Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 8 (2010), No 4, p 213-226
DOI 10.2478/v10136-009-28-2
Chronobiologic study of the GH-IGF1 axis and the ageing immune system
Gianluigi Mazzoccoli, Francesco Giuliani, Michele Inglese, Nunzia Marzulli, Mariangela Pia Dagostino, Angelo De Cata, Antonio Greco, Stefano Carughi, Roberto Tarquini
Address: Gianluigi Mazzoccoli, Department of Internal Medicine, Scientific Institute and Regional General Hospital "Casa Sollievo della Sofferenza", Cappuccini Avenue, 71013 S. Giovanni Rotondo (FG), Italy
g.mazzoccoli@tin.it
Received 22nd March 2010.
Revised 21nd April 2010.
Published online 14th June 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
References
SUMMARY
One of the many systems that weakens as we age is our immune system and there is a reduction in the GH-IGF1 axis activity with increasing age. In this study we evaluated the immune system and the GH-IGF1 axis function in healthy ageing. CD3, CD4, CD20, CD25, HLA-DR and GH showed acrophase during the night, whereas CD8, CD16 and TCRgamma delta expressing cells showed acrophase during the day. MESOR of CD3 was higher in the old aged subjects, MESOR of CD20 and CD20 values at 14:00h and at 02:00h were higher in the young middle aged subjects, MESOR of CD25 and CD25 values at 10:00 were higher in the elderly subjects, MESOR of HLA-DR was higher in the young middle aged subjects, whereas MESOR of DR+ T cells and HLA-DR at 02:00h were higher in the elderly subjects, MESOR of TCRgamma delta bearing cells was higher in the elderly subjects, GH value at 18:00h was also higher in the elderly subjects, and MESOR of IGF1 was higher in the young middle aged subjects. There was a statistically significant difference for the acrophases of CD25, HLA-DR and IGF1. There were different and opposing correlations among lymphocyte subpopulations and GH-IGF1 axis hormones in young and middle aged subjects in comparison with old aged subjects. Linear regression evidenced a statistically significant positive trend between age and the 24h mean of CD3 and CD25 and a statistically significant negative trend between age and the 24h mean of CD20 and GH. In conclusion, ageing is associated with an altered GH and IGF1 secretion, with decreased peripheral B cell compartment, increased peripheral T cell compartment and alterations of circadian rhythmicity.
KEY WORDS
GH; IGF1; ageing; neuro-endocrine-immune system; circadian; lymphocyte; somatopause; immunomodulation
INTRODUCTION
The age-associated decrease in growth hormone (GH)
secretion and insulin-like growth factor (IGF) 1
production by the liver and other tissues in response
to GH has been termed the somatopause or
hyposomatotropism of ageing (Gusenoff et al. 2001,
Rehman et al. 2001, Russel-Aulet et al. 2001). IGF1
is one of the most important growth factors for
normal cell proliferation: it acts as an endocrine
hormone via the blood and as a paracrine and
autocrine growth factor locally. An autocrine or
paracrine GH-IGF1 system has been found in
lymphoid tissues, capable of influencing
lymphopoiesis and the immune function and in
particular, IGF1 assists the maturation of lymphocytes
in bone marrow and their function in the periphery
(Auernhammer et al. 1995).
A characteristic phenomenon of ageing is the
involution or disappearance of the thymus gland, that
is the director and activator of the immune system. It
secretes hormones such as thymosin and thymopietin,
which regulate the immune system. GH is able to
reverse the thymic atrophy of old rats so that their
thymus glands became as large and robust as the
thymus glands of healthy young rats (Morrhaye et al.
2009). It has been demonstrated that GH and IGF1
promote hematopoiesis, particularly the
megakaryocyte and erythroid lineages, both in vitro
and in vivo and promote early B cell and natural killer
(NK) cell development, which occurs in the bone
marrow, induce B cell proliferation and
immunoglobulin (Ig) production and promote the
survival of T cell progenitors and T cell development
in the thymus. GH and IGF1 have been found to
promote T cell chemotaxis and therefore may play a
role in normal lymphocyte circulation to the
lymphnodes and spleen (Clark 1997). The rate of GH
secretion from the anterior pituitary is highest around
puberty, and declines progressively thereafter. The
cause of the normal age-related decrease in GH
secretion is not well understood. Evidence suggests
the existence of a relationship between declining GH
and IGF1 levels and age-related changes in body
composition and physical function. Associated with
these physiological changes is a clinical picture often
referred to as the somatopause: frailty, muscle
atrophy, relative obesity, increased frequency of
fractures, disordered sleep, decreased immune
function (Toogood 2003). Immunological response
decreases in most elderly people and
immunosenescence is a process that affects all cell
compartments of the immune system. Ageing
associated changes have been demonstrated not only
in T lymphocytes but also in different aspects of the
innate immunity including natural killer (NK) cells
(Born et al. 1995, Ginaldi et al. 2000).
The aim of our study was to evaluate differences
among healthy young-middle aged subjects and old
aged subjects in the GH-IGF1 axis and lymphocyte
subpopulations.
MATERIALS AND METHODS
Subjects gave written informed consent and the study
was approved by the local Scientific and Ethical
Committee. Peripheral blood samples were collected
at intervals of four hours for twenty four hours from
fifteen healthy young-middle aged male subjects
(YMA subjs, age range 36-55 years, mean age±SE
44.1±1.8) and fifteen healthy old aged male subjects
(OA subjs, age range 67-79 years, mean age±SE
68.5±1.3). Inclusion criteria were age (<65 years for
YMA subjs, 65 and <80 years for OA subjs), BMI
(>25 and <30), non smoker, normal physical activity
level, no psychiatric disorder, no alcohol intake, no
chronic conditions, and normal blood pressure level)
In all subjects healthy status was assessed by medical
history and physical examination, basal screening
blood and urine tests, ECG, chest X-ray, and upper
and lower abdominal ultrasound scan. All subjects
were studied in our Department and were submitted
to the same social routine (light/dark cycle and
mealtimes). Sleep was allowed between 23:00h
(lights off) and 07:00h (lights on). During daytime
(between 07:15h and 20:15h), subjects stayed in the
Department and standardized meals were provided at
appropriate times for breakfast (07:30h), lunch
(12:30h), and dinner (18:30h).
In each blood sample we measured GH and total
IGF1 on serum and analyzed lymphocyte
subpopulations: CD3 (total T cells), CD4 (T
helper/inducer), CD8 (T cytotoxic/suppressor), CD16
(natural killer), CD20 (total B cells), CD25 (activated
T cells with the expression of the alpha chain of the
IL2 receptor), HLA-DR (B cells and activated T
cells), TcRdelta1 (TCRgammadelta expressing cells) on peripheral
blood anticoagulated with sodium ethylenediamine
tetraacetic acid (EDTA). To measure hormone serum
concentrations blood samples were centrifuged
immediately after collection and frozen at -20 °C for
later determination. All samples were analyzed in
duplicate in a single assay; the intrassay and
interassay coefficients of variation were below 5%
and 3% for GH, and 3% and 8% for IGF1. We
measured GH by immunoenzymometric assay
(AIA-PACK HGH, Tosoh, Japan), IGF1 by
radioisotopic assay (IGF I 100T Kit, Nichols Institute
Diagnostics, San Clemente, USA). Analyses of
lymphocyte subpopulations were performed on
unfixed cell preparations with a multicolor
fluorescence activated cell sorter (FACScan,
Becton-Dickinson FACS Systems, Sunnyvale, USA)
and a panel of monoclonal antibodies (mAbs) to
lymphocyte surface antigens (Ortho Diagnostic
Systems, Inc., Raritan, USA: OKT3, OKT4, OKT8,
OK-NK, OKB20, OKT26a, OK-DR; Medical
Systems, Thermo Fisher Scientific Inc. Rockford,
USA: TcRdelta1). Briefly, mAbs were directly
conjugated with phycoerythrin (PE) and to
fluorescein isothiocyanate (FITS) and 10 microl mAbs
were added to 100 ml EDTA blood in Trucount tubes
(BD Biosciences, San Jose;, USA). After a 15-min
incubation at room temperature the erythrocytes were
disintegrated and after centrifugation the supernatants
were washed with PBS. Non-lymphocytic cells
contaminating the preparations were excluded from
analysis using scatter gates set on the 90° light scatter
profile. At least 10,000 cells were acquired on the
FACScan. Absolute counts of T cell subsets were
calculated based on the proportion of the respective T
cell subpopulation and on absolute counts obtained by
the procedure. The number of fluorescent cells was
expressed as a percentage of the total lymphocytes.
Statistical analysis
The statistical evaluation of hormone serum levels
and lymphocyte subpopulation values was performed
by non-inferential descriptive biometric analyses,
including one-way ANOVA performed between the
timepoints for each variable and each group on
original data and on data transformed as a percentage
of their individual 24h mean to look for a time-effect,
Pearson's product moment correlation coefficients
calculated for hormone serum levels at each sampling
time to assess temporal relationships between their
variations, linear regression between age and the 24h
mean of each variable, Student's t test and
Mann-Whitney rank sum test, where appropriate, on
MESOR, amplitude and acrophase values. The data
were also analyzed by an inferential temporal
descriptive biometric analysis using the methods
named Single Cosinor and Population Mean Cosinor,
based on a least-squares fit of a cosine curve to
individual and grouped time series data, testing the
occurrence of a 24h rhythm and quantifying the
parameters MESOR, amplitude and acrophase of the
rhythm. MESOR is the acronym for Midline
Estimating Statistic of Rhythm and defines the
rhythm-determined average. Amplitude is the
measure of one half the extent of rhythmic change in
a cycle estimated by the function used to approximate
the rhythm. Acrophase, measure of timing, is the
phase angle of the crest time in the function
appropriately approximating a rhythm, in relation to
the specified reference timepoint (Nelson et al. 1979).
Chronobiologic analysis was performed and
chronobiologic graphs were created with Cosinor 2.2.
ANOVA, Pearson's product moment correlation,
linear regression, Student's t test and Mann-Whitney
rank sum test were performed with SigmaPlot11.0.
We used the significance level 2alpha=0.05.
RESULTS
In YMA subjects a clear circadian rhythm was
validated for the time-qualified changes of all the
factors studied, with the exception of IGF1, with
Cosinor analysis, and a time effect for all the factors
studied was evidenced with ANOVA performed on
data transformed as a percentage of their individual
24h mean. CD3, CD4, CD20, CD25, HLA-DR and
GH showed acrophase during the night, whereas
CD8, CD16 and TCRgammadelta expressing cells showed
acrophase during the day. In OA subjs a clear
circadian rhythm was validated with Cosinor analysis
for the nyctohemeral changes of CD3, CD8, CD16,
CD20 and GH and a time effect was evidenced with
ANOVA performed on data transformed as a
percentage of their individual 24h mean for CD4,
CD8, CD16, CD20, CD25, DR+T cells and GH.
Our data evidenced that MESOR of CD3 was
higher in OA subjects (statistically significant),
MESOR of CD20 and CD20 values at 14:00h and at
02:00h were higher in YMA subjects (statistically
significant), MESOR of CD25 and CD25 values at
10:00 were higher in elderly subjects (statistically
significant), MESOR of HLA-DR was higher in
YMA subjectss (statistically significant) whereas
MESOR of DR+ T cells and HLA-DR at 02:00h were
higher in elderly subjects (statistically significant),
MESOR of TCRgammadelta bearing cells was higher in elderly
subjects (statistically significant), GH value at 18:00h
was higher in elderly subjects (statistically
significant), MESOR of IGF1 was higher in YMA
subjects (statistically significant). There was a
statistically significant difference for the acrophases
of CD25 (statistically significant), HLA-DR
(statistically significant) and IGF1 (statistically
significant). Pearson's product moment correlations
showed that in YMA subjects at 06:00h HLA-DR
correlated positively with GH (r=0.882, statistically
significant); at 22:00h CD8 correlated positively with
IGF1 (r=0.972, statistically significant); at 02:00h
CD8 correlated positively with IGF1 (r=0.992,
statistically significant). In OA subjects at 06:00h
HLA-DR correlated negatively with GH (r=-0.914,
statistically significant), TcRdelta1 correlated negatively
with GH (r=-0.875, p=0.05); at 22:00h CD8
correlated negatively with IGF1 (r=-0.938,
statistically significant); at 02:00h CD20 correlated
negatively with GH (r=-0.941, statistically
significant), CD25 correlated positively with IGF1
(r=0.913, statistically significant), TcRdelta1 correlated
negatively with GH (r=-0.876, statistically
significant). Linear regression evidenced a
statistically significant trend between age and the 24h
mean of CD3, CD20, CD25 and GH.
Table 1. Chronobiological summary of data derived from best fitting (fitted period: 24 hours=360°) and F and p value from ANOVA performed between the time points for each variable.
|
 Young middle aged subjects Young middle aged subjects
|
 Old aged subjects Old aged subjects
| |
p |
MESORa |
Amplitude |
Acrophase |
ANOVA |
p |
MESORa |
Amplitude |
Acrophase |
ANOVA | | Original units |
%
of mean |
Original
units |
%
of mean | | F |
p |
F |
p |
F |
p |
F |
p | | CD3 |
0.002 |
78.0±0.1a |
1.1±0.2 |
01:40±0:50 |
0.08 |
0.99 |
2.3 |
0.015 |
0.002 |
84.9±0.2* |
1.0±0.0 |
04:45±0:12 |
0.71 |
0.61 |
2.1 |
0.090 | | CD4 |
0.001 |
45.2±0.8 |
3.1±1.1 |
00:13±1:38 |
0.42 |
0.82 |
5.6 |
0.001 |
0.145 |
45.1±0.8 |
3.1±1.2 |
02:13±1:28 |
0.89 |
0.50 |
2.6 |
0.047 | | CD8 |
0.003 |
29.5±0.2 |
1.9±0.2 |
12:05±0:10 |
0.13 |
0.98 |
4.2 |
0.001 |
0.005 |
29.2±0.2 |
3.2±0.5 |
12:36±0:49 |
1.50 |
0.22 |
4.3 |
0.001 | | CD16 |
0.030 |
6.2±0.4 |
0.8±0.2 |
14:08±1:25 |
0.32 |
0.89 |
2.7 |
0.030 |
0.001 |
8.0±0.3 |
2.4±0.4 |
12:45±0:30 |
0.68 |
0.69 |
3.1 |
0.020 | | CD20 |
0.002 |
13.2±0.2* |
1.5±0.1 |
22:27±0:49 |
0.25 |
0.93 |
1.0 |
0.005 |
0.001 |
8.3±0.1 |
1.1±0.1 |
19:09±1:26 |
0.79 |
0.56 |
3.0 |
0.029 | | CD25 |
0.002 |
3.8±0.0 |
0.6±0.2 |
00:37±0:28 |
0.29 |
0.91 |
2.9 |
0.033 |
0.060 |
7.1±0.1* |
1.0±0.2 |
16:45±0:49* |
0.17 |
0.96 |
1.7 |
0.045 | | DR+T cells |
0.005 |
3.2±0.3 |
0.8±0.2 |
00:49±3:25 |
0.24 |
0.34 |
1.1 |
0.020 |
0.057 |
5.1±0.3* |
1.7±0.2 |
9:24±0:45* |
0.34 |
0.42 |
2.7 |
0.040 | | HLA-DR |
0.010 |
16.2±0.2* |
1.3±0.3 |
22:10±0:45 |
0.74 |
0.60 |
2.9 |
0.050 |
0.297 |
13.2±0.1 |
1.2±0.9 |
12:20±2:13* |
0.73 |
0.60 |
1.8 |
0.150 | | TcRdelta1 |
0.002 |
2.1±0.0 |
0.6±0.1 |
10:38±1:57 |
0.55 |
0.73 |
1.0 |
0.020 |
0.210 |
4.2±0.1* |
0.3±0.1 |
12:48±2:02 |
0.39 |
0.54 |
0.9 |
0.176 | | GH |
0.001 |
0.3±0.1 |
0.3±0.1 |
01:01±0:54 |
2.41 |
0.06 |
2.2 |
0.018 |
0.015 |
0.3±0.0 |
0.3±0.0 |
23:57±0:13 |
1.34 |
0.28 |
4.2 |
0.01 | | IGF1 |
0.086 |
229.4±1.3* |
17.2±1.2 |
08:28±0:25 |
1.39 |
0.26 |
3.6 |
0.013 |
0.267 |
212.3±3.1 |
2.5±4.7 |
12:04±8:01* |
0.06 |
0.99 |
1.1 |
0.388 |
* a mean±SE
Units: % for lymphocyte subpopulations, ng/ml for GH, ng/ml for IGF1, hours:minutes for acrophase; all parameters analyzed in all the subjects. p # value from an F-test of the
null amplitude rejection hypothesis (for a rhythm with a chosen period tau); statistically significant.
Table 1 shows chronobiological data derived from
best fitting cosine curves, F and p value from
ANOVA performed between the timepoints for each
variable and p values from t-test and Mann-Whitney
rank sum test, as indicated performed between
MESOR, amplitude and acrophase values. Figs 1 and
2 show the fitting cosine curves of rhythm of
lymphocyte subpopulations, GH and IGF1 in YMA
and OA subjects, Figs 3 and 4 show the 24-hour
profiles of lymphocyte subpopulations, GH and IGF1
in YMA subjects and OA subjects, Fig. 5 shows the
regression lines between age and the 24h mean of
CD3, CD20, CD25 and GH.
DISCUSSION
Biological rhythms in different frequency ranges
characterize the mammalian body and this
phenomenon is particularly evident when we consider
the neuro-endocrine and immune system functions,
characterized by a multifrequency time structure
(Besedovski and del Rey 1996, Haus 1996, Haus and
Smolensky 1999). In the healthy organism, rhythms
of the same frequency may have the same phase or
different phases and usually show a well defined
time-relation to each other. The loss of the array of
rhythms or a change of their functional interactions
may alter the organism's time structure leading to
chronodisruption and internal desynchronization. The
alteration of the organism's time structure may lead to
functional disturbances and to alteration of the
anatomic integrity (Haus et al. 1983).
Circadian rhythmicity of variation characterizes
innate and adaptive immunity as well as their humoral
factors and cellular effectors (Abo and Kumagai
1978, Abo et al. 1981, Canon et al. 1985, Born et al.
1997, Arjona et al. 2004). The nyctohemeral
variations of physiological phenomena are controlled
by a complex system comprising of a master circadian
clock in the suprachiasmatic nuclei (SCN), which are
entrained by environmental timing cues
(light-darkness and/or the activity-rest pattern),
extra-SCN cerebral clocks and peripheral oscillators.
At a molecular level circadian rhythms are regulated
by transcriptional and post-translational feedback
loops generated by a set of interplaying clock proteins
and as many peripheral tissues and cells, leukocytes
also rhythmically express clock genes (Teboul et al.
2005, Fukuya et al. 2007, Berger 2008). Lymphocyte
subpopulations present circadian variation of some of
their subsets and this variation may influence
magnitude and expression of the immune responses
(Levi et al. 1985, Mazzoccoli et al. 1997). The
circadian variation of lymphocyte subsets has been
related to circadian changes in cell production, release
and destruction and to cortisol and epinephrine
influence on cell redistribution to the bone marrow,
mobilization and migration to lymphoid and non
lymphoid organs and peripheral tissues (Dimitrov et
al. 2009). The phenomenon of lymphocyte
subpopulation redistribution may be more complex
and may involve other hormones, monoamines and
cyto/chemochines. The contribution of the immune
system to healthy ageing and longevity is still an open
question and immunosenescence is a process that
affects all cell compartments of the immune system.
Aging of the immune system function may be related
to alteration of circadian rhythmicity with loss of
interaction among key lymphocyte subsets,
immunomodulating hormones and cytokines/
chemokines as well.
The results obtained in our study show interesting
differences between the studied groups in hematic
levels and temporal organization of some investigated
factors. Young and middle aged subjects have higher
levels of total B cells and show a clear circadian
rhythm and a customary temporal architecture of
many studied factors. As evidenced in our study,
peripheral blood lymphocytes show circadian
variations of specific subpopulations and the T
helper/inducer and the T suppressor/cytotoxic subsets
change with circadian rhythmicity but in an opposite
phase, showing a temporal organization of
lymphocyte functions. The variations of total T cells,
T helper/inducer subset, DR+ B cells and activated T
cells, total B cells and activated T cells with
expression of the alpha chain of IL-2 receptor show
circadian rhythmicity with acrophase at night,
synchronized with those of GH, in antiphase with the
rhythm of T suppressor/cytotoxic lymphocytes,
natural killer cells and TCRgammadelta expressing cells.
There is a general agreement in the international
literature about the presence of circadian rhythmicity
of variation of the total number of lymphocytes, with
the zenith during the night in antiphase with the
rhythm of cortisol secretion, whereas the reports
concerning the single lymphocyte subsets are
conflicting. Some papers describe a circadian rhythm
only for CD4 with acrophase during the night
(Kawate et al. 1981, Ritchie et al. 1983, Kronfol et al.
1997, Suzuki et al. 1997), whereas other reports
describe an ultradian rhythm of CD8 (Levi et al.
1988a) and a clear circadian rhythm of CD4/CD8
ratio (Levi et al. 1983). This discrepancy may be
related to the large interindividual and seasonal
variability of lymphocyte rhythmicity that may
hamper the statistical interpretation of the data from
different studies and from different groups of subjects
(Canon et al. 1986, Levi et al. 1988b).
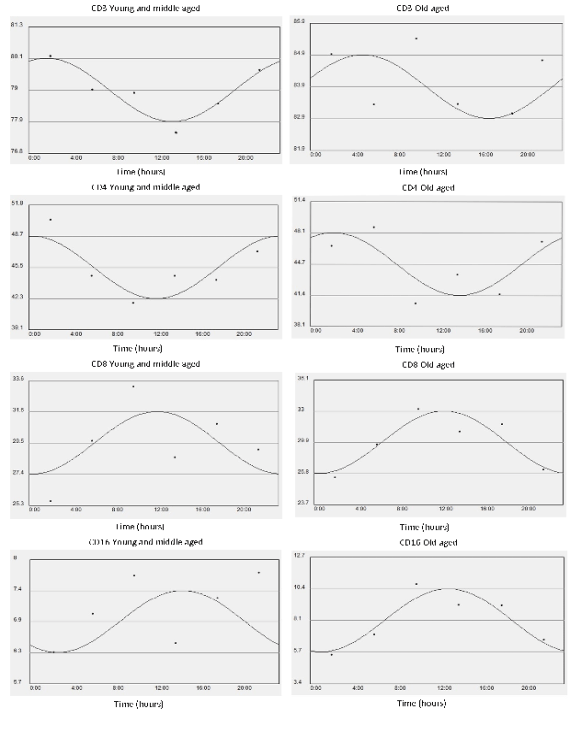
Fig. 1a. x-y plots showing from top to bottom the fitted cosine curve of rhythm (continous line) superimposed on raw data
(dots), the MESOR and 95% confidence limits of CD3, CD4, CD8, CD16 expressing cells in Young middle aged subjects (left)
and Old aged subjects (right). Units: % for lymphocyte subpopulation on y axis.
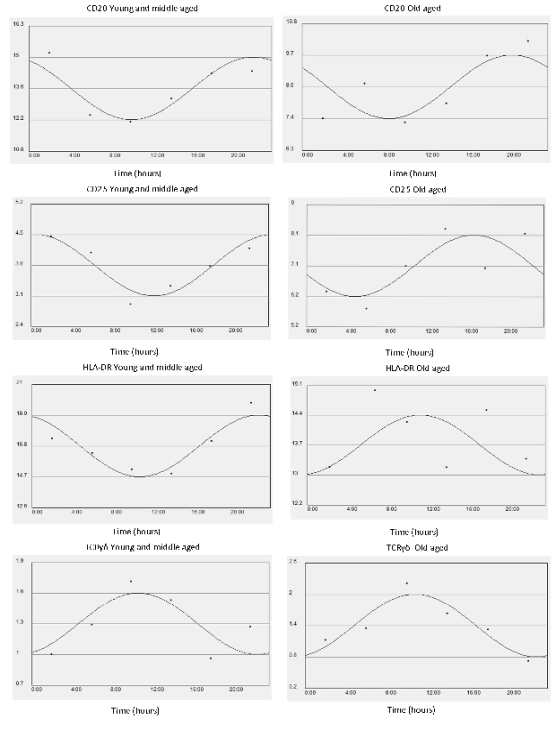
Fig. 1b. x-y plots showing from top to bottom the fitted cosine curve of rhythm (continous line) superimposed on raw data
(dots), the MESOR and 95% confidence limits of CD20, CD25, HLA-DR, gammadeltaTCR expressing cells in Young middle aged subjects
(left) and Old aged subjects (right). Units: % for lymphocyte subpopulation on y axis.
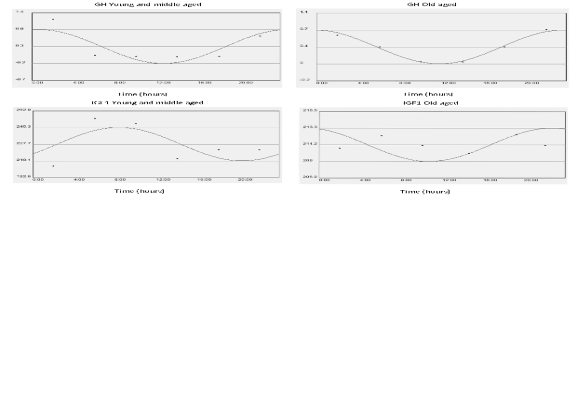
Fig. 2. x-y plots showing from top to bottom the fitted cosine curve of rhythm (continous line) superimposed on raw data
(dots), the MESOR and 95% confidence limits of GH and IGF1in Young middle aged subjects (left) and Old aged subjects
(right). Units: ng/ml for GH and IGF1 on y axis.
Our study found significant differences in the
pattern of GH and IGF1secretion between the studied
groups with old aged subjects presenting higher GH
levels in the evening, but decreased mean levels and
different acrophase of IGF1 in comparison with
younger subjects. This phenomenon may be caused
by an alteration in the GH-IGF1 axis regulation.
Elderly subjects in our study had higher levels of
CD3+ lymphocytes, DR+ T cells, activated T cells
with expression of the alpha chain of IL-2 receptor
and gammadeltaTCR expressing cells and we have found that
in these subjects the circadian rhythm of CD25 subset
is phase advanced, the circadian rhythm of total T
cells is phase delayed and the nyctohemeral variations
of T helper/inducer subset, DR+ B cells and activated
T cells, total B cells, TCRgammadelta expressing cells do not
show circadian periodicity. The MESOR of TCRgammadelta
bearing cells is increased in our elderly subjects and
this might be an important finding, because TCRgammadelta
complex is mainly expressed at the cell surface of
cellular elements temporally and maybe functionally
related to cytotoxic T lymphocytes. Previous studies
have shown that this complex is involved in T cell
activation and that activated gammadelta expressing cells
frequently exhibit cytotoxic activity against multiple
target cell lines including neoplastic cells, thereby
playing a key role in immunosurveillance (Bensussan
et al. 1989, Macintyre and Sigaux 1989, Scott et al.
1990, Sleasman et al. 1990).
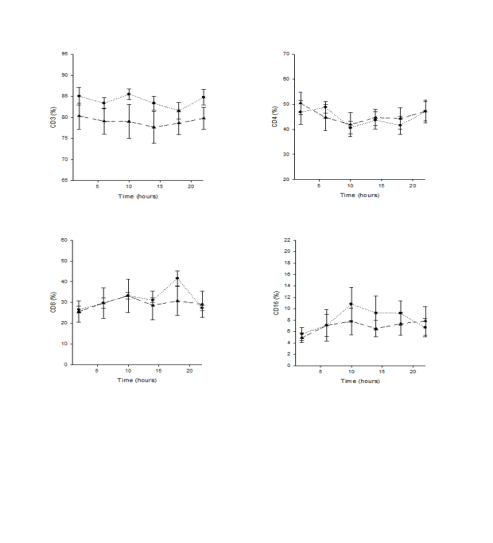
Fig. 3a. x-y plots showing 24-hour profiles of CD3, CD4, CD8 and CD16 expressing cells in fifteen Young middle aged and
fifteen Old aged subjects; * statistically significant.
The alteration of circadian rhythmicity found in
our old aged subjects may be responsible for altered
correlations among the lymphocyte subpopulation and
hormone time-related variations, such as are found for
the altered correlation of CD8+ lymphocytes with
IGF1 and HLA-DR+ cells with GH, and maybe the
expression of the loss of physiological timed windows
of interaction with occurrence of new anomalous
interactions. Nocturnal GH levels were lower in our
old aged subjects, but this result does not reach
statistical significance (p=0.06), maybe because in
humans GH secretory dynamics decline from high
values in young adults to be virtually absent after the
age of 50-60 years (Corpas et al. 1993, Touitou and
Haus 2000) and we preferred to include in the study middle aged (45-65 years) and to leave out more than
80 years aged volunteers to evaluate age ranged
subjects with closer physiological characteristics. We
enrolled able-bodied and generally healthy old
subjects and their slightly lower GH serum levels
confirm the need to distinguish GH deficient from
non GH deficient old individuals. Studies conducted
on GH-deficient patients have demostrated that
different time treatment schedules of GH
administration have different effects on IGF1 serum
levels and the closest similarity to normal hormone
and metabolite patterns and relationships is reached
by GH injection in the evening (Copeland et al. 1980,
Jorgensen et al. 1988, 1990, Laursen et al. 1995,
Oscarsson et al. 1997). In the young, GH is secreted
in a pulsatile fashion mainly during the first hours of
sleep (stages 3 and 4), whereas ageing is associated
with a severe decrease in both frequency and
amplitude of the pulses, leading to a decline of
plasma GH levels, in part as a consequence of
decreased responsiveness of the pituitary to
GH-releasing factor and leading to reduced peripheral
IGF1 production. In our elderly subjects IGF1 levels
are significantly decreased and the GH secretion
seems to be higher in the late afternoon than during
the night (phase advanced), maybe in relationship to an advance in the phase of the sleep-wakefulness
cycle, the most common change of sleep pattern with
age (Duffy and Czeisler 2002). This finding is in
agreement with the alteration of GH secretion in the
elderly reported in precedent studies (Touitou and
Haus 1994, Touitou et al. 1997) and confirms the
importance of normal circadian rhythmicity of GH
secretion for the preservation of hormone action.

Fig. 3b. x-y plots showing 24-hour profiles of CD20, CD25, HLA-DR, TcR expressing cells in fifteen Young middle aged
and fifteen Old aged subjects; * statistically significant.
Increased classical signs of T cell activity are the
level of soluble interleukin 2 receptor in serum and
up-regulation of HLA-DR and interleukin 2 receptor
on circulating T lymphocytes. IL-2 plays a pivotal
role in regulating the adaptive immune system by
controlling the survival and proliferation of regulatory
T cells, which are required for the maintenance of
immune tolerance. Of crucial importance for the
delivery of IL-2 signals to regulatory T cells is the
expression of CD25, which confers high affinity
binding to IL-2 (Schwartz 2003, Letourneau et al.
2009). Our data show that important alterations of the
immune system function occur during ageing. The
decrease of B cells (CD20 and HLA-DR+ B cells),
lymphocytes that play a key role in the humoral
immune response, may be responsible for a decreased
response to exogenous antigens, included vaccines
and adjuvants (Saurvein-Teissla et al. 1998, de Bruijn et al. 2004). In addition, the increase of activated T
cells (CD25 and DR+ T cells) may be associated with
an increased frequency of autoimmune phenomena
and to an altered regulation of immune function and
in our study we have documented that the circadian
rhythmicity of these subsets is severely altered in
elderly subjects.
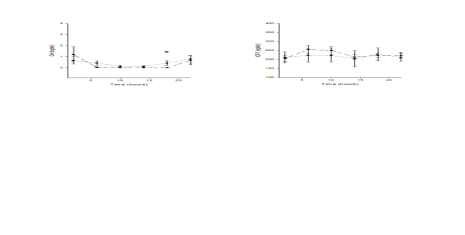
Fig. 4. x-y plots showing 24-hour profiles of GH and IGF1 serum levels in fifteen Young middle aged and fifteen Old aged
subjects; * statistically significant.
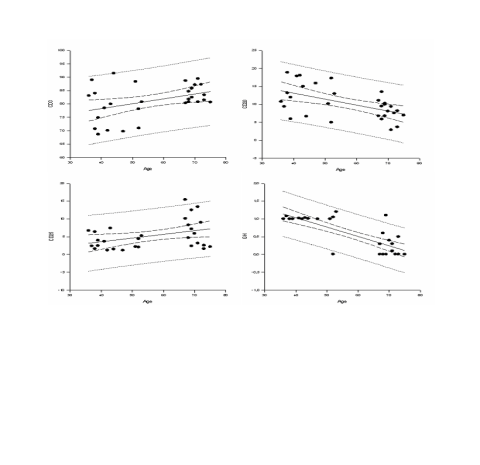
Fig. 5. x-y plots showing regression lines with 95% confidence limits between age and the 24h mean of CD3, CD20, CD25,
GH (all statistically significant).
In conclusion, elderly people present a decrease of
peripheral B cell compartment, an increase of the
peripheral T cell compartment, an alteration of
circadian rhythmicity and an alteration of GH-IGF1
axis function, that may be responsible for altered
integration between the neuro-endocrine and immune
system.
REFERENCES
Abo T, Kumagai K: Studies of surface immunoglobulins on human B lymphocytes. III. Physiological variations of SIg+ cells in peripheral blood. Clin Exp Immunol 33:441-452, 1978.
Abo T, Kawate T, Itoh K, Kumagai K: Studies on the bioperiodicity of the immune response. 1. Circadian rhythms of human T, B, and K cell traffic in the peripheral blood. J Immunol 126:1360-1363, 1981.
Arjona A, Boyadjieva N, Sarkar DK: Circadian rhythms of granzyme B, perforin, IFN-gamma, and NK cell cytolytic activity in the spleen: effects of chronic ethanol. J Immunol 172:2811-2817, 2004.
Auernhammer CJ, Strasburger CJ: Effects of growth hormone and insulin-like growth factor on the immune system. Eur J Endocrinol 133:635-645, 1995.
Bensussan R, Lagabrielle JF, Degos L: TcRgamma delta bearing lymphocyte clones with lymphokine activated killer activity against autologous laeukemic cells. Blood 15:135-139, 1989.
Berger J: A two-clock model of circadian timing in the immune system of mammals. Pathol Biol 56:286–291, 2008.
Besedovski HO, del Rey A: Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev 17:64-102, 1996.
Born J, Uthgenannt D, Dodt C, Nunninghoff D, Ringvolt E, Wagner T, Fehm HL: Cytokine production and lymphocyte subpopulations in aged humans. An assessment during nocturnal sleep. Mech Ageing Dev 84:113-126, 1995.
Born J, Lange T, Hansen K, Molle M, Fehm H-L: Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol 158:4454-4464, 1997.
Canon C, Levi FA, Reinberg A, Mathe G: Circulating CALLA-positive lymphocytes exhibit circadian rhythms in man. Leuk Res 9:1539-1546, 1985.
Canon C, Levi FA, Touitou Y, Sulon J, Demey-Ponsart E, Reinberg A, Mathe G: Circadian and seasonal variation of inducer/suppressor ratio in venous blood of healthy human donors. CR Acad Sci Paris 302:519-524, 1986.
Clark R: The somatogenic hormones and insulin-like growth factor-1: stimulators of lymphopoiesis and immune function. Endocr Rev 18:157-179, 1997.
Copeland KC, Underwood LE, Van Wyk JJ: Induction of immunoreactive somatomedin C in human serum by growth hormone: dose-response relationships and effect on chromatographic profiles. J Clin Endocrinol Metab 50:690-697, 1980.
Corpas E, Harman SM, Blackman MR: Human growth hormone and human aging. Endocr Rev 14:20-39, 1993.
de Bruijn IA, Nauta J, Gerez L, Palache AM: Virosomal influenza vaccine: a safe and effective influenza vaccine with high efficacy in elderly and subjects with low pre-vaccination antibody titers. Virus Res 103:139-145, 2004.
Dimitrov S, Benedict C, Heutling D, Westermann J, Born J, Lange T: Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood 113:5134-5143, 2009.
Duffy JF, Czeisler CA: Age-related change in the relationship between circadian period, circadian phase, and diurnal preference in humans. Neurosci Lett 318:117-120, 2002.
Fukuya H, Emoto N, Nonaka H, Yagita K, Okamura H, Yokoyama M: Circadian expression of clock genes in human peripheral leukocytes. Biochem Biophys Res Commun 354:924-928, 2007.
Ginaldi L, De Martinis M, Modesti M, Loreto F, Quaglino D: Immunophenotypical changes of T lymphocytes in the elderly. Gerontology 46:242-248, 2000.
Gusenoff JA, Harman S, Veldhuis JD, Jayme JJ, St. Clair C, Munzer T, Christmas C, O'Connor KG, Stevens TE, Bellantoni MF, Pabst K, Blackman MR: Cortisol and GH secretory dynamics, and their interrelationships, in healthy aged women and men. Am J Physiol Endocrinol Metab 280:E616–E625, 2001.
Haus E: Biologic rhythms in hematology. Pathol Biol (Paris) 44:618-630, 1996.
Haus E, Smolensky MH: Biologic rhythms in the immune system. Chronobiol Int 16:581-622, 1999.
Haus E, Lakatua DJ, Swoyer J, Sackett-Lundeen L: Chronobiology in hematology and immunology. Am J Anat 168:467-517, 1983.
Jorgensen JOL, Flyvbyerg A, Lauritzen T, Alberti KGMM, Orskov H, Christiansen JS: Dose-response studies with biosynthetic human growth hormone (GH) in GH-deficient patients. J Clin Endocrinol Metab 67:36-40, 1988.
Jorgensen JOL, Moller N, Lauritzen T, Alberti KGMM, Orskov H, Christiansen JS: Evening versus morning injections of growth hormone (GH) in GH-deficient patients: effects on 24-hour patterns of circulating hormones and metabolites. J Clin Endocrinol Metab 70:207-214, 1990.
Kawate T, Abo T, Hinuma S, Kumagai K: Studies of the bioperiodicity of the immune response: II. Co-variations of murine T and B cells and a role of corticosteroid. J Immunol 126:1364-1367, 1981.
Kronfol Z, Nair M, Zhang Q, Hill EE, Brown MB: Circadian immune measures in healthy volunteers: relationship to hypothalamic-pituitary-adrenal axis hormones and sympathetic neurotransmitters. Psychosom Med 59:42-50, 1997.
Laursen T, Jorgensen JOL, Jakobsen G, Hansen B, Christiansen JS: Continuous infusion versus daily injections of growth hormone (GH) for 4 weeks in GH-deficient patients. J Clin Endocrinol Metab 80:2410-2418, 1995.
Letourneau S, Krieg C, Pantaleo GO, Boyman O: IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. J Allergy Clin Immunol 123:758-762, 2009.
Levi F, Canon C, Blum JP, Reinberg A, Mathe G: Large-amplitude circadian rhythm in helper:suppressor ratio of peripheral blood lymphocytes. Lancet 20:462-463, 1983.
Levi FA, Canon C, Blum JP, Mechkouri M, Reinberg A, Mathe G: Circadian and/or circahemidian rhythms in nine lymphocyte-related variables from peripheral blood of healthy subjects. J Immunol 134:217-222, 1985.
Levi FA, Canon C, Touitou Y, Sulon J, Mechkouri M, Ponsart ED, Touboul JP, Vannetzel JM, Mowzowicz I, Reinberg A, Mathe G: Circadian rhythms in circulating T lymphocyte subtypes and plasma testosterone, total and free cortisol in five healthy men. Clin Exp Immunol 71:329-335, 1988a.
Levi FA, Canon C, Touitou T, Reinberg A, Mathe G: Seasonal modulation of the circadian time structure of circulating T and natural killer lymphocyte subsets from healthy subjects. J Clin Invest 81:407-413, 1988b.
Macintyre EA, Sigaux F: T cell receptor gamma delta: current state of knowledge and potential clinical applications in haematology. Br J Haematol 73:2-5, 1989.
Mazzoccoli G, Correra M, Bianco G, De Cata A, Balzanelli M, Giuliani A, Tarquini R: Age-related changes of neuro-endocrine-immune interactions in healthy humans. J Biol Regul Homeost Agents 11:143-147, 1997.
Morrhaye G, Kermani H, Legros JJ, Baron F, Beguin Y, Moutschen M, Cheynier R, Martens HJ, Geenen V: Impact of Growth Hormone (GH) Deficiency and GH Replacement upon Thymus Function in Adult Patients. PLoS One 4:E5668, 2009.
Nelson W, Tong YL, Halberg F: Methods for cosinorrhytmometry. Chronobiologia 6:305-323, 1979.
Oscarsson J, Johansson G, Johansson J-O, Lundberg P-A, Lindstedt G, Bengtsson B-A: Diurnal variation in serum insulin-like growth factor (IGF)-I and IGF binding protein-3 concentrations during daily subcutaneous injections of recombinant human growth hormone in GH-deficients adults. Clin Endocrinol (Oxf) 46:63-68, 1997.
Rehman HU, Masson EA: Neuroendocrinology of ageing. Age Ageing 30:279-287, 2001.
Ritchie AWS, Oswald I, Micklem HS, Boyd JE, Elton RA, Jazwinska E, James K: Circadian variation of lymphocyte subpopulations: a study with monoclonal antibodies. Br Med J 286:1773-1775, 1983.
Russell-Aulet M, Dimaraki EV, Jaffe CA, DeMott-Friberg R, Barkan AL: Aging-related growth hormone (GH) decrease is a selective hypothalamic GH-releasing hormone pulse amplitude mediated phenomenon. J Gerontol A Biol Sci Med Sci 56:M124-M129, 2001.
Saurwein-Teissla M, Stegera MM, Gluckb R, Cryzb S, Grubeck-Loebensteina B: Influenza vaccination in a healthy geriatric population: preferential induction of antibodies specific for the H3N2 influenza strain despite equal T cell responsiveness to all vaccine strains. Vaccine 16:196-200, 1998.
Schwartz RS: Diversity of the Immune Repertoire and Immunoregulation The New England. J Med 348:1017-1026, 2003.
Scott CS, Richards SJ, Roberts BE: Patterns of membrane TcR alpha beta and TcR gamma delta chain expression by normal blood CD4+CD8-, CD4-CD8+,CD4-CD8dim+ and CD4-CD8- lymphocytes. Immunology 70:351-356, 1990.
Sleasman JW, Morimoto C, Schlossman SF, Tedder TF: The role of functionally distinct helper T lymphocyte subpopulations in the induction of human B cell differentiation. Eur J Immunol 20:1357-1366, 1990.
Suzuki S, Toyabe S, Moroda T: Circadian rhythm of leucocytes and lymphocytes subsets and its possible correlation with the function of the autonomic nervous system. Clin Exp Immunol 110:500-508, 1997.
Teboul M, Barrat-Petit M-A, Mei Li X, Claustrat B, Formento J-L, Delaunay F: Atypical patterns of circadian clock gene expression in human peripheral blood mononuclear cells. J Mol Med 83:693–699, 2005.
Toogood AA: Growth Hormone (GH) Status and Body Composition in Normal Ageing and in Elderly Adults with GH Deficiency. Horm Res 60:105-111, 2003.
Touitou Y, Haus E: Aging of the human endocrine and neuroendocrine time structure. Ann N Y Acad Sci 719:378-397, 1994.
Touitou Y, Haus E: Alterations with aging of the endocrine and neuroendocrine circadian system in humans. Chronobiol Int 17:369-390, 2000.
Touitou Y, Bogdan A, Haus E, Touitou C: Modifications of circadian and circannual rhythms with aging. Exp Gerontol 32:603-614, 1997.
|
BACK
|








