Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 4, p 199-208
DOI 10.2478/v10136-009-0027-3
Arsenic trioxide as an anti-tumour agent: mechanisms of action and strategies of sensitization
Yolanda Sanchez, Donna Amran, Elena de Blas, Patricio Aller
Address: Patricio Aller, Centro de Investigaciones Biologicas, CSIC, Ramiro de Maeztu 9, 28040-Madrid, Spain
aller@cib.csic.es
Received 4th March 2010.
Revised 11th May 2010.
Published online 26th May 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Induction of moderate oxidative stress as a sensitizing strategy
Protein kinase modulators as pro-oxidant and sensitizing agents
Dietary polyphenols as pro-oxidant and sensitizing agents
Acknowledgements
References
SUMMARY
Arsenic trioxide (As2O3, ATO) is a very efficacious, clinically established agent for the treatment of acute promyelocytic leukaemia, and also potentially useful against other haematological and non-haematological malignancies. Nonetheless, the relative resistance of many tumour cell types requires the generation of sensitizing strategies. One of the properties of ATO which might be exploited for therapeutic purposes is its sensitivity to the intracellular oxidant state, as revealed by increased apoptosis production under conditions of reduced glutathione (GSH) depletion and/or elevated reactive oxygen species (ROS) content. This review summarizes some studies from our laboratory demonstrating that experimental modulation of protein kinase activities (PI3K/Akt, JNK, MEK/ERK) potentiates ATO-provoked apoptosis in relatively resistant human acute myeloid leukaemia (U937, HL60) cell lines by mechanisms involving GSH depletion and/or increased ROS content. In a similar manner, co-treatment with dietary flavonoides such as genistein, normally considered as anti-oxidants, may potentiate apoptosis via generation of moderate oxidative stress and activation of ROS-inducible protein kinases. Finally, co-treatment with ATO may sensitize otherwise refractory leukaemia cells to TNFalpha-family cytokine-produced apoptosis, by mechanisms involving the interplay between the "intrinsic" (mitochondrial) and "extrinsic" (death receptor-mediated) pathways.
KEY WORDS
arsenic trioxide; apoptosis; oxidative stress; protein kinases; phenolic agents; TNFalpha; leukaemia cells
INTRODUCTION
Although the medical use of arsenicals is very ancient
(for review see Miller et al. 2002), it was only
recently that the anti-tumour importance of some
these compounds was demonstrated beyond all doubt.
Clinical assays carried out during the 1990s at
Chinese (Harbin and Shanghai) medical institutions
revealed that treatment with low, non-toxic doses of
arsenic trioxide (As2O3, ATO) caused high rates of
complete remission in patients suffering from acute
promyelocytic leukaemia (APL), either newly
diagnosed or relapsed after treatment with the more
classical agent all-trans retinoic acid (ATRA)
(reviewed by Wang and Chen 2008). Under the
trademark of TRISENOX, ATO was later approved
by the Food and Drug Administration in USA, and
also recently by some European countries, as a highly
efficacious and relatively safe agent to treat
ATRA-refractory or relapsed APLs. Recent studies
indicated that higher rates of durable remission may
be still obtained using adequate ATO plus ATRA
combinations (Wang and Chen 2008).
It is normally accepted that ATO efficacy is due
to the capacity of the drug to disrupt the PML-RARalpha
fusion onco-protein, derived from the t(15;17)
chromosomal translocation, characteristic of most
APLs. Since trivalent arsenicals easily react with
vicinal sulfhydryl (SH) residues in proteins, ATO
probably binds a cysteine-rich region at the PML
moiety, leading to PML-RARalpha degradation. This
would in turn relieve the APL cells from the
differentiation blockade imposed by the fusion
protein, driving them into terminal differentiation, the
main response at low ATO concentrations (approx.
0.1-0.5 microM in in vitro assays). At higher but still
physiologically acceptable concentrations (approx.
0.5-2 microM) ATO preferentially induces apoptosis
instead of differentiation. Nonetheless, it is doubtful
that PML-RARalpha disruption is the main cause for
apoptosis generation. Actually, the drug exerts many
other effects which, derived or not from ATO-cystein
protein interactions, are potentially deleterious. As a
few examples, ATO may cause reactive oxygen
species (ROS) over-production, either from
mitochondrial or extra-mitochondrial origin
(respiratory chain inhibition, or activation of NADPH
oxidase and NO synthase isozymes). ATO may also
directly bind the adenine nucleotide translocator
(ANT) at the mitochondrial permeability transition
pore (mPTP) complex. Both ROS over-accumulation
and direct binding may lead to mPTP opening,
mitochondria dysfunction and apoptosis. In addition,
ATO may elicit death receptor (DR5, TNF-R1)
expression which facilitates apoptosis along the
death-receptor mediated pathway (vide infra). ATO
binds tubulin, disrupting the microtubule assembly
and spindle formation, affecting cell cycle
progression and viability. ATO may facilitate
apoptosis by inhibiting IkappaB kinase and NF-kappaB
activation, by inhibiting JAK/STAT pathways, and by
activating pro-apoptotic (JNK, p38) MAPK
pathways. ATO may also disrupt other oncogenic
fusion proteins, such as Bcr-Abl, characteristic of
chronic myelogenous leukaemia, or
AML1/MDS1/EVI1, present in some forms of
leukaemia (for reviews see Miller et al. 2002, Wang
and Chen 2008, Platanias 2009, among many others).
Due to these and other effects, ATO causes apoptosis
in cells from haematologic malignancies other than
APL, such as myelodisplastic syndromes (MDS),
multiple myeloma (MM), acute myeloid leukaemia
(AML) and chronic myelogenous leukaemia (CML)
(Amadori et al. 2005) and in solid tumour-derived cell
lines. While the potential value of ATO is supported
by clinical assays in some cases (e.g., MDS, MM),
the applicability is restricted in other cases by the
requirement of high toxic doses to effectively induce
apoptosis. Thus, extending the therapeutic application
of ATO would require the generation of sensitizing
strategies, allowing decreasing the effective drug
concentrations.
There are many excellent original studies and
review articles dealing with historical, clinical, and
mechanistic aspects of ATO, which are accessible to
the reader but will not be commented on here.
Instead, following editorial advice, the attention in
this review will focus on some sensitizing approaches
recently assayed in our laboratory.
INDUCTION OF MODERATE OXIDATIVE STRESS AS A SENSITIZING STRATEGY
A common characteristic of tumour cells is the
presence of elevated ROS levels and oxidative
damage to macromolecules, which are probably
responsible for their oncogenic transformation and
increased metabolic activity. On the other hand the
intrinsic elevated level of oxidative stress makes
tumour cells more susceptible than normal cells to the
action of ROS-inducing cytotoxic agents, a property
which may be exploited in anti-tumour therapies
(Ozben 2007, Lau et al. 2008).
In addition to acting as a pro-oxidant agent itself,
ATO is an oxidative stress- responsive drug - i.e., its
toxicity depends very much on the oxidant state of the
cells. For instance, ATO sensitivity in tumour cells
inversely correlates with the inherent levels of GSH
or GSH-associated enzymes (Dai et al. 1999, Yang et
al. 1999). Due to the multiplicity of functions of
glutathione, decreased GSH content may compromise
essential defensive functions, such as ROS
scavenging by glutathione peroxidases (leading to
ROS over-accumulation) or ATO detoxification via
glutathione S-transferases. Moreover, since GSH may
directly bind and quench trivalent arsenic (Scott et al.
1993), a decrease in GSH may result in increased free
intracellular ATO content and toxicity. In addition,
the cell susceptibility to ATO toxicity directly
correlates with the inherent intracellular ROS content
(Yi et al. 2002). There are many studies
demonstrating that experimental modulation of the
oxidant environment, either by provoking GSH
depletion (e.g. by treatment with the
glutamate-cysteine ligase (gamma-GCS) inhibitor
D,L-buthionine-(S,R)-sulfoximine (BSO), or with
ascorbic acid), or by co-treatment with exogenous
H2O2 or ROS increasing agents, enhances
ATO-provoked apoptosis in cultured cells and animal
cell models (Dai et al. 1999, Pelicano et al. 2003,
Maeda et al. 2004, Lau et al. 2008, and our
unpublished observations) (see also Fig. 1).
Interestingly, the potential efficacy of ATO/ascorbic
acid combinations has been clinically assessed in
phase II trials (Abou-Jawde et al. 2006).
The following paragraphs are dedicated to the
description of two sensitizing strategies assayed in
our laboratory in which oxidative stress seems to play
a decisive regulatory role, namely modulation of
protein kinase activities and co-treatment with dietary
polyphenols. Experiments were mostly carried out in
U937 and HL60 cells, two AML cell lines considered
as relatively resistant to ATO toxicity.
PROTEIN KINASE MODULATORS AS PRO-OXIDANT AND SENSITIZING AGENTS
A frequent characteristic of tumour cells is the
constitutive activation of defensive signalling
pathways (e.g. PI3K/Akt, Raf/MEK/ERK,
JAK/STAT), a property which may contribute to their
abnormal elevated survival capacity and increased
resistance to anticancer treatments. Hence, these
pathways represent potential important targets for
pharmacological intervention, and actually
considerable efforts are being directed to the
development of new compounds combining good
inhibitory potency with physiological tolerance.
There are many bibliographic examples
demonstrating that experimental down-regulation of
kinase activities increase the apoptotic efficacy of
anti-tumour agents, including ATO (Tabellini et al.
2005, Ye et al. 2005, Altman et al. 2008, Lunghi et al.
2008, among others).
With these precedents in mind, we analyzed the
capacity of some pharmacological PI3K/Akt and
MAPK inhibitors to modulate the toxicity of ATO in
U937 and other human acute myeloid leukaemia cell
lines. In two successive articles (Ramos et al. 2005,
2006) we reported that co-treatment with non-toxic
concentrations of PI3K/Akt (LY294002, wortmannin,
AktiV), MEK/ERK (PD98059, U0126) and JNK
(SP600125) inhibitors greatly increased
ATO-provoked apoptosis, while the effects were poor
or null when the inhibitors were combined with other
anti-tumour drugs (e.g., the DNA-targeting agents
cisplatin and camptothecin). Although not originally
contemplated, we could also state that the kinase
inhibitors decreased the intracellular GSH content
(and when determined, the GSH/GSSG ratio) and
increased intracellular ROS over-accumulation, which
were at least in part responsible for apoptosis
potentiation (see some results, Fig. 1). Mechanistic
studies revealed that GSH depletion could be
regulated at different levels. Thus, PI3K and JNK
inhibitors down-regulated gamma-GCS activity, in similar
manner as the canonical GSH suppressor BSO. In
contrast, MEK/ERK inhibitors caused
gamma-GCS-independent, N-acetyl-L-cysteine
(NAC)-preventable GSH depletion, suggesting
regulation at the level of substrate (cysteine)
availability. It must be noted that most current
bibliographic information indicates modulation
(generally stimulation) of protein kinase activities by
oxidative stress, although some reports suggest that
the inverse cause-effect relationship, namely
modulation of oxidative stress parameters by protein
kinases, is also possible. For instance, it was
described that gamma-GCS activity is susceptible of
modulation by changes in phosphorylation (Sun et al.
1996), and that PI3K inhibitors suppressed
insulin-provoked increase in gamma-GCS synthesis in rat
hepatocytes (Kim et al. 2004), two observations that
are fully compatible with our results. Of note, our
findings revealing that the potentiation of
ATO-provoked apoptosis by MEK/ERK and JNK
inhibitors is mediated by GSH depletion have recently
been corroborated by other authors (Lin et al. 2009).
Of course, multiple factors other than oxidative
stress may participate in the potentiation of
ATO-provoked apoptosis by protein kinase
modulators. For instance, Lunghi et al. (2008)
reported that MEK/ERK inhibitors potentiated
ATO-provoked apoptosis in multiple myeloma cells
deficient for p53 (a situation similar to HL60 and
U937 AML cells) via increased expression of
pro-apoptotic Bim and death receptors (DRs) 4 and 5.
Nonetheless, some relationship of these findings with
oxidative stress might be possible, since other authors
also reported involvement of DR5 over-expression in
ATO-provoked apoptosis under conditions of GSH
depletion in U937 and other leukaemia cells (Chen et
al. 2006). In addition, the relationship linking protein
kinase down-regulation with generation of oxidative
stress is not univocal, but may depend on the
experimental conditions. For instance, Sordet et al.
(2001) indicated that 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-treated, differentiated U937
cells exhibited increased susceptibility to apoptosis
induction by ATO, and that this effect was associated
with a decrease in GSH content and an increase in
ROS accumulation. In a later study (Fernandez et al.
2004) we corroborated these results using two PKC
activators, TPA and bryostatin 1, and also
demonstrated that TPA- or bryostatin 1-provoked
GSH decrease and apoptosis potentiation were
preceded and mediated by ERK activation. Thus,while these results corroborate the importance of
oxidative stress in cell sensitization to ATO-provoked
apoptosis, they also indicate that oxidative stress is
compatible with two opposite modes of ERK
modulation, namely down-regulation by
pharmacological kinase inhibitors and up-regulation
by PKC activators.
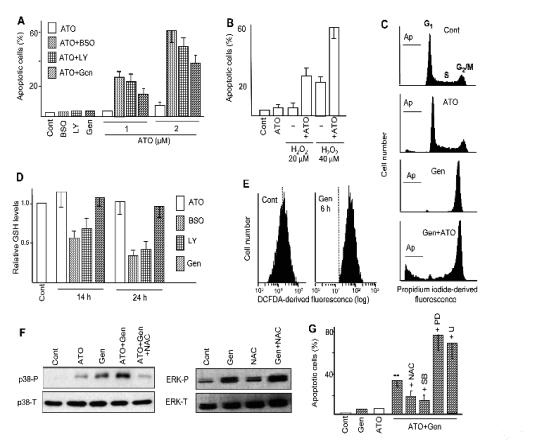
Fig. 1. Potentiation of ATO-provoked apoptosis by pro-oxidant agents. (A, B) Frequency of apoptosis, as determined by
chromatin condensation/fragmentation, in U937 cell cultures treated for 24 h with the GSH suppressing agent
DL-buthione-(S,R)-sulfoximine (BSO, 1 mM), the PI3K inhibitor LY294002 (LY, 30 M), the isoflavone genistein (Gen, 50 microM),
low H2O2 concentrations, and ATO (2 microM, except when otherwise indicated), alone and in combination. Observe that the effects
in combined treatments are higher than those caused by each agent alone. (C) Cell cycle distribution and frequency of apoptotic
cells (Ap) at 24 h of treatment, as determined by propidium iodide staining and flow cytometry assays, in cultures treated with
ATO and genistein, alone and in combination. (D) Relative intracellular GSH content, as measured by monochlorobimane
derivatization and fluorimetry determination, 14 and 24 h of treatment with the indicated agents. The value 1 was arbitrarily given
to untreated (Cont) cells. (E) Increase intracellular ROS content upon treatment with genistein, as determined by labelling with
H2DCFDA and fluorescence measurement by flow cytometry. (F) Relative levels of phosphorylated (P) and total (T) p38-MAPK
and ERK, as determined at 16 h of treatment by immunoblot. Observe that genistein stimulates phosphorylation/activation of
both kinases, but only p38-MAPK is dependent on oxidative stress, as indicated by the inhibitory action of the antioxidant agent
N-acetyl-L-cysteine (NAC, 10 mM). (G) Modulation of ATO plus genistein-provoked apoptosis by NAC, the p38-MAPK
inhibitor SB203580 (SB, 10 microM), and the MEK/ERK inhibitors PD98059 (PD, 20 microM) and U0126 (U, 2.5 microM). Observe that
NAC and SB203580 exert a protective action, indicating that apoptosis induction is mediated by oxidative stress and p38-MAPK.
On the other hand PD98059 and U0126 over-increase apoptosis generation, indicating a protective action of ERKs.
DIETARY POLYPHENOLS AS PRO-OXIDANT AND SENSITIZING AGENTS
Flavonoids and other plant-derived phenolic agents
represent a large group of compounds present in our
daily diet. In the low concentrations obtainable from
food, polyphenols normally exert beneficial
chemo-preventive and anti-inflammatory effects,
which are attributed to their anti-oxidant capacities
(Rahman et al. 2006). Nonetheless at higher (but still
physiologically tolerable) concentrations these agents
may cause cytoreduction by inducing tumour cell
apoptosis. The anti-tumour capacity of polyphenols
was demonstrated under cell culture conditions, but
also corroborated in vivo using mouse models (e.g.,
using genistein, Mohammad et al. 2006) and clinical
assays in humans (e.g., curcumin, reviewed by Anand
et al. 2008). Because of this, and also because they
are better tolerated by the organism than most
conventional anticancer drugs, the possibility of
incorporating natural phenolic agents in clinical
protocols has attracted great attention. Anyway, rather
than as monotherapy, polyphenols may be more
efficacious when used as radio- and
chemo-sensitizing agents, in combination with
conventional antitumour agents (Garg et al. 2005,
Sarkar and Li 2006, Limtrakul 2007). The
pro-apoptotic action of polyphenols is normally
attributed to their capacity to down-regulate
defensive, anti-apoptotic factors, such as NF-kappaB and
the PI3K/Akt pathway (Aggarwall and Shishodia
2006, Ramos 2008). Nonetheless, it must be noted
that in spite of their normal anti-oxidant functions,
some polyphenols may act as pro-oxidant agents,
causing ROS over-production from either
mitochondrial (Salvi et al. 2002) or
extra-mitochondrial (Galati et al. 2002) sources. The
physiological and perhaps therapeutical consequences
of polyphenol-provoked oxidative stress have been
insufficiently explored.
In a recent publication (Sanchez et al. 2008) we
demonstrated that co-treatment with the soy-derived
isoflavone genistein greatly increased ATO-provoked
apoptosis in human myeloid and lymphoid leukaemia
cell lines. This represented a somewhat drug- and
tumour cell-selective response, since isoflavone-
provoked sensitization was poor or null in
combination with other anti-tumour agents (e.g., the
DNA-targeting drug cisplatin), or when combined
with ATO in non-tumour proliferating peripheral
blood lymphocytes. Time-course studies revealed that
within the time-period required for apoptosis
execution (16-24 h) genistein did not affect NF-kappaB or
Akt activation, which were only down-regulated at
later times (48 h and thereafter). On the other hand,
the isoflavone caused a rapid (3-6 h) and moderate
ROS over-accumulation which, as indicated by the
protective action of anti-oxidant agents, was at least
in part responsible for the pro-apoptotic response.
Moreover, genistein caused ROS-dependent activation of p38-MAPK and AMPK kinases which,
as indicated by the protective action of
pharmacological inhibitors or specific siRNAs, also
mediated apoptosis potentiation (some of these results
are presented in Fig. 1). It is noteworthy that genistein
also caused ROS-independent activation of the
Raf-1/MEK/ERK pathway. However this pathway did
not promote but instead restrained apoptosis, since
co-treatment with pharmacological MEK/ERK
inhibitors further increased the toxicity ATO plus
genistein (Sanchez et al. 2009a). While ROS
over-production also mediate the potentiation of
ATO-provoked apoptosis by other polyphenols - e.g.,
epigallocatechin-3-gallate [EGCG], naringenin and
curcumin (Nakazato et al. 2005, Sanchez et al. 2009b,
and our unpublished results) - this is not the only
possible mechanism responsible for ATO
sensitization. For instance, co-treatment with the
flavonol quercetin greatly potentiated ATO-provoked
apoptosis in U937 leukaemia cells, in the absence of
measurable ROS over-production, and without
apparent protection by ROS-scavenging agents.
Nevertheless, quercetin rapidly caused Akt
dephosphorylation/inactivation (16 h) together with
slight GSH depletion (Ramos and Aller 2008), a
response which as demonstrated above suffices to
increase ATO toxicity.
Although not directly within the scope of this
review, it may be worthy of mention that phenolic
agents cause multiple molecular and cellular effects
other than apoptosis, but also with potential
therapeutic benefits. For instance, genistein inhibits
cell proliferation by causing G2/M cycle phase arrest.
Indirect determinations suggested that
genistein-provoked G2/M blockade is probably
derived from its action as a DNA topoisomerase II
inhibitor, while the pro-oxidant and pro-apoptotic
responses of the isoflavone are apparently associated
with its capacity to inhibit tyrosine kinases (Sanchez
et al. 2009b, and references therein). In addition,
genistein induces differentiation in human AML cell
lines, a response which in contrast to the
pro-apoptotic action of the isoflavone is not regulated
by ROS and p38-MAPK, but is dependent on
persistent Raf-1/MEK/ERK activation (Sanchez et al.
2009a). Importantly, this study also indicated
cooperative effects between genistein and ATRA,
namely potentiation of ATRA-provoked
differentiation by low genistein concentrations, and
prevention by ATRA of the long-term toxic effects of
the isoflavone. Hence, the potential application of
genistein and perhaps other related phenolic agents in
differentiation therapies deserves to be considered.
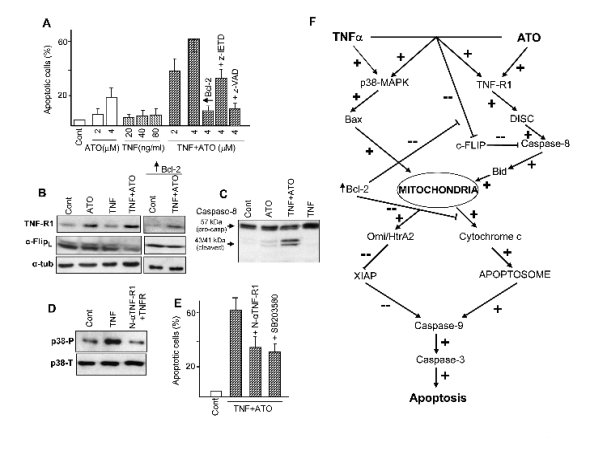
Fig. 2. Cooperative effects between ATO and TNFalpha. (A) Frequency of apoptosis in U937 cells upon treatment with the
indicated concentrations of ATO and TNFalpha, alone and in combination. + Bcl-2, + z-IETD and + z-VAD indicate the degree of
protection obtained by Bcl-2 transfection or co-treatment with the caspase-8 inhibitor z-IETD-fmk or the pan-caspase inhibitor
z-VAD-fmk (50 microM). (B, C) Expression of TNF-R1 and c-FLIPL (B) and caspase-8/cleavage activation (C) in cells subjected
to the indicated treatments. Observe that Bcl-2 transfection prevents c-FLIPL down-regulation by TNFalpha plus ATO. (D)
Stimulation of p38-MAPK phosphorylation/activation by TNFalpha, and its prevention by co-treatment with anti-TNF-R1
neutralizing antibody (N-alphaTNF-R1). (E) Attenuation of TNF+ATO-provoked apoptosis by co-treatment with N-TNF-R1 or
the p38-MAPK inhibitor SB203580. Except when otherwise indicated, ATO was used at 4 microM and TNFalpha at 40 ng/ml.
Determinations of apoptosis (A, E) were carried out at 24 h of treatment, and protein analysis (B-D) at 16 h of treatment. (F)
These and other (not shown) results were used to elaborate the present scheme, which describes the interaction between the
extrinsic and intrinsic pathways. In summary ATO, alone or with TNFalpha, elicits TNF-R1 expression and c-FLIP inhibition. This
facilitates cytokine-provoked activation of the extrinsic (receptor-mediated) pathway, involving caspase-8. Complementary
TNFalpha, alone or with ATO, causes p38-MAPK activation, which in turn facilitates Bax-mediated activation of the intrinsic
(mitochondrial) pathway. Both pathways are mutually interdependent. Thus, caspase-8 activation engages the mitochondrial
pathway via Bid cleavage/activation; and enforced Bcl-2 expression not only prevents mitochondrial activation (e.g., inhibition
of cytochrome c release), but also restrains caspase-8 activation by preventing c-FLIP inhibition. Mitochondrial activation
involves cytochrome c release, which facilitates apoptosome assembly, and Omi/HtrA2 release, which causes degradation of the
inhibitory protein XIAP, both effects leading to the activation of the caspase-9/caspase-3 pathway. Symbols + and - indicate
up-regulation and down-regulation, respectively, of either expression or activity. These results were adapted from Amran et al.
(2007), Ph.D. dissertation, Universidad Complutense de Madrid.
ARSENIC TRIOXIDE AS A SENSITIZING AGENT
It is known that apoptosis is executed throughout two
main discernible pathways, termed "intrinsic"
(mitochondrial) and "extrinsic" (death
receptor-mediated) (Fulda and Debatin 2006). In
short, the intrinsic pathway, induced by most
chemotherapeutic agents, is characterized by the
release of cytochrome c and other
mitochondria-located proteins to the cytosol,
assembly and activation of the apoptosome,
degradation of caspase-restraining IAPs ("inhibitor of
apoptosis proteins"), and sequential activation of
initiator caspase-9 and effector caspases (-3 and/or -7). On the other hand the extrinsic pathway,
triggered by cytokines of the tumour necrosis factor
alpha (TNFalpha) family, is initiated by cytokine binding
and activation of death receptors (TNF-Rs), followed
by DISC ("death-inducing signalling complex")
formation and activation of initiator caspase-8 (or
-10) and effector caspases. In some cell types (termed
"type I" cells), in which DISC formation is strong
enough, intense caspase-8 activation may suffice to
directly elicit effector caspase activation and final
apoptosis. By contrast, in cells in which DISC
formation is weak (termed "type II" cells), the
apoptotic signal requires amplification by
mitochondrial engagement, via caspase-8-mediated
activation of the Bcl-2-family pro-apoptotic protein
Bid.
While the preceding paragraphs were aimed at
considering strategies of sensitization to
ATO-provoked apoptosis, ATO itself may in turn
operate as a sensitizing agent. In fact, in addition to
the capacity of ATO to trigger mitochondria-dependent apoptosis by direct binding to
mPTP, low drug concentrations may induce
death-receptor expression (e.g., Liu et al 2003,
Szegezdi et al 2006, Kim et al 2008), thus rendering
the cells more responsive to TNFalpha family cytokines.
In a recent study (Amran et al. 2007), we proposed
that co-treatment or pre-treatment with ATO
sensitized the otherwise TNFalpha-refractory U937
myeloid leukaemia cells to cytokine-provoked
apoptosis, and that sensitization was the result of a
complex mechanism involving the interplay of both
receptor-mediated and mitochondrial pathways (see
some selected results and a schematic representation
in Fig. 2). Thus, the combination of ATO plus TNFalpha
efficaciously activated typical events of the
mitochondrial executioner pathway, such as release of
mitochondrial-located proteins (cytochrome c and
Omi/HtrA2) to the cytosol, Bax translocation to
mitochondria, XIAP degradation, and activation of
the caspase-9/-3 pathway, while the effects of ATO
and TNFalpha separately were negligible. When upstream
regulatory events were investigated, it was observed
that ATO (alone or in combination with TNFalpha)
stimulated receptor type 1 (TNF-R1) expression,
decreased the caspase-8 antagonist c-FLIP ("FLICE
inhibitory protein") expression, and elicited
cleavage/activation of caspase-8 and Bid.
Determinations using appropriate anti-TNF-R1
neutralizing antibody and caspase-8-specific inhibitor
demonstrated that activation of the
TNF-R1/caspase-8/Bid axis was required for final
apoptosis execution. Conversely and importantly, we
could observe that enforced Bcl-2 over-expression
prevented c-FLIP inhibition and caspase-8 activation,
indicating that proper activation of the extrinsic
pathway is under mitochondrial control. Finally (and
also importantly), TNFalpha caused TNF-R1-dependent
phosphorylation/activation of p38-MAPK, and this
activation was required for proper activation of the
mitochondrial executioner pathway.
While the use of TNFalpha in anti-tumour therapies is
questionable (actually we selected this cytokine only
for mechanistic reasons, not for therapeutic
considerations), we and others also demonstrated
cooperation to induce apoptosis between ATO and
the TNF-alpha family member Apo2L/TRAIL, both in
leukaemic (Liu t al. 2003, Szegezdi et al. 2006) and
non-leukaemic (Kim et al. 2008) tumour cell models.
Apo2L/TRAIL is considered to be a promising
anticancer agent because of its capacity to induce
apoptosis in cancer cells with lower toxicity towards
normal cells (Kimberley et al. 2004), but its efficacy
as a single agent is frequently limited. Hence, priming
with ATO might represent a valuable strategy to
improve the clinical efficacy of this cytokine.
CONCLUSIONS
In summary, the variety of molecular effects with
evident pro-apoptotic consequences makes of ATO a
promissory drug for the treatment of multiple
neoplastic diseases. Nonetheless, adequate
combination with sensitizing agents, often desirable
in clinical protocols, may be a sine qua non condition
in the case of ATO to conciliate clinical efficacy with
low, tolerable side-toxic effects. The well-known
susceptibility of ATO toxicity to moderate oxidative
stress - either intracellular GSH depletion or
increased ROS accumulation - offers an adequate
framework for the generation of sensitizing strategies.
Among many other examples presented in the
literature, we have demonstrated that co-treatment
with pharmacological protein kinase inhibitors and
dietary polyphenols may offer excellent possibilities,
and that the sensitizing capacity of these agents is in
part mediated by the generation of moderate oxidative
stress, at least under in vitro conditions. Conversely,
the capacity of ATO to activate apical events of
"extrinsic" apoptotic pathway might represent a way
of improving the efficacy of cytokines with potential
clinical application.
ACKNOWLEDGEMENTS
This work was supported by the Ministerio de Ciencia
e Innovacion, Spain, Grant SAF2007-64721 (Plan
Nacional de Investigacion Cientifica, Desarrollo e
Innovacion Tecnologica, Direccion General de
Investigacion), and Grant CSD2007-00020 (Programa
Consolider-Ingenio 2010).
REFERENCES
Abou-Jawde RM, Reed J, Kelly M, Walker E, Andresen S, Baz R, Karam MA, Hussein M: Efficacy and safety results with the combination therapy of arsenic trioxide, dexamethasone, and ascorbic acid in multiple myeloma patients: a phase 2 trial. Med Oncol 23:263-272, 2006.
Aggarwall BB, Shishodia S: Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71:1397-1421, 2006.
Altman JK, Yoon P, Katsoulidis E, Kroczynska B, Sassano A, Redig AJ, Glaser H, Jordan A, Tallman MS, Hay N, Platanias LC: Regulatory effects of mammalian target of rapamycin-mediated signals in the generation of arsenic trioxide responses. J Biol Chem 283:1992-2001, 2008.
Amadori S, Fenaux P, Ludwig H, O'Dwyer M, Sanz M: Use of arsenic trioxide in haematological malignancies: insight into the clinical development of a novel agent. Curr Med Res Opin 21:403-411, 2005.
Amran D, Sanchez Y, Fernandez C, Ramos AM, de Blas E, Breard D, Calle C, Aller P: Arsenic trioxide sensitizes promonocytic leukaemia cells to TNFalpha-induced apoptosis via p38-MAPK-regulated activation of both receptor-mediated and mitochondrial pathways. Biochim Biophys Acta 1773:1653-1663, 2007.
Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Agarwall BB: Curcumin and cancer: An "old-age" disease with an "age-ol" solution. Cancer Lett 267:133-164, 2008.
Chen D, Chan R, Waxman S, Jing Y: Buthionine sulfoximine enhancement of arsenic trioxide-induced apoptosis in leukaemia and lymphoma cells is mediated via activation of c-Jun NH2-terminal kinase and up-regulation of death receptors. Cancer Res 66:11416-11423, 2006.
Dai J, Weinberg RS, Waxman S, Jing Y: Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood 93:268-277, 1999.
Fernandez C, Ramos AM, Sancho P, Amran D, de Blas E, Aller P: 12-O-tetradecanoylphorbol-13-acetate may both potentiate and decrease the generation of apoptosis by the antileukemic agent arsenic trioxide in human promonocytic cells. Regulation by extracellular signal-regulated protein kinases and glutathione. J Biol Chem 279:3877-3884, 2004.
Fulda S, Debatin KM: Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25:4798-4811, 2006.
Galati G, Sabzevari O, Wilson JX, O'Brien PJ: Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 177:91-104, 2002.
Garg AK, Buchholz TA, Aggarwall BB: Chemosensitization and radiosensitization of tumours by plant polyphenols. Antioxid Redox Signal 7:1630-1647, 2005.
Kim EH, Yoon MJ, Kim SU, Kwon TK, Sohn S, Choi KS: Arsenic trioxide sensitizes human glioma cells, but not normal astrocytes, to TRAIL-induced apoptosis via CCAAT/enhancer - binding protein homologous protein-dependent DR5 up-regulation. Cancer Res 68:266-275, 2008.
Kim SK, Woodcroft KJ, Khodadadeh SS, Novak RF: Insulin signaling regulates gamma-glutamylcysteine ligase catalytic subunit expression in primary cultured rat hepatocytes. J Pharmacol Exp Ther 311:99-108, 2004.
Kimberley FC, Screaton GR: Following a TRAIL: update on a ligand and its five receptors. Cell Res 14:359-372, 2004.
Lau AT, Wang Y, Chiu JF: Reactive oxygen species: Current knowledge and applications in cancer research and therapeutic. J Cell Biochem 104:657-667, 2008.
Limtrakul P: Curcumin as a chemosensitizer. Adv Exp Med Biol 595:269-270, 2007.
Lin TH, Lu FJ, Yin YF, Tseng TH: Enhancement of esculetin on arsenic trioxide-provoked apoptosis in human leukaemia U937 cells. Chem Biol Interact 180:61-68, 2009.
Liu Q, Hilsenbeck S, Gazitt Y: Arsenic trioxide-induced apoptosis in myeloma cells: p53-dependent G1 or G2/M cell cycle arrest, activation of caspase-8 or caspase-9, and synergy with APO2/TRAIL. Blood 101:4078-4087, 2003.
Lunghi P, Giuliani N, Mazzera L, Lombardi G, Ricca M, Corradi A, Cantoni AM, Salvatore L, Riccioni R, Constanzo A, Testa U, Levrero M et al.: Targeting the MEK/MAPK signal transduction module potentiates ATO-induced apoptosis in multiple myeloma cells through multiple signalling pathways. Blood 112:2450-2462, 2008.
Maeda H, Hori S, Ohizumi H, Segawa T, KAkehi Y, Ogawa O, Kakizuka A: Effective treatment of advanced solid tumours by the combination of arsenic trioxide and L-buthionine-sulfoximine. Cell Death Differ 11:737-746, 2004.
Miller WH, Jr., Schipper HM, Lee JS, Singer J, Waxman S: Mechanisms of action of arsenic trioxide. Cancer Res 62:3893-3903, 2002.
Mohammad RM, Banerjee S, Li Y, Aboukameel A, Kucuk O, Sarkar FH: Cisplatin-induced antitumour activity is potentiated by the soy isoflavone genistein in BxPC-3 pancreatic tumor xenografts. Cancer 106:1260-1268, 2006.
Nakazato T, Ito K, Ikeda Y, Kizaki M: Grean tea component, catechin, induces apoptosis of human malignant B cells via production of reactive oxygen species. Clin Cancer Res 11:6040-6049, 2005.
Ozben T: Oxidative stress and apoptosis: Impact on cancer therapy. J Pharm Sci 96:2181-2196, 2007.
Pelicano H, Feng L, Zhou Y, Carew JS, Hileman EO, Plunkett W, Keating MJ, Huang P: Inhibition of mitochondrial respiration. A novel strategy to enhance drug-induced apoptosis in human leukemia cells by reactive oxygen species-mediated mechanism. J Biol Chem 278:37832-37839, 2003.
Platanias LC: Biological responses to arsenic compounds. J Biol Chem 284:18583-18587, 2009.
Rahman I, Biswas SK, Kirkham PA: Regulation of inflammation and redox signalling by dietary polyphenols. Biochem Pharmacol 72:1439-1452, 2006.
Ramos AM, Aller P: Quercetin decreases intracellular GSH content and potentiates the apoptotic action of the antileukemic drug arsenic trioxide in human leukemia cell lines. Biochem Pharmacol 75:1912-1923, 2008.
Ramos AM, Fernandez C, Amram D, Sancho P, de Blas E, Aller P: Pharmacologic inhibitors of PI3K/Akt potentiate the apoptotic action of the antileukemic drug arsenic trioxide via glutathione depletion and increased peroxide accumulation in myeloid leukaemia cells. Blood 105:4013-4020, 2005.
Ramos AM, Fernandez C, Amran D, Esteban D, de Blas E, Palacios MA, Aller P: Pharmacologic inhibitors of extracellular signal-regulated kinase (ERKs) and c-Jun NH2-terminal kinase (JNK) decrease glutathione content and sensitize human promonocytic leukaemia cells to arsenic trioxide-induced apoptosis. J Cell Physiol 209:1006-1015, 2006.
Ramos S: Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res 52:507-526, 2008.
Salvi M, Brunati AM, Clari G, Toninello A: Interaction of genistein with the mitochondrial electron transport chain results in opening of the membrane transition pore. Biochim Biophys Acta 1556:187-196, 2002.
Sanchez Y, Amran D, Fernandez C, de Blas E, Aller P: Genistein selectively potentiates arsenic trioxide-induced apoptosis in human leukaemia cells via reactive oxygen species generation and activation of reactive oxygen species-inducible protein kinases (p38-MAPK, AMPK). Int J Cancer 123:1205-1214, 2008.
Sanchez Y, Amran D, de Blas E, Aller P: Regulation of genistein-induced differentiation in human acute myeloid leukaemia cells (HL60, NB4). Protein kinase modulation and reactive oxygen species generation. Biochem Pharmacol 77:384-396, 2009a.
Sanchez Y, Calle C, de Blas E, Aller P: Modulation of arsenic trioxide-induced apoptosis by genistein and functionally related agents in U937 human leukaemia cells. Regulation by ROS and mitogen-activated protein kinases. Chem Biol Interact 182:37-44, 2009b.
Sarkar FH, Li Y: Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res 66:3347-3350, 2006.
Scott N, Hatlelid KM, MacKenzie NE, Carter DE: Reactions of arsenic(III) and arsenic(V) species with glutathione. Chem Res Toxicol 6:102-106, 1993.
Sordet O, Rebe C, Leroy I, Bruey JM, Garrido C, Miguet C, Lizard G, Plenchette S, Corcos L, Solary E: Mitochondria-targeting drugs arsenic trioxide and lonidamine bypass the resistance of TPA-differentiated leukemic cells to apoptosis. Blood 97:3931-3940, 2001.
Sun WM, Huang ZZ, Lu SC: Regulation of gamma-glutamylcyteine synthetase by protein phosphorylation. Biochem J 320:321-328, 1996.
Szegezdi E, Cahill S, Meyer M, O'Dwyer M, Samali A: TRAIL sensitisation by arsenic trioxide is caspase-8 dependent and involves modulation of death receptor components and Akt. Br J Cancer 94:398-406, 2006.
Tabellini G, Cappellini A, Tazzari PL, Fala F, Billi AM, Manzoli L, Cocco L, Martelli AM: Phosphoinositide 3-kinase/Akt involvement in arsenic trioxide resistance of human leukaemia cells. J Cell Physiol 202:623-634, 2005.
Wang ZY, Chen Z: Acute promylocytic leukaemia: from highly fatal to highly curable. Blood 111:2505-2515, 2008.
Yang CH, Kuo ML, Chen JC, Chen YC: Arsenic trioxide sensitivity is associated with low level of glutathione in cancer cells. Br J Cancer 81:796-799, 1999.
Ye J, Li A, Liu Q, Wang X, Zhou J: Inhibition of mitogen-activated protein kinase kinase enhances apoptosis induced by arsenic trioxide in human breast cancer MCF-7 cells. Clin Exp Pharmacol Physiol 32:1042-1048, 2005.
Yi J, Gao F, Shi G, Li H, Wang Z, Shi X, Tang X: The inherent cellular level of reactive oxygen species: one of the mechanisms determining apoptotic susceptibility of leukaemia cells to arsenic trioxide. Apoptosis 7:209-215, 2002.
|
BACK
|



