Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 9 (2011), No 1, p 43-48
DOI 10.2478/v10136-009-0034-4
Nycthemeral rhythms of total locomotor activity and oxidative markers in horse
Giuseppe Piccione, Claudia Giannetto, Stefania Casella, Simona Marafioti, Vanessa Messina, Anna Assenza, Caterina Faggio, Francesco Fazio
Address: Giuseppe Piccione, Dipartimento di Scienze Sperimentali e Biotecnologie Applicate, Laboratorio di Cronofisiologia Veterinaria, Facolta di Medicina Veterinaria, Universita degli Studi di Messina, Polo Universitario Annunziata, 98168, Messina, Italy
giuseppe.piccione@unime.it
Received 14th September 2010.
Revised 6th October 2010.
Published online 11th November 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Materials and Methods
Results
Discussion
References
SUMMARY
The aim of this study was to investigate the nycthemeral rhythm of total locomotor activity (TLA) in horse and the possible involvement of the daily organization of rest/activity cycles in the fluctuation of the redox state. For this purpose we recorded TLA and determined oxidative markers in ten clinically healthy Italian Saddle horses. TLA was continuously recorded by means of an actigraphy-based data logger Actiwatch-Mini®. For the assessment of free radicals (dROMs), the antioxidant barrier (Oxy-ads) and the thiol-antioxydant barrier (SHp), blood samples were collected every 4 hours over a 48 h period. One-way repeated measures analysis of variance (ANOVA) showed a statistically significant effect of time of day on all studied parameters. The application of the periodic model and the statistical analysis of cosinor indicate, in horses, the existence of a daily rhythm of the studied parameters during the 48 h of monitoring of the horses. The results show that nycthemeral rhythms of TLA and oxidative markers have different trends in horse. dROMs and Oxy-ads showed a nycthemeral rhythm with an acrophase in the middle of the photophase, and an acrophase of SHp nycthemeral rhythm preceded them. In contrast, TLA showed its acrophase only after the middle of the photophase. TLA showed a lower robustness of rhythms (16.3 and 20.3%) and in respect to the robustness values of the rhythms of oxidative markers (67.3-86.2%). In conclusion, the results of the present investigation showed that oxidative markers have different patterns than locomotor activity, and further studies could be necessary to determine whether other external stimuli, such as solar radiation, food administration or physical exercise are able to influence redox state rhythms in this species.
KEY WORDS
nycthemeral rhythm; free radicals; horse; oxidative power; locomotor activity
INTRODUCTION
In living beings numerous biological functions exhibit
circadian rhythms whose generation is realised by a
complex system with a central pacemaker located
within the suprachiasmatic nuclei (Refinetti 2006,
Berger 2008). Rapid progress in the elucidation of the
mechanism of the circadian clock has been made over
the last century. This has shown the circadian
rhythmicity to be adaptations that allow organisms to
prepare for relatively predictable events in their
environment. The appearance of reproducible and
stable circadian rhythms of high amplitude, and with
a characteristic phasing with respect to other
biological processes and the external environment, is
believed to guarantee an optimal functioning of the
biological system, with maximum efficiency,
performance and wellbeing (Weinert and Waterhouse
2007). In fact, changes in the behavioural activity of
animals are widely used as an indicator for the
assessment of their welfare (Muller and Schrader
2003).
Among these indicators, the total locomotor
activity (TLA) and the oxidative markers have
received considerable attention. TLA has been
documented in a large number of species of mammals
(Piccione et al. 2010a). Most studies have been
carried out on rodents, including laboratory and mole
rats, domestic mice, hamsters, squirrels, voles and
guinea pigs, but circadian patterns of TLA
particularly, including different behaviour such as
feeding, drinking, walking, grooming, ruminating, as
well as all conscious and unconscious movements,
have been well described in rabbits, cats, dogs, sheep
(Piccione et al. 2006, 2007, 2010a, Refinetti 2006),
goats (Piccione et al. 2008b, c) and horses (Bertolucci
et al. 2008, Piccione et al. 2008a). The evaluation of
total locomotor activity has been also studied in
relation to the daily rhythms of the redox state in
sheep (Piccione et al. 2010b) and dairy cattle
(Giannetto et al. 2010).
Oxidative markers have also been documented,
showing that more than one rhythm can be controlled
by a single oscillator, and that multiple rhythms may
be driven by different oscillators (Johnson 2001). In
rats, for example, the circadian variations of the total
antioxidant status are related to the circadian
melatonin rhythm (Benot et al. 1998) whereas in
humans it has been observed that free
radical-scavenging activity is affected by physical
activity and ingestive behaviours (Atsumi et al. 2008).
With this in mind, and considering the relevant
interest in the redox state as a mediator of stress and
pathological conditions and in TLA as an indicator
for the assessment of animal welfare, the aim of this
study was to investigate the nycthemeral rhythm of
TLA in the horse and its possible influence on the
fluctuations of free radicals and anti-oxidant power.
MATERIALS AND METHODS
Animals and housing
Ten Italian Saddle geldings (mean body weight
470±30 kg, 7-9 years old) were used. Before the start
of the study, all subjects underwent a heart
examination, respiratory auscultations, and routine
haematology and plasma biochemistry. Only
clinically healthy animals were used. Horses were
kept in individual boxes under a natural photoperiod
(12/12 LD cycle, sunrise at 06:00, sunset at 18:00)
and a natural environmental temperature (18-21 °C;
60% relative humidity) in Sicily, Italy (latitude 37°
28' N, longitude 14° 37' E). Horses were fed ad
libitum with hay (first cut meadow hay, sun cured,
late cut 8 kg/horse/day) and a mix of cereals (oats and
barley, 50% each, about 3.5 kg/horse/day, divided
into two meals - 07:00 and 19:00). Water was
available ad libitum.
All the treatment, housing and animal care
reported above conformed to the standards
recommended by the Guide for the care and use of
animals (D.L. 27/1/1992, n 116) and EU (Directive
86/609/CEE).
Total locomotor activity recording
The total locomotor activity of horses, which includes
behaviours such as feeding, drinking, walking,
grooming and small movements during sleep, was
recorded for two days of the experimental period.
Each horse was equipped with an actigraphy-based
data logger (Actiwatch-Mini®, Cambridge Neurotechnology Ltd, UK), that recorded a digitally
integrated measure of motor activity. This activity
acquisition system is based on miniaturized
accelerometer technologies, currently used for human
activity monitoring, but also tested for activity
monitoring in small non-human mammals
(Munoz-Delgrado et al. 2004, Mann et al. 2005).
Actiwatch utilizes a piezo-electric accelerometer that
is set up to record the integration of the intensity,
amount and duration of movement in all directions.
The corresponding voltage produced is converted and
stored as an activity count in the memory unit of the
Actiwatch. The maximum sampling frequency is
32Hz. Actigraphs were placed by means of collars
that were accepted without any apparent disturbance.
Activity was monitored with a sampling interval of
5 minutes. The total daily amount of activity, the
amount of activity during the photophase and
scotophase were calculated using Actiwatch Activity
Analysis 5.06 (Cambridge Neurotechnology Ltd,
UK). The Cosine peak of a rhythm (that is, the time of
the daily peak) was computed by cosinor
rhythmometry (Nelson et al. 1979) using the
Actiwatch Activity Analysis 5.06 program.
Blood sampling
Blood samples (10 ml) were collected every 4 h over
a 48 h period, starting at 8:00 on day 1 and finishing
at 8:00 on day 3, in vacutainer tubes without an
anticoagulant (Terumo Corporation, Japan) via
intravenous cannulas inserted into the jugular vein.
Blood samples were centrifuged (ALC 4235 A Milan,
Italy) at 3000gx20 min. The obtained serum was
immediately analyzed by means of a UV
spectrophotometer (model Slim SEAC, Firenze, Italy)
for the assessment of the following parameters:
reactive oxygen species (dROMs), antioxidant barrier
(Oxy-adsorbent) and thiol antioxidant barrier (SHp).
These techniques are based on the "spin traps"
system, in which molecules react with free radicals,
creating complexes revealed by spectrophotometry.
The dROMs test is a colorimetric test that assesses
the levels of hydroperoxides (R-OOH), the "markers"
and "amplifiers" of tissue damage generated by
peroxidation of lipids, amino acids, proteins and
nucleic acids. In this test, these molecules, after
reaction with a properly buffered chromogen, develop
a coloured derivative, which is photometrically
detected. The concentration of ROMs, which directly
parallels changes in colour intensity, is expressed in
Carratelli Units (1 CARR U=0.08 mg% hydrogen
peroxide). Increased values directly correlate to
increased levels of oxidative stress.
The oxy-adsorbent test evaluates the ability of
plasma to oppose the massive oxidant action of an
excess of hypochlorous acid in water solution by
assessing photometrically the residual unreacted
radicals of the acid. Decreased values directly
correlate with the injury severity of "plasma barrier
to oxidation". When the "excess" of radicals of
hypochlorous acid after massive oxidation is high, the
plasma barrier is reduced and vice versa.
The SHp test is a colorimetric determination of the
plasma/serum thiol antioxidant barrier, which opposes
peroxidative processes inhibiting both alkoxyl and
hydroxyl radicals. This test is based on the ability of
thiol groups to develop a coloured complex when
reacted with DTNB (5,5-dithiobis-2-nitrobenzoic
acid). The "titre" of thiols directly parallels colour
intensity. Decreased values directly correlate with a
lower efficacy of the thiols antioxidant barrier.
Statistical analysis
One-way repeated measures analysis of variance
(ANOVA) was used to determine a statistically
significant effect of time of day on total locomotor
activity and oxidative markers at the significant level
2alpha=0.05. The data was analysed using the software
STATISTICA 7 (StatSoft Inc., USA).
Using cosinor rhythmometry (Nelson et al. 1979),
four rhythmic parameters were determined: mesor
(mean level), amplitude (half the range of oscillation),
acrophase (time of peak), and robustness (strength of
rhythmicity). Rhythm robustness (stationarity of a
rhythm) was computed as the quotient of the variance
associated with sinusoidal rhythmicity and the total
variance of the time series (Refinetti 2004).
Robustness greater than 10% is above noise level and
indicates statistically significant rhythmicity.
RESULTS
One-way repeated measures analysis of variance
showed a significant effect of time of day on all
studied parameters on both days of monitoring.
The application of the periodic model and the
statistical analysis of cosinor indicated the existence
of a daily rhythm of the studied parameters in horse
during 48 h of monitoring, and enabled us to define
the periodic parameters and their acrophase during the
two days of monitoring (Table 1). TLA and oxidative
parameters showed a stable diurnal daily rhythm both
of characterized by a different pattern (Figs 1-2).
dROMs and Oxy-ads showed nycthemeral rhythms
with acrophases in the middle of the photophase, and
acrophase of SHp nycthemeral rhythm preceded
them. In contrast, the TLA showed its acrophase only
after the mid-point of the photophase. The TLA
showed a lower robustness of rhythms (16.3 and
20.3%) in respect to robustness values of rhythms of
oxidative markers (67.3-86.2%).
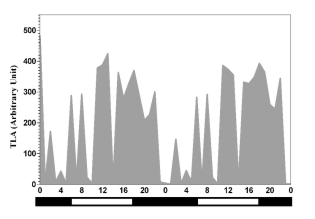
Fig. 1. Plexogram of horses kept in individual boxes under natural photoperiod (12/12 LD cycle, sunrise at 06:00, sunset
at 18:00) and natural environmental temperature. Locomotor activity is indicated by vertical grey marking. White and black bars
indicate photophase and scotophase.
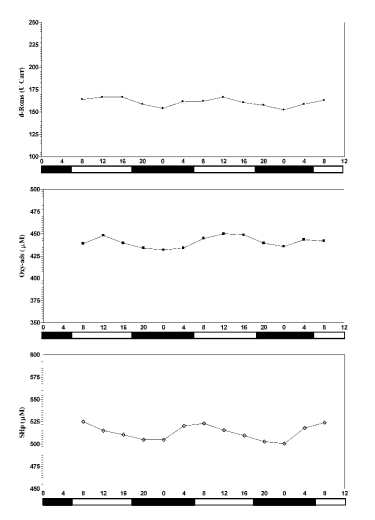
Fig. 2. Patterns of oxidative stress markers. Each point represents the mean of ten horses. White and black bars indicate
photophase and scotophase.
Table 1. Mean values ± SD of four rhythmic parameters of total locomotor activity (TLA), free radicals (dRoms), antioxidant barrier (Oxy-ads) and thiol-antioxydant barrier (SHp), recorded during 48 hours of monitoring in horse.
| Characteristics |
Days of
monitoring |
Mesor |
Amplitude |
Acrophase
(hours) |
Robustness
(%) | | TLA |
day 1
day 2 |
195.54
206.22 |
118.79
133.77 |
15:56
16:37 |
16.3
20.3 | | dRoms (U Carr) |
day 1
day 2 |
161.71
159.71 |
5.67
5.87 |
12:17
11:32 |
67.3
85.7 | | Oxy-ads (microM) |
day 1
day 2 |
438.22
443.58 |
7.54
6.41 |
11:43
12:07 |
76.2
76.3 | | SHp (microM) |
day 1
day 2 |
513.42
511.79 |
9.93
11.22 |
08:03
08:39 |
84.2
86.2 |
DISCUSSION
Our results showed that nycthemeral rhythms of TLA
and oxidative markers have different trends in horse;
this is not in agreement with previous studies in
which the circadian oscillations of many behavioural
processes and physiological parameters are paralleled
(Langmesser and Albrecht 2006). These different
patterns were accompanied by different robustness of
rhythm values, and it is unlikely that a rhythm with
low robustness might influence the rhythm with high
robustness. Thus, the rhythm of TLA cannot be the
cause of the redox state rhythm in horse. Whether the
rhythm of activity is the cause of the other rhythms
cannot be determined from the data on the robustness
of this rhythm. Nevertheless, it was shown that the
circadian organization of rest/activity cycles implies
fluctuations in the level of free radicals oxygen
species that are generated as by-products of the
fluctuations in activity and metabolic rates
(Langmesser and Albrecht 2006). So, the rhythmicity
in radical formation should relate to that in oxygen consumption, which is widely documented in many
animals and which should, in turn, depend on the
circadian rhythms of locomotor activity (Hardeland et
al. 2000).
Moreover, endogenous circadian and exogenously
driven daily rhythms of antioxidative molecules have
been described in various phylogenetically distant
organisms. Substantial amplitudes were observed in
several cases, suggesting the significance of
rhythmicity in avoiding excessive oxidative stress
(Hardeland et al. 2003).
Our finding, in which different variables exhibit
different degrees of rhythmicity, is not surprising
because it has been observed in other studies in which
simultaneous recording of many variables was carried
out (Refinetti 1999, Piccione et al. 2005, Giannetto
and Piccione 2009). However, the finding is
important because of its implications for the defense
of the organism and in maintaining the redox state.
In conclusion, the results of the present
investigation confirm that the monitoring of oxidative
stress parameters contribute to the clinical evaluation
of the horse but underline that oxidative markers are
not affected by locomotor activity, and further studies
are necessary to determine whether other external
stimulus, such as solar radiation, food administration
or physical exercise are able to influence redox state
rhythms in this species.
REFERENCES
Atsumi T, Tonosaki K, Fujisawa S. Salivary free radical-scavenging activity is affected by physical and mental activity. Oral Dis. 14: 490-496, 2008.
[CrossRef]
[PubMed]
Benot S, Molinero P, Soutto M, Goberna R, Guerrero JM. Circadian variations in the rat serum total antioxidant status: Correlation withmelatonin levels. J Pineal Res. 25: 14, 1998.
[CrossRef]
[PubMed]
Berger J. A two-clock model of circadian timing in the immune system of mammals. Pathol Biol. 56: 286, 2008.
[CrossRef]
[PubMed]
Bertolucci C, Giannetto C, Fazio F, Piccione G. Seasonal variations in daily rhythms of activity in athletic horses. Animal. 2: 1055-1060, 2008.
[CrossRef]
Giannetto C, Piccione G. Daily rhythms of 25 physiological variables in Bos taurus maintained under natural conditions. J Appl Biomed. 7: 55-61, 2009.
[JAB]
Giannetto C, Fazio F, Assenza A, Caola G, Pennisi P, Piccione G. Circadian rhythms of redox states and total locomotor activity in dairy cattle. Czech J Anim Sci. 55: 183-189, 2010.
Hardeland R, Coto-Montes A, Burkhardt S, Zsizsik BK. Circadian rhythms and oxidative stress in non-vertebrate organisms. In Vanden Driessche T. (ed.): The redox state and circadian rhythms. Kluwer Academic Publishers, Dordrecht 2000, pp. 121-126.
Hardeland R, Coto-Montes A, Poeggeler B. Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol Int. 20: 921-962, 2003.
[CrossRef]
[PubMed]
Johnson CR. Endogenous timekeepers in photosynthetic organisms. Annu Rev Physiol. 63: 695-728, 2001.
[CrossRef]
[PubMed]
Langmesser S, Albrecht U. Life time-circadian clock, mitochondria and metabolism. Chronobiol Int. 23: 151-157, 2006.
[CrossRef]
[PubMed]
Mann TM, Williams KE, Pearce PC, Scott EA. A novel method for activity monitoring in small non-human primates. Lab Anim. 39: 169-177, 2005.
[CrossRef]
[PubMed]
Muller R Schrader L. A new method to measure behavioural activity levels in dairy cows. Appl Anim Behav. Sci 83: 247-258, 2003.
[CrossRef]
Munoz-Delgrado J, Corsi-Cabrera M, Canales-Espinosa D, Santillan-Doherty AM, Erket HG. Astronomical and meteorological parameters and rest-activity rhythm in the spider monkey, Ateletes geoff royi. Physiol Behav. 83: 101-117, 2004.
Nelson K, Tong JL, Lee JK, Halberg F. Methods for cosinor rhythmometry. Chronobiologia. 6: 305-323, 1979.
[PubMed]
Piccione G, Caola G, Refinetti R. Temporal relationship of 21 physiological variables in horse and sheep. Comp Biochem Physiol A Comp Physiol. 142: 389-396, 2005.
Piccione G, Bertolucci C, Caola G, Foa A: Effects of restricted feeding on circadian activity rhythms of sheep - A brief report. Appl Anim Behav Sci. 107: 233-238, 2006.
[CrossRef]
Piccione G, Giannetto C, Costa A, Caola G. Daily rhythms of total activity in rabbits during different light/dark schedules. Trends Appl Sci Res. 2: 360-364, 2007.
[CrossRef]
Piccione G, Costa A, Giannetto C, Caola G. Daily rhythms of activity in horses housed in different stabling conditions. Biol Rhythm Res. 39: 79-84, 2008a.
[CrossRef]
Piccione G, Giannetto C, Assenza A, Fazio F, Caola G. Locomotor activity and serum tryptophan and serotonin in goats: daily rhythm. J Appl Biomed. 6: 73-79, 2008b.
[JAB]
Piccione G, Giannetto C, Casella S, Caola G. Seasonal change of daily motor activity rhythms in Capra hircus. Can J Anim Sci. 88: 351–355, 2008c.
[CrossRef]
Piccione G, Giannetto C, Casella S, Caola G. Daily locomotor activity in five domestic animals. Anim Biol Leiden Neth. 60: 15-24, 2010a.
[CrossRef]
Piccione G, Giannetto C, Fazio F, Pennisi P, Caola G. Evaluation of total locomotor activity and oxidative markers daily rhythms in sheep. Biol Rhythm Res. 41: 433-439, 2010b.
[CrossRef]
Refinetti R. Relationship between the daily rhythms of locomotor activity and body temperature in eight mammalian species. Am J Physiol. 277: R1493-R1500, 1999.
[PubMed]
Refinetti R. Non-stationary time series and the robustness of circadian rhythms. J Theoret Biol. 227: 571-581, 2004.
[CrossRef]
[PubMed]
Refinetti R. Circadian physiology. 2nd Ed. Taylor & Francio Group, Boca Raton 2006, pp. 153-213.
Weinert D, Waterhouse J. The circadian rhythm of core temperature: Effects of physical activity and aging, Physiol Behav. 90: 246-256, 2007.
[CrossRef]
|
BACK
|



