Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 9 (2011), No 1, p 35-41
DOI 10.2478/v10136-009-0030-8
Inhibition of blood and tissue cholinesterases by soman in guinea pigs in vivo
Jiri Bajgar, Jiri Kassa, Miroslav Pohanka, Jana Zdarova Karasova, Ladislav Novotny, Josef Fusek, Vaclav Blaha
Address: Jiri Bajgar, Department of Toxicology, Faculty of Military Health Sciences, University of Defence, Trebesska 1575, 500 01 Hradec Kralove,
Czech Republic
bajgar@pmfhk.cz
Received 28th April 2010.
Revised 18th May 2010.
Published online 4th November 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Material and Methods
Results
Discussion
Acknowledgement
References
SUMMARY
Guinea pigs were intoxicated intramuscularly with different doses of soman, and cholinesterase activities were determined in the blood, diaphragm
and parts of the brain - the pontomedullar area, the frontal cortex and the basal ganglia. The time course of poisoning following low doses (1,3,
5 microg/kg) and a dose equal to 1xLD50 (28.5 microg/kg) were studied. The dose having a negligible effect on cholinesterases in the
tissues studied was assessed at 1-3 microg/kg, and, following administration of a dose of 5 microg/kg, statistically significant blood
cholinesterase inhibition was demonstrated.
KEY WORDS
blood; brain parts; cholinesterases; guinea pig; soman; inhibition in vivo
INTRODUCTION
The most important chemical warfare agents are sarin
(O-isopropyl methylphosphonofluoridate), soman
(O-pinacolyl methylphosphonofluoridate) (these two
compounds belong to so called G-compounds) and
VX (O-ethyl S-2-diisopropylaminoethyl methyl
phosphonothiolate) (V-compounds) (Bajgar 2004,
Patocka 2004). Many organophosphorus compounds
are produced in civilian facilities and evaluated in
industry, agriculture, human and veterinary medicine
etc. The threat of their use not only in military
conflicts but also therefore in civilian life cannot be
excluded as is evident from the terroristic attacks in
Japan (Morita et al. 1995, Okumura et al. 1996,
Nozaki et al. 1997) and extensive knowledge of their
effects is a necessary basis for studies searching for
the effective treatment of intoxication following their
use.
From the pharmacodynamics perspective, soman
is the most serious poison: its toxicity and inhibition
potency to AChE is relatively high and comparable
with that of VX (Clement et al. 1981, Clement 1989,
Shih et al. 1990, Bajgar 1991, 1992, Patocka et al.
2005, Fawcett et al. 2009). Soman is quickly resorbed
at all routes of administration (Bajgar 1991) and
inhibits cholinesterases (particularly acetylcholinesterase, AChE, EC 3.1.1.7) in the central and
peripheral nervous system. The high lipophility of
soman leads to a higher affinity to the central nervous
system with subsequent strong inhibition of the brain
AChE in vivo (Bajgar 1991, 1992). The inhibition of
the blood and brain AChE by soman is very fast,
attaining 50% activity within minutes (Bajgar 2004).
Soman and sarin are detoxified in the liver plasma
(Jokanovic 1990, Bajgar 1991, Skopec and Bajgar
1993) and, therefore, this part is excluded from the
toxic effect. Also losses of G-compounds in the
organism are caused by their binding to non specific
esterases, cholinesterases and detoxification, so this
part of soman is not able to produce a toxic effect. It
has been estimated that only 1-3% of the dose
administered inhibited AChE in the brain; i.e. 1-3%
of the dose administered produced the basic toxic
effect (Kadar et al. 1985, Bajgar 1991). Sterri and
Fonnum (1981) have reported that only about 5% of
the given dose of soman reacts with AChE, while the
remainder is detoxified. On the other hand, V
compounds are not detoxified (by direct
decomposition) in the organism (Bajgar 1991), but
VX is detoxified by other routes, e.g. a decrease in
the effective level of VX in the organism can be
caused via its binding to the enzymes mentioned.
Soman is also bound to other proteins and a decrease
in its level can also be produced by tissue depots
(Jokanovic 2009).
The mechanism of AChE inhibition is practically
the same for all nerve agent compounds:
phosphorylation or phosphonylation of serin in the
catalytic triad - the so-called esteratic site
(Ser200-His440-Glu327) at the bottom of a deep and
narrow cavity of the enzyme. The rate of spontaneous
dephosphorylation is very low and it can be omitted
in most cases. However, it can be improved/increased
using cholinesterase reactivators (oximes) able to
reactivate nerve agent-inhibited AChE (Kuca et al.
2004, Patocka et al. 2005, Musilek et al. 2007).
Depending on the structure of the inhibitor, inhibited
AChE is dealkylated (aged) and the complex formed
is resistant to reactivation. This reaction is very fast
for soman-inhibited AChE (the half-life is about
10 min) (Bajgar 1991, 2004). Therefore it can be
concluded that AChE activity inhibited by soman
cannot be changed spontaneously after a short period
of exposure. Thus, soman can be used as a model of
stable inhibition of AChE. Data are available on
cholinesterase inhibition following relatively high
doses of soman applied to experimental animals
(Bajgar 1992, Patocka et al. 2005, Shih et al. 2005,
2009a, b, Zdarova Karasova et al. 2009a), but there
are no detailed data dealing with the effect of low
doses of soman by parenteral administration. Some
attempts have been made using soman inhalation
exposure, to assess the minimal dose of soman
causing negligible changes of AChE activity (Bajgar
et al. 2004).
The question of choice of experimental animal is
of great interest. Most experiments have been
performed on rats (Clement 1989, Patocka et al. 2005,
Bajgar et al. 2007, 2009, Novotny et al. 2009, Zdarova Karasova et al. 2009a, b and others) and
guinea pigs have been used in a few studies (Shih et
al. 2005, 2009a, b, Fawcett et al. 2009, Mamczarz et
al. 2010) but the presence of carboxylesterases in rats
influences toxicity and therapeutic studies (Kadar et
al. 1985). Different levels of organophosphate
metabolizing carboxylesterases in the blood of
various species contribute to their differential
sensitivity to these agents. Primates (including
humans) have almost none, whereas guinea pigs have
low levels, and rats and mice have high levels of
blood carboxylesterases. Thus, to standardize the
development of antidotes to nerve agents, it was
recommended that these studies should be carried out
on guinea pigs (Koplovitz et al. 1992, Fawcett et al.
2009). Soman toxicity is not dependent on the sex or
age of the guinea pigs (Fawcett et al. 2009).
This study is focused on the dose dependent and
time dependent changes in AChE activities in
different tissues of guinea pig, and an estimation of
the minimal dose of soman causing negligible
changes in AChE activity. To compare our results
more precisely with data from relevant literature (time
course of AChE inhibition following s.c. soman
administration; Shih et al. 2005), the same dose
(1×LD50) was also used.
MATERIAL AND METHODS
Animals
Female guinea pigs (Tricolor, BIO.TEST, s.r.o.,
Konarovice, Czech Republic) weighing 350±30 g
were used in groups of 6 animals. The animals were
housed in the Central Vivarium of the Faculty of
Military Health Sciences under veterinary control. All
the experiments were performed with the permission
of and under the supervision of the Ethics Committee
of the Faculty of Military Health Sciences, Hradec
Kralove; (permission No 153/06) according to § 17 of
Czech law No 207/2004, permission of responsible
person 0001/94 - M 699.
Chemicals
Soman was obtained from the Military Technical
Institute of Protection (Brno, Czech Republic). It was
of minimally 98% purity and stored in glass ampullas
(0.33 ml). The solutions for the experiments were
prepared immediately before use.
Intoxication - dose dependence
The control group received an i.m. injection of saline
(0.1 ml/100 g body weight) whereas the study groups
were given soman i.m. in doses of 1, 3, 5, 20, 24, 27,
28.5 and 30 microg/kg; the LD50 of soman determined in
previous experiments was 28.5 microg/kg. The animals
were killed following aether anaesthesia 30 min after
intoxication.This procedure does not influence
cholinesterase activity (Novotny et al. 2009). The
blood and organs were removed and haemolysates or
homogenates were prepared. The animals injected
with saline served as the control group.
Intoxication - time dependence
The animals in groups were intoxicated with soman in
doses of 1, 3, 5 and 28.5 microg/kg (i.m.) and blood and
organs were removed at different time intervals (1, 5,
10, 30, 60 and 120 min) after intoxication except for
the last dose used, where the sampling was made
30 min after intoxication or immediately after death.
Preparation of samples
The blood was haemolysed with distilled water (1:10)
and AChE activity was determined immediately in
haemolysates. The tissues were frozen at -40 °C.
After thawing, the frontal cortex (FC), basal ganglia
(BG) and pontomedullar part (PM) of the brain were
prepared. Then the brain parts and diaphragm were
homogenised (Janke and Kunkel homogeniser,
Germany) 1:10 with distilled water. AChE activity
was determined in homogenates.
Determination of cholinesterase activity
AChE activity was determined according to Ellman et
al. (1961) as follows: 100 microl of the haemolysate was
mixed with 1700 microl DTNB solution (1 mM solution
of DTNB in 0.1 M TRIS-HCl buffer, pH 7.6) and the
enzymatic reaction was started by adding 200 ul of
substrate solution (1 mM acetylthiocholine in distilled
water). The mixture was stabilized for 3 min and then
the absorbancy at 436 nm/min was monitored (this
delay is necessary for exclusion of false positive
results by titration of free SH-groups interfering with
the activity determination). The activity was
expressed as microkat/l or g wet weight tissue or as % of
control values.
Statistical evaluation
The homogeneity of the experimental groups was
tested by Bartlett's test. The differences between
groups were calculated using means + SD and
differences were tested by Student's test at the
significance level 2alpha=0.05.
RESULTS
Absolute values of cholinesterase activity in the
guinea pig blood varied from 77.0 to 98.5 microkat/l, with
an average of 83.3±12.5 microkat/l. It is of interest that
the activity determined in the diaphragm is very low.
In the brain, AChE activity varied from the highest of
435.3±47.2 microkat/g, BGto the lowest of 90.2±10.9
microkat/g, FC. For better comparison following soman
intoxication, all the data were expressed as a % of the
control values.
Changes in cholinesterase activity following
different doses of soman are shown in Fig. 1. It is
clear that the decrease of activity is dose dependent.
The sensitivity of various tissues is different:
cholinesterase activity in the blood is the most
sensitive, followed by the diaphragm, and the relative
resistance of AChE activity in the basal ganglia is
evident. The dose causing 50% of inhibition in
particular tissues can be assessed to be about
15 microg/kg in the blood, while the dose causing 50% of
inhibition in the basal ganglia is 25 microg/kg. At doses
approaching LD50 value, all activities decrease to
0-10% of control values for all tissues studied.
Statistically significant inhibition was observed for
cholinesterases in the blood from the 5 microg/kg dose;
for other tissues studied, this inhibition was registered
from the 10 microg/kg dose except cholinesterases in the
basal ganglia. Following administration of soman at
the highest dose (20 microg/kg), all activities were
significantly different from the control values. As for
the time course of cholinesterase inhibition, very fast
changes for all tissues studied were found. The
changes in blood cholinesterase inhibition following
administration of three low doses of soman is shown
in Fig. 2; the time course of cholinesterase activity
decrease following administration of the dose equal to
LD50 is also demonstrated. The activity reaches a
steady state within 5-10 min and it remains
practically without changes for 2 h after soman
intoxication. The changes in cholinesterase activities
following three low doses of soman (120 min after
intoxication) are statistically significant at the dose of
5 microg/kg for the blood cholinesterases only. A
summary of the changes in cholinesterase activities
following low doses of soman 120 min after the
intoxication is given in Fig. 3.

Fig. 1. Cholinesterase activities in the blood and different organs following soman intoxication (i.m.) in different doses.
The results are means only; SD were not higher than ± 15%; example of SD. Cholinesterase determination was made 30 min after
the soman injection or immediately after death. Animals injected with saline served as the control group; diaph - diaphragm;
FC - frontal cortex; PM - pontomedullar area; BG - basal ganglia.
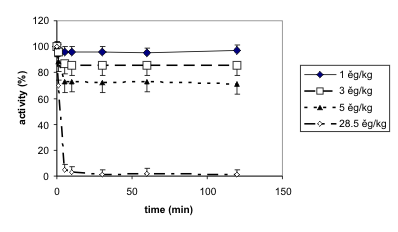
Fig. 2. Time-course of changes in cholinesterase activities in the blood following soman intoxication (i.m.) in different
doses. The results are means with their SD. The animals in groups were intoxicated with soman in doses of 1, 3, 5 and 28.5 microg/kg
(i.m.), and blood and organs were removed in different time intervals after the intoxication except for the last dose used (the
sampling was made 30 min after the intoxication or immediately after death).
DISCUSSION
A comparison of AChE activity in various tissues as
reported in the literature is difficult due to the
different methodical details adopted in the
determination of enzyme activity (e.g. different
expression of activity per ml of wet weight tissue or
mg of protein). However, when the activity of the
structure having the highest activity is compared relatively (in %, the highest activity relative to
100%), then comparisons show a good relationship
(Fig. 4).
The toxicity of soman in guinea pigs given
subcutaneously has been reported variously as
28 microg/kg (Shih et al. 2005) or from 25.1 to 27 microg/kg
(Fawcett et al. 2009); very close to the toxicity at i.m.
administration. Our results showed toxicity from i.m.
administration (for female guinea pigs) as 28.5 g/kg.
In contrast, percutaneous administration showed
approximately 400 times higher toxicity (DeMar et al.
2010). It has been demonstrated recently in guinea
pigs, that the lethal potencies of nerve agents VX and
sarin are sex-and age dependent, whereas the lethal
potency of soman is not so dependent (Fawcett et al.
2009).
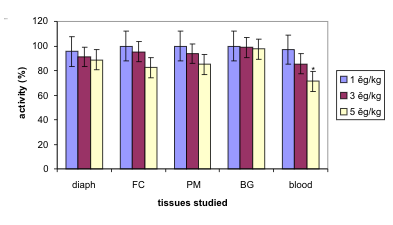
Fig. 3. Changes in cholinesterase activities (%) 120 min following intramuscular soman administration at low doses. The
results are means with their SD. Statistically significant difference was observed for the blood and highest dose, 5 microg/kg;
indicated by asterisk; abbreviations as in Fig. 1.
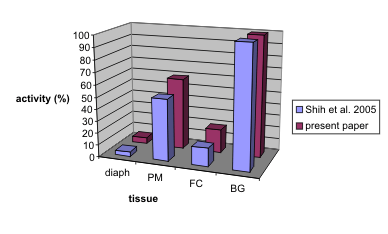
Fig. 4. Comparison of our present results and results described by Shih et al. (2005). AChE activity in the basal ganglia was
expressed as 100%, the activities of other structures was expressed in % (highest activity in each paper related to 100 %); abbreviations as in
Fig. 1.
The minimal dose of soman causing negligible
changes in blood cholinesterase activity can be
considered to be 1-3 microg/kg; very roughly, this dose is
equivalent to the dose of 1.2 g/l as demonstrated in our previous studies of the exposure of guinea pigs to
soman inhalation (Bajgar et al. 2004), and
corresponds to 0.3xLCt50 or approximately 0.1xLD50
for i.m. administration as in our present experiments.
The dose most significantly influencing the activity is
5 microg/kg, corresponding to 0.2xLD50. For inhalation
exposure, this concentration was 1.5 microg/l i.e.
0.4xLCt50, respectively. Thus, a small increase in the
dose of soman can be followed by a fast decrease in
cholinesterase activity in the brain; it is of interest
that small changes in AChE activities in the brain
have been described as critical for survival or
non-survival of experimental animals - in this case
rats (Bajgar et al. 2008) and thus for treatment. Partial
reactivation caused by different oximes in
reactivation experiments was found to be important
to their therapeutic effectiveness (Bajgar 2004, Kuca
et al. 2004, Patocka et al. 2005, Musilek et al. 2007).
These fine changes are of great interest for further
studies. Moreover, this approach could lead to
improvement in our knowledge of the mechanisms of
the action of organophosphates and soman poisoning.
At the same time, it could contribute to a better
understanding of cholinergic nerve transmission and
thus to pharmacology and neuropharmacology in
general.
CONCLUSIONS
Low doses of soman (1,3 and 5 microg/kg) administered
i.m. caused statistically significant (p<0.05) inhibition
of cholinesterases at a dose of 5 microg/kg in the blood
only but not in the diaphragm and brain parts.
Following a dose of soman close to LD50 (28.5
microg/kg), changes in cholinesterase activity were very
fast attaining a steady rate 10 min after soman
injection.
ACKNOWLEDGEMENT
The authors are indebted to Mrs E. Vodakova and M.
Zechovska for skilful technical assistance. Financial
support of the Ministry of Defence, grant No
OVUOFVZ 200905 (MORCE) is gratefully
acknowledged.
REFERENCES
Bajgar J. The influence of inhibitors and other factors on cholinesterases. Sbor Ved Pr LFUK (Hradec Kralove). 34: 3-75, 1991.
Bajgar J. Biological monitoring of exposure to nerve agents. Brit J Ind Med. 49: 648-653, 1992.
[PubMed]
Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis and treatment. Adv Clin Chem. 38: 151-216, 2004.
[CrossRef]
Bajgar J, Sevelova L, Krejcova G, Fusek J, Vachek J, Kassa J, Herink J, de Jong LPA, Benschop HP. Biochemical and behavioral effects of soman vapours in low concentrations. Inhal Toxicol. 16: 497-507, 2004.
[CrossRef]
[PubMed]
Bajgar J, Hajek P, Slizova D, Krs O, Fusek J, Kuca K, Jun D, Bartosova L, Blaha V. Changes of acetylcholinesterase activity in different brain areas following intoxication with nerve agents: biochemical and histochemical study. Chem Biol Interact. 165: 14-21, 2007.
[CrossRef]
[PubMed]
Bajgar J, Fusek J, Kassa J, Jun D, Kuca K, Hajek P. An attempt to assess functionally minimal acetylcholinesterase activity necessary for survival of rats intoxicated with nerve agents. Chem Biol Interact. 175: 281-285, 2008.
[CrossRef]
[PubMed]
Bajgar J, Jun D, Kuca K, Fusek J, Zdarova Karasova J, Kassa J, Cabal J, Blaha V. Inhibition of blood cholinesterase by nerve agents in vitro. J Appl Biomed. 7: 201-206, 2009.
[JAB]
Clement JG. Survivors of soman poisoning: recovery of the soman LD50 to control value in the presence of extensive acetylcholinesterase inhibition. Arch Toxicol. 63: 150-154, 1989.
[CrossRef]
[PubMed]
Clement JG, Hand BT, Shilhoff JD. Differences in the toxicity of soman in various strains of mice. Fundam Appl Toxicol. 1: 419-420, 1981.
[CrossRef]
DeMar JC, Clarkson ED, Ratcliffe RH, Campbell AJ, Thangavelu SG, Herdman CA, Leader H, Schulz SM, Marek E, Medynets MA, Ku TC, Evans SA, Khan FA, Owens RR, Nambiar MP, Gordon RK. Pro-2-PAM therapy for central and peripheral cholinesterases. Chem Biol Interact. 187: 191-198, 2010.
[CrossRef]
[PubMed]
Ellman GL, Courtney DK, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7: 88-95, 1961.
[CrossRef]
Fawcett WP, Aracava Y, Adler M, Pereira EFR, Albuquerque EX. Acute toxicity of organophosphorus compounds in guinea pigs is sex- and age-dependent and cannot be solely accounted for by acetylcholinesterase inhibition. J Pharmacol Exp Ther. 328: 516-524, 2009.
[CrossRef]
[PubMed]
Jokanovic M. Liver esterases and soman toxicity in the rat following partial hepatectomy. Biochem Pharmacol. 39: 797-799, 1990.
[CrossRef]
Jokanovic M. Current understanding of the mechanisms involved in metabolic detoxification of warfare nerve agents. Toxicol Lett. 188: 1-10, 2009.
[CrossRef]
[PubMed]
Kadar T, Raveh L, Cohen G, Oz N, Baraness I, Balan A, Ashani Y, Shapira S. Distribution of 3H-soman in mice. Arch Toxicol. 58: 45-49, 1985.
[CrossRef]
[PubMed]
Koplovitz I, Gresham VC, Dochterman LW, Kaminskis A, Stewart JR. Evaluation of the toxicity, pathology, and treatment of cyclohexylmethylphosphonofluoridate (CMPF) poisoning in rhesus monkeys. Arch Toxicol. 66: 622-628, 1992.
[CrossRef]
[PubMed]
Kuca K, Picha J, Cabal J, Liska F. Synthesis of the three monopyridinium oximes and evaluation of their potency to reactivate acetylcholinesterase inhibited by nerve agents. J Appl Biomed. 2: 51-56, 2004.
[JAB]
Mamczarz J, Pereira EFR, Aracava Y, Adler M, Albuquerque EX. An acute exposure to a sub-lethal dose of soman triggers anxiety-related behavior in guinea pigs: interaction with acute restraint. Neurotoxicology. 31: 77-84, 2010.
[CrossRef]
[PubMed]
Morita H, Yanagisava N, Nakajima T, Shimizu M, Hirabayashi H, Okudera H, Nohara M, Midorikawa Y, Mimura S. Sarin poisoning in Matsumoto, Japan. Lancet. 346: 290-293, 1995.
[CrossRef]
Musilek K, Kuca K, Jun D, Dolezal M. In vitro reactivation potency of bispyridinium (E)-but-2-ene linked acetylcholinesterase reactivators against tabun-inhibited acetylcholinesterase. J Appl Biomed. 5: 25-30, 2007.
[JAB]
Novotny L, Misik J, Zdarova Karasova J, Kuca K, Bajgar J. Influence of different ways of euthanasia on the activity of cholinesterases in the rat. J Appl Biomed. 7: 133-136, 2009.
[JAB]
Nozaki H, Hori S, Shinozawa Y, Fujishima S, Takuma K, Kimura H, Suzuki M, Aikawa N. Relationship between pupil size and acetylcholinesterase activity in patients exposed to sarin vapor. Intensive Care Med. 23: 1005-1007, 1997.
[CrossRef]
[PubMed]
Okumura T, Takasu N, Ishimatsu S, Miyanoki S, Mitsuhashi A, Kumada K, Tanaka K, Hinohara S: Report on 640 victims of the Tokyo subway sarin attack. Ann Emerg Med 28:129-135, 1996.
[CrossRef]
Patocka J. Military Toxicology (in Czech). Grada Publishing and Avicenum, Prague, 2004, 178 pp.
Patocka J, Cabal J, Kuca K, Jun D. Oxime reactivation of acetylcholinesterase inhibited by toxic phosphorus esters: in vitro kinetics and thermodynamics. J Appl Biomed. 3: 91-99, 2005.
[JAB]
Shih TM, Penetar DM, McDonough JH, Romano JA, King JM. Age-related differences in soman toxicity and in blood and regional cholinesterase activity. Brain Res Bull. 24: 429-436, 1990.
[CrossRef]
Shih TM, Kan RK, McDonough JH. In vivo cholinesterase inhibitory specificity of organophosphorus nerve agents. Chem Biol Interact. 157-158: 293-303, 2005.
[CrossRef]
[PubMed]
Shih TM, Skovira JW, McDonough JH. Effects of 4-pyridine aldoxime on nerve agent inhibited acetylcholinesterase activity in guinea pigs. Arch Toxicol. 83: 1083-1089, 2009a.
[CrossRef]
[PubMed]
Shih TM, Skovira JW, O'Donnell JC, McDonough JH. Evaluation of nine oximes on in vivo reactivation of blood, brain, and tissue cholinesterase activity inhibited by organophosphorus nerve agents at lethal dose. Toxicol Mech Methods. 19: 386-400, 2009b.
[CrossRef]
[PubMed]
Skopec F, Bajgar J. Anticholinesterase action of organophosphates: importance of the liver. Sbor Ved Pr LFUK (Hradec Kralove). 36: 83-92, 1993.
Sterri SH, Fonnum F. Detoxification of organophosphorus compounds. Acta Pharmacol Toxicol (Copenh). 49 (Suppl. 1): 89, 1981.
Zdarova Karasova J, Bajgar J, Novotny L, Kuca K. Is a high dose of Huperzine A really suitable for pretreatment against high doses of soman? J Appl Biomed. 7: 93-99, 2009a.
[JAB]
Zdarova Karasova J, Bajgar J, Jun D, Pavlikova R, Kuca K. Time-course changes of acetylcholinesterase activity in blood and some tissues in rats after intoxication by Russian VX. Neurotox Res. 16: 356-360, 2009b.
[CrossRef]
[PubMed]
|
BACK
|





