of intestinal antimicrobial peptides by covalent dimer formation. Nature
Immunol 5:836-843, 2004.
Jerala R, Porro M: Endotoxin neutralizing peptides. Curr Top Med Chem 4:1173-1184, 2004.
Koczan G, Ghose AC, Mookerjee A, Hudecz F: Methotrexate conjugate with branched polypeptide influences Leishmania donovani infection in
vitro and in experimental animals. Bioconjug Chem 13:518-524, 2002.
Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME: Interaction of human defensins with Escherichia coli. Mechanism of
bactericidal activity. J Clin Invest 84:553-561, 1989.
Lemaitre B, Hoffmann J: The host defense of Drosophila melanogaster. Annu Rev Immunol 25:697-743, 2007.
Lupetti A, Welling MM, Pauwels EK, Nibbering PH: Radiolabelled antimicrobial peptides for infection detection. Lancet Infect Dis 3:223-229,
2003.
Luque-Ortega JR, van't Hof W, Veerman EC, Saugar JM, Rivas L: Human antimicrobial peptide histatin 5 is a cell-penetrating peptide targeting
mitochondrial ATP synthesis in Leishmania. FASEB J 22:1817-1828, 2008.
Marcos JF, Munoz A, Perez-Paya E, Misra S, Lopez-Garcia B: Identification and rational design of novel antimicrobial peptides for plant protection.
Annu Rev Phytopathol 46:273-301, 2008.
Matsuzaki K: Control of cell selectivity of antimicrobial peptides. Biochim Biophys Acta 1788:1687-1692, 2009.
Melo MN, Ferre R, Castanho MA: Antimicrobial peptides: linking partition, activity and high membrane-bound concentrations. Nat Rev Microbiol
7:245-250, 2009.
Metz-Boutigue MH, Shooshtarizadeh P, Prevost G, Haikel Y, Chich JF: Antimicrobial peptides present in mammalian skin and gut are multifunctional
defence molecules. Curr Pharm Des 16:1024-1039, 2009.
Mitev GM, Mellbye BL, Iversen PL, Geller BL: Inhibition of intracellular growth of Salmonella enterica serovar Typhimurium in tissue
culture by antisense peptide-phosphorodiamidate morpholino oligomer. Antimicrob Agents Chemother 53:3700-3704, 2009.
Mogi T, Kita K: Gramicidin S and polymyxins: the revival of cationic cyclic peptide antibiotics. Cell Mol Life Sci 66:3821-3826, 2009.
Niyonsaba F, Nagaoka I, Ogawa H, Okumura K: Multifunctional antimicrobial proteins and peptides: natural activators of immune systems. Curr Pharm
Des 15:2393-2413, 2009.
Otte JM, Zdebik AE, Brand S, Chromik AM, Strauss S, Schmitz F, Steinstraesser L, Schmidt WE: Effects of the cathelicidin LL-37 on intestinal
epithelial barrier integrity. Regul Pept 156:104-117, 2009.
Otto M: Bacterial evasion of antimicrobial peptides by biofilm formation. Curr Top Microbiol Immunol 306:251-258, 2006.
Otto M: Bacterial sensing of antimicrobial peptides. Contrib Microbiol 16:136-149, 2009.
Otvos L, Jr.: Antibacterial peptides and proteins with multiple cellular targets. J Pept Sci 11:697-706, 2005.
Peschel A: How do bacteria resist human antimicrobial peptides? Trends Microbiol 10:179-186, 2002.
Powers JP, Tan A, Ramamoorthy A, Hancock REW: Solution structure and interaction of the antimicrobial polyphemusins with lipid membranes.
Biochemistry 44:15504-15513, 2005.
Rao KS, Ghorpade A, Labhasetwar V: Targeting anti-HIV drugs to the CNS. Expert Opin Drug Deliv 6:771-784, 2009.
Rodriguez-Hernandez MJ, Saugar JM, Docobo-Perez F, de la Torre BG, Pachon-Ibanez ME, Garcia-Curiel A, Fernandez-Cuenca F, Andreu D, Rivas L, Pachon
J: Studies on the antimicrobial activity of cecropin A-melittin hybrid peptides in colistin-resistant clinical isolates of Acinetobacter
baumannii. J Antimicrob Chemother 58:95-100, 2006.
Rosenfeld Y, Shai Y: Lipopolysaccharide (Endotoxin)-host defense antibacterial peptides interactions: role in bacterial resistance and prevention
of sepsis. Biochim Biophys Acta 1758:1513-1522, 2006.
Santra S, Yang H, Stanley JT, Holloway PH, Moudgil BM, Walter G, Mericle RA: Rapid and effective labeling of brain tissue using TAT-conjugated
CdS:Mn/ZnS quantum dots. Chem Commun (Camb) 25:3144-3146, 2005.
Schittek B, Paulmann M, Senyurek I, Steffen H: The role of antimicrobial peptides in human skin and in skin infectious diseases. Infect Disord Drug
Targets 8:135-143, 2008.
Selsted ME: theta-defensins: cyclic antimicrobial peptides produced by binary ligation of truncated alpha-defensins. Curr Protein Pept Sci
5:365-371, 2004.
Steinstraesser L, Kraneburg UM, Hirsch T, Kesting M, Steinau HU, Jacobsen F, Al-Benna S: Host defense peptides as effector molecules of the innate
immune response: a sledgehammer for drug resistance? Int J Mol Sci 10:3951-3970, 2009.
Sun X, Salih E, Oppenheim FG, Helmerhorst EJ: Kinetics of histatin proteolysis in whole saliva and the effect on bioactive domains with
metal-binding, antifungal, and wound-healing properties. FASEB J 23:2691-2701, 2009.
Torchilin VP: Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers 90:604-610,
2008.
Vives E, Schmidt J, Pelegrin A: Cell-penetrating and cell-targeting peptides in drug delivery. Biochim Biophys Acta 1786:126-138, 2008.
Wade D, Boman A, Wahlin B, Drain CM, Andreu D, Boman HG, Merrifield RB: All-D amino acid-containing channel-forming antibiotic peptides. Proc Nat
Acad Sci USA 87:4761-4765, 1990.
Watkins CL, Brennan P, Fegan C, Takayama K, Nakase I, Futaki S, Jones AT: Cellular uptake, distribution and cytotoxicity of the hydrophobic cell
penetrating peptide sequence PFVYLI linked to the proapoptotic domain peptide PAD. J Control Release 140:237-244, 2009.
Wu Z, Hoover DM, Yang D, Boulegue C, Santamaria F, Oppenheim JJ, Lubkowski J, Lu W: Engineering disulfide bridges to dissect antimicrobial and
chemotactic activities of human beta-defensin 3. Proc Nat Acad Sci USA 100:8880-8885, 2003.
Yang D, de la Rosa G, Tewary P, Oppenheim JJ: Alarmins link neutrophils and dendritic cells. Trends Immunol 30:531-537, 2009.
Zasloff M: Antimicrobial RNases of human skin. J Invest Dermatol 129:2091-2093, 2009.
Zhang L, Falla TJ: Host defense peptides for use as potential therapeutics. Curr Opin Investig Drugs 10:164-171, 2009.
|
BACK
|