Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 9 (2011), No 1, p 49-56
DOI 10.2478/v10136-009-0031-7
Caffeine-suppressed ATM pathway leads to decreased p53 phosphorylation and increased programmed cell death in gamma-irradiated leukaemic molt-4
cells
Ales Tichy, Darina Muthna, Jirina Vavrova, Jaroslav Pejchal, Zuzana Sinkorova, Lenka Zarybnicka, Martina Rezacova
Address: Ales Tichy, Department of Radiobiology, Faculty of Military Health Sciences, University of Defence, Trebesska 1575, 500 01 Hradec
Kralove, Czech Republic
tichy@pmfhk.cz
Received 20sup>th April 2010.
Revised 9th June 2010.
Published online 5th November 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Materials and Methods
Results and Discussions
Conclusion
Acknowledgement
References
SUMMARY
Ionising radiation (IR) is one of the main treatment modalities in oncology. However, we still search for substances which can radio-sensitize
tumour cells. In this study we used caffeine, a non-specific ataxia-telangiectasia mutated kinase (ATM) inhibitor, and studied its effect on the
activation of the proteins involved in cell cycle control and the induction of apoptosis in human T-lymphocyte leukaemic MOLT-4 cells (p53 wt). We
evaluated the expression of the tumour-suppressor p53 (itself and phosphorylated on Ser15 and Ser392), the cell cycle
regulator p21, and the anti-apoptotic protein myeloid cell leukemia 1 (Mcl-1). After treatment with 2 mM caffeine, the cells were irradiated by 1
or 3 Gy, lysed and the proteins detected by Western-blotting. Apoptosis was determined by flow-cytometric annexin V/propidium iodine detection.
Irradiation by 1 or 3 Gy induced p53 phosphorylation at Ser15 and Ser392 after 2 h with maximum after 4 h. Adding caffeine
significantly inhibited Ser15 phosphorylation, which is ATM-dependent but surprisingly also Ser392 phosphorylation, which is
ATM-independent, suggesting that caffeine might have another cellular target (protein kinase). Similarly, caffeine caused a substantial decrease
in p21 in combination with both doses of IR and also Mcl-1 was down-regulated. Three days after irradiation, caffeine significantly increased
induction of apoptosis. The ATM/p53 pathway was suppressed by caffeine, which led to increased apoptosis accompanied by a p53-independent decrease
in Mcl-1. It also caused down-regulation of p21, which possibly contributed to the shortened cell cycle arrest necessary for effective DNA repair
and thus impeded radio-resistance. Caffeine promotes the cytotoxic effect of ionising radiation and provides a possible platform for the
development of new anti-cancer therapeutics known as radio-sensitizers.
KEY WORDS
ATM; p53; p21; Mcl-1; caffeine; ionising radiation; MOLT-4
Abbreviations: IR, ionising radiation; ATM, ataxia telangiectasia mutated kinase; ATR, ATM- and Rad3-related
protein kinase; chk-2, checkpoint kinase-2
INTRODUCTION
Exposure of mammalian cells to ionising radiation
(IR) leads to DNA damage such as double strand
breaks (DSB). A very early step in the DNA repair is
rapid intermolecular autophosphorylation and
activation of ataxia-telangiectasia mutated (ATM)
kinase (Lavin et al. 1995, Bakkenist and Kastan
2003). The importance of ATM as a central controller
of cellular responses to DSB is undeniable; it
regulates all three cell cycle checkpoints, DNA repair,
and apoptosis (Khanna et al. 2001). ATM functions in
the signalling pathways as a co-ordinator of the key
components, since it regulates its substrates by
phosphorylation. These are e.g. p53, murine-double
minute protein (mdm2), and checkpoint kinase-2
(chk-2) managing the G1 checkpoint (Matsuoka et al.
2000, Maya et al. 2001) and many others for the
transient S-phase arrest and the G2/M checkpoint.
IR is one of the main treatment modalities in
oncology. However, it can generate a series of
unwanted events inside the cells and therefore it is
recommended that doses be minimised as much as
possible. This might be achieved by the combination
of irradiation with substances which are capable of
sensitising the target cells towards radiation;
radio-sensitisers.
In a number of cells, ATM and its regulatory
function can be inhibited by many protein kinase
inhibitors (Blasina et al. 1999, Kaufmann et al. 2003).
In this work we focused on caffeine, which could act
on cell cycle checkpoints and affect the progression
of the cell cycle, but its impact on the apoptosis of
tumour cells has not yet been properly identified.
Steinmann et al. (1991) stated that caffeine is capable
of abrogating cell cycle checkpoints in several
different mammalian cell lines.
Caffeine is a methylxanthine possessing many
different cellular effects. A number of in vitro and in
vivo studies have demonstrated that caffeine
modulates both innate and adaptive immune
responses via inhibition of cAMP-phosphodiesterase;
another group of effects induced by caffeine is
mediated through its inhibitory action on adenosine
receptors, which could have a direct impact on
neovascularization of human tumours, and finally,
some studies have proved that caffeine affects the
anti-tumour activity of DNA-intercalating drugs
(reviewed in Sabisz and Skladanowski 2008). The
precise mechanism is still under debate, but some
have shown that the radio-sensitising effects of
caffeine are associated with the disruption of multiple
DNA damage-responsive cell cycle checkpoints and
DNA repair (e.g. Asaad et al. 2000, Deplanque et al.
2001, Block et al. 2004). Undoubtedly, the effects of
caffeine are to a great extent cell-type dependent.
In this paper we describe the effect of caffeine on
T-lymphocyte leukaemic MOLT-4 cells in the
perspective of ATM inhibition and subsequent
inhibition of its targets involved in DNA repair, cell
cycle control, and apoptosis. We conclude that
pre-treatment of MOLT-4 cells with caffeine prior to
gamma-irradiation has a radio-sensitising effect.
MATERIALS AND METHODS
Cell culture and culture conditions
The human T-lymphocyte leukaemia MOLT-4 cells
were obtained from the American Type Culture
Collections (Manassas, USA). The cells were cultured
in Iscove's modified Dulbecco's medium (Sigma, St.
Louis, USA) supplemented with 20% fetal calf serum,
0.05% L-glutamine, 150 UI/ml penicillin, 50 microg/ml
streptomycin in a humidified incubator at 37 °C and
controlled 5% CO2 atmosphere. The cultures were
divided every second day by dilution to a
concentration of 2x105 cells/ml. Cell lines in the
maximal range of up to 20 passages were used for this
study.
Gamma-irradiation
Exponentially growing cells were suspended at a
concentration of 2,105/ml. Aliquots of 10 ml of cell
suspension were plated into 25 cm2 flasks (Nunc,
Wiesbaden, Germany) and irradiated at room
temperature using a 60Co gamma-ray source with a dose-rate
of 0.4 Gy/min, at a distance of 1 m from the source.
After irradiation, the flasks were placed in a 37 °C
incubator with 5% CO2 atmosphere and aliquots of the
cells were removed at various times after irradiation
by 1 or 3 Gy for further analysis. The cells were
counted and cell viability was determined with the
Trypan blue exclusion assay.
Caffeine exposure
We added 2 mM caffeine (Sigma) to the cells 1 h
prior to irradiation.
Flow-cytometry
For apoptosis detection we used an Apoptest-FITC kit
(Dako-Cytomation, Brno, Czech Republic). During
apoptosis, cells expose phosphatidylserine at the cell
surface. Annexin V (A) is a phospholipid binding
protein, which (in the presence of calcium ions) binds
selectively and with high affinity to phospha-tidylserine. Cells with a permeable cell membrane
(late apoptotic or necrotic) were detected by
propidium iodide (PI) staining. Flow-cytometric
analysis was performed on a Coulter Epics XL flow
cytometer equipped with a 15mW argon-ion laser
with excitation capabilities at 488 nm (Beckman
Coulter, Fullerton, USA). A minimum of 10,000 cells
was collected for each two-colour sample in a list
mode file format. List mode data were analysed using
Epics XL System II software (Beckman Coulter).
Electrophoresis and Western blotting
At various times after irradiation (2, 4, 16, and 24 h),
the cells were washed with phosphate buffer saline
and lysed. Whole cell extracts were prepared by lysis
in 500 microl of lysis buffer (137 mM NaCl, 10%
glycerol, 1% n-octyl-beta-glucopyranoside, 50 mM NaF,
20 mM Tris, 1 mM Na3VO4, pH 8 - all from Sigma,
St. Louis, USA - and 1 tablet of CompleteTM Mini,
Roche, Manheim, Germany). The lysates containing
equal amount of protein (30 microg) were loaded onto a
12% SDS (sodium dodecyl sulphate) polyacrylamide
gel. After electrophoresis, the proteins were
transferred to a PVDF (polyvinylidene difluoride)
membrane (BioRad, Hercules, USA), and hybridized
with an appropriate antibody: anti-p53 and anti-p53
Ser392 (1:1000 and 1:500, Exbio, Prague, Czech
Republic); anti-p53 Ser15 (1:1000, Calbiochem, San Diego, USA), Mcl-1, p21, and anti-beta-actin
(1:1000, 1:500 and 1:10000, Sigma). After washing,
the blots were incubated with secondary
peroxidase-conjugated antibody - 1:1000/1:10000
(Dako, High Wycombe, UK) and the signal was
developed with an ECL detection kit (BM
Chemiluminescence - POD, Roche, Manheim,
Germany) by exposure to a film.
RESULTS AND DISCUSSION
Tumour cells exposed to caffeine tend to be more
sensitive to IR and other genotoxic agents. In spite of
the fact that the radio-sensitising effects of caffeine
have been studied for over last two decades, the
underlying mechanism is still in debate. Nevertheless,
it is more than likely that this phenomenon is linked
to signalling pathways critical for adequate recovery
from irradiation, namely DNA damage checkpoints.
Accumulating evidence indicates that under normal
conditions the crucial role in checkpoint control is
played by the members of phosphatidylinositol
3-kinase related kinases, especially by ATM
(Hoekstra 1997, Pandita 2003). ATM derives from
ataxia-telangiectasia (A-T), human autosomal
recessive disorder, in which a gene is mutated. A-T
cells display specific genotype and in addition to
increased radio-sensitivity they suffer from genomic
instability, cancer predispositions and increased
sensitivity to inhibitors of topoisomerase (Lavin and
Shiloh 1997). ATM integrates the cellular response to
DSB caused by these agents via phosphorylation of
the key proteins involved in cell cycle regulation,
DNA repair, and apoptosis. This was studied in our
previous work on -irradiated MOLT-4 cells, which
proved ATM/chk-2/p53 signalling pathway to be
functional (ATM, chk-2, p53, and mdm2 were
phosphorylated rapidly after irradiation) and both of
the p53 phosphorylations on Ser15 and Ser392 were
detected after irradiation (Tichy et al. 2007).
Moreover, Sarkaria et al. (1999) showed on lung
adenocarcinoma cells that caffeine inhibits the
catalytic activity of both ATM and ATM and
Rad3-related kinase (ATR). Kaufmann et al. (2003)
have stated that a caffeine concentration that inhibits
ATM by 50% is 1mM and the 50% inhibitory
concentration for ATR is about 3mM (in vitro). These
concentrations define the range at which caffeine
effectively reverses cell cycle checkpoints and
enhances cytotoxicity in carcinogen-damaged cells
without significant toxicity of its own. In this study
we used a caffeine concentration of 2 mM. Lower
concentrations are less effective at reversing
checkpoint function and higher concentrations cause
a reduction in DNA synthesis associated with
cytotoxicity.
Phosphorylations of p53 are inhibited by caffeine
In this study, we focused on one of the typical
molecules, whose activation is linked to IR - protein
p53. The specific mechanisms that determine whether
p53-dependent cell cycle arrest or p53-dependent
apoptosis prevails in response to specific DNA
damage are poorly understood. Sarkaria et al. (1999)
reported that caffeine inhibits gamma- and UV
radiation-induced phosphorylation of p53 on Ser15, a
modification that may be directly mediated by ATM.
However, Kaufmann et al. (2003) in their work on
human fibroblasts described the ATM-dependent
phosphorylation of p53 after gamma-irradiation as resistant
to caffeine. Importantly, in our present work, we
examined the implication of caffeine on
phosphorylations on Ser15 and Ser392 and we found
them strongly affected by this agent. As a matter of
fact, both of the phosphorylations were substantially
inhibited throughout the whole period of the
experiment.
P53 was present in the intact cells and its amount
dramatically increased after irradiation in a
dose-dependent manner. It was up-regulated 2 h after
irradiation by doses of 1 and 3 Gy with maximum
after 16 h. Caffeine caused a mild decrease in p53 in
irradiated and partially even in non-irradiated cells
(Fig. 1).
Phosphorylation on Ser15 is crucial for IR-induced
DNA damage response. ATM, ATR, and
DNA-dependent protein kinase are responsible for
this phosphorylation, whereby p53 stability is
maintained (Shieh et al. 1997, Tibbetts et al. 1999,
Helt et al. 2005). The phosphorylation is important for
activation of p53 after exposure to IR, since it impairs
the ability of mdm2 to inhibit p53-dependent
transactivation (Shieh et al. 1997). Phosphorylation of
p53 on Ser15 was detected only in irradiated cells after
2 h with a maximum after 4 h. Caffeine caused a
substantial decrease in this post-translational
modification after irradiation (Fig. 1).
Phosphorylation of p53 on Ser392 enhances its
sequence-specific DNA binding (Criswell et al.
2003). Sakaguchi et al. (1997) showed that
phosphorylation on Ser392 stabilizes the tetramer
formation of p53 that is critical for its ability to
activate transcription because it facilitates
phosphorylation of the transactivation domain due to
a more favourable conformation. Casein kinase 2
(CK2) phosphorylates p53 on Ser392, but Claudio et al.
(2006) demonstrated that Cdk9 itself is capable of
such phosphorylation independently of CK2. Saito et
al. (2002) proposed the N-terminal p53
phosphorylation sites to be grouped into two
categories with respect to dependence on ATM for
rapid phosphorylation in response to IR. One group,
consisting of Ser6, Ser33, Ser315, and Ser392, is
independent of ATM and constitutively
phosphorylated at low levels; the other group,
consisting of Ser9, Ser15, Ser20, and Ser46, is
ATM-dependent, typically with a rapid response to
IR-induced damage, presumably DSB. Notably, our
data show that caffeine treatment suppressed Ser392
phosphorylation and as long as it is
ATM-independent, caffeine may have another
cellular target. We suggest that caffeine inhibits
another protein kinase, which executes this
phosphorylation.
Sarkaria et al. (1999) also stated that the
radio-sensitizing effects of caffeine are related to the
inhibition of ATM and ATR and that both proteins
are relevant targets for the development of novel
anti-cancer agents. After gamma-irradiation the pivotal role
is played by ATM, whereas ATR is indispensable
after UV-radiation or cellular damage caused by
intercalating cytostatic agents. Nevertheless, these
data must be perceived in the light of in vitro studies,
because other groups reported multiple ATM-ATR
substrates including chk-1 and chk-2 to be
hyper-phosphorylated in cells treated with caffeine
and IR, and although caffeine is an inhibitor of
ATM-ATR kinase activity in vitro, it can block
checkpoints without inhibiting ATM-ATR in vivo
(reviewed in Cortez, 2003).
Caffeine causes down-regulation of p21
Besides providing the evidence that caffeine inhibits
ATM-dependent phosphorylation of p53 in
gamma-irradiated leukaemic cells we also report on the
inhibition of p21. This protein functions as inhibitor
of cyclin-dependent kinases and thus can stop the cell
cycle (reviewed in Schwartz, 2002).
Protein p21 was not detected in non-irradiated or
caffeine-treated cells. It was up-regulated 4 h after
irradiation by doses of 1 and 3 Gy with maximum
after 24 h, but this effect was markedly inhibited by
caffeine (Fig. 1). This is in accordance with other
studies describing caffeine-induced ATM-dependent
inhibition of p21 (Hill et al. 2008, Meng and Jiang
2009). We observed a link between radio-resistance
and p21 up-regulation also in the promyelocytic
leukaemic cell line HL-60 (p53-negative). After 2 Gy
we did not detect an increase in p21, but in the
radio-resistant line HL-60-R (which received a
fractionated dose of 60 Gy before the experiment), an
increase in p21 was already obvious 4 h after
irradiation (unpublished data).
Caffeine causes down-regulation of Mcl-1
We evaluated the downstream effect of caffeine and
we checked the expression of the anti-apoptotic
member of the Bcl-2 protein family, myeloid cell
leukemia 1 (Mcl-1), which consists of the key
regulators of cell death. Mcl-1 acts as an apical
molecule in control of apoptosis and propagation of
cell survival (Borner 2003). A decrease in Mcl-1 is
associated with apoptosis induction, since it blocks
IR-induced apoptosis and inhibits clonogenic cell
death by maintaining mitochondrial integrity via
interaction with pro-apoptotic partners (Borner 2003,
Skvara et al. 2005). Also, a novel role for this protein
has been explored; Jamil et al. (2005) concluded that
Mcl-1 is essential in ATR-mediated chk-1
phosphorylation, perhaps acting as an adaptor protein.
We detected the anti-apoptotic protein Mcl-1 in
non-irradiated cells. Its amount in caffeine-treated
cells did not change during the whole 24 h period of
the experiment. Sixteen h after irradiation by the dose
of 3 Gy we observed a partial decrease in Mcl-1. A
very pronounced decrease in Mcl-1 was observed 24 h
after irradiation. In our previous work on HL-60 we
found that the amount of Mcl-1 initially increased
after irradiation by a sublethal but not lethal dose and
later (when apoptosis occurred) it decreased in a
dose-dependent manner (Tichy et al. 2008). In the
recent experiments we did not observe any
up-regulation of Mcl-1, but this protein was markedly
down-regulated 24 h after irradiation by a
combination of gamma-radiation with caffeine.
Moreover, this obvious decrease in Mcl-1 was
p53-independent (Fig. 1).
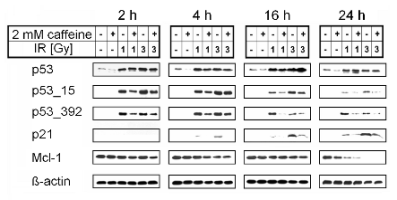
Fig. 1. Inhibitory effect of caffeine on proteins involved in cell cycle regulation and apoptosis. The MOLT-4 cells were irradiated and
lysed as indicated; proteins were electrophoretically separated and detected by immunoblotting. Combination of
caffeine (added 1 h prior irradiation) and ionising radiation caused substantial decrease in phosphorylation of p53 and
down-regulation of p21 and Mcl-1. Representative Western blots are shown.
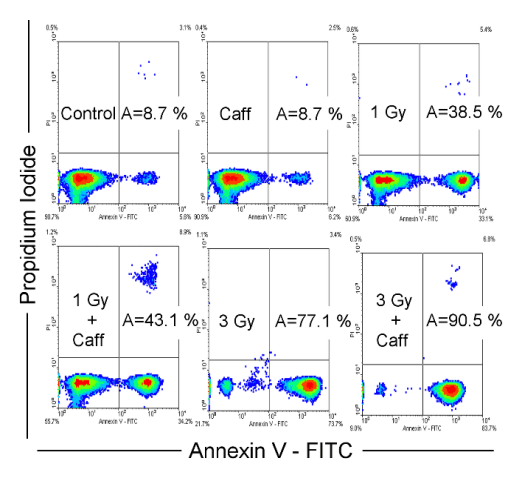
Fig. 2. Effect of caffeine and ionising radiation on induction of apoptosis. The MOLT-4 cells were treated with caffeine (added 1 h prior
to irradiation and washed out 24 h post irradiation), irradiated, stained with annexin V/propidium iodide and then,
apoptosis was measured by flow-cytometry 72 h after irradiation. Combination of caffeine and ionising radiation leads to
increased apoptosis.
Caffeine increases induction of apoptosis
The phase at which leukemia cells enter apoptosis
depends on the nature of the insult. In the case of UV- or gamma-radiation it is G1-phase (Feng et al.
2007). This group also demonstrated on MOLT-4
cells that most, if not all, apoptosis is initiated in this
specific cell cycle phase. Moreover, cells progressing
through the cell cycle without stopping at checkpoints
could avoid apoptosis. The apoptotic pattern and
schedule varies with changes in the cell cycle and the
uncoupling of apoptosis from cell cycle progression
may lead to uncontrolled proliferation. Cellular
proliferation is a manifestation of passage through the
cell cycle, which is regulated at various checkpoints
(Bartek and Lukas 2001). We evaluated induction of
apoptosis 72 h after irradiation by annexin
V/propidium iodide staining. The annexin
V/propidium iodide staining revealed 8.7% of
apoptotic cells in the control and the same amount
(8.7%) in caffeine-treated cells. After irradiation by
1 or 3 Gy we found 38.5 or 77.1% of apoptotic cells,
respectively. Adding caffeine induced an increase in
the number of apoptotic cells to 43.1 or 90.5%,
respectively (Fig. 2). It is likely that caffeine
abrogated G2 cell cycle arrest, as previously
confirmed by others (Kastan et al. 1991, Sarkaria et
al. 1999, Vavrova et al. 2003) and therefore we
observed a higher proliferation rate in caffeine- and
IR-treated cells than in irradiated cells during the first
days after irradiation (unpublished data). In our
previous work on HL-60 cells, caffeine-induced
apoptosis was observed in the late intervals after
irradiation - 7 to 10 days (Vávrová et al. 2003).
Linkage of radio-resistance with the time of DNA
damage repair was also apparent in our experiments
on cells irradiated by low dose-rate. When the cells
were irradiated in the G2-phase, their radio-resistance
was higher than after single irradiation. On the other
hand, when the cells entered mitosis their
radio-resistance decreased (Vavrova et al. 2004).
It seems that, DNA damage can result in the
activation of cell cycle checkpoints and subsequent
cell cycle arrest, which may lead either to DNA repair
or in the case of extensive DNA lesions, to apoptosis.
It is likely that caffeine effectively abrogates DNA
damage G2/M checkpoint activation and could permit
entry of arrested cells into M-phase by inhibiting
ATM. And so we observed increased proliferation
24 h after irradiation and not apoptosis immediately
after Mcl-1 decrease; nevertheless, later (72 h after
irradiation), the cells with propagated unrepaired
DNA died by increased apoptosis.
CONCLUSION
In this work, we show that caffeine suppresses
ATM/p53 signalling pathway in human T-lymphocyte
leukaemic MOLT-4 cells. P21 up-regulation was
suppressed as a result of inhibited p53
phosphorylation on Ser15 and surprisingly also Ser392
phosphorylation, which is ATM-independent,
suggesting that caffeine might have another cellular
target (protein kinase). Notably, we observed a
significant p53-independent decrease in anti-apoptotic
Mcl-1 expression. These data suggest that caffeine
increases the G2/M block override, diminishes repair
of gamma-irradiation induced DNA damage, and
subsequently contributes to induction of apoptosis.
Thus caffeine promotes the cytotoxic effect of
ionising radiation and provides a platform for the
development of new anti-cancer therapeutics known
as radio-sensitisers.
ACKNOWLEDGEMENT
This work was supported by Ministry of Defence,
Czech Republic (project MO0FVZ0000501) and by
Ministry of Education, Czech Republic (project MSM
0021620820). The authors would like to thank Mrs.
Nada Mazankova, Bc. Lenka Mervartova, Mrs.
Jaroslava Prokesova, and Mrs. Eva Vodakova for
their excellent technical support.
Declaration of interest: The authors report no
conflicts of interest. The authors alone are responsible
for the content and writing of the paper.
REFERENCES
Asaad NA, Zeng ZC, Guan J, Thacker J, Iliakis G. Homologous recombination as a potential target for caffeine radiosensitization in mammalian cells: reduced caffeine radiosensitization in XRCC2 and XRCC3 mutants. Oncogene. 19: 5788-5800, 2000.
[CrossRef]
[PubMed]
Bakkenist C, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimmer dissociation. Nature. 421: 499-506, 2003.
[CrossRef]
[PubMed]
Bartek J, Lukas J. Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 490: 117-122, 2001.
[CrossRef]
Blasina A, Price BD, Turenne GA, McGowan CH. Caffeine inhibits the checkpoint kinase ATM. Curr Biol. 9: 1135-1138, 1999.
[CrossRef]
Block WD, Merkle D, Meek K, Lees-Miller SP. Selective inhibition of the DNA-dependent protein kinase (DNA-PK) by the radiosensitizing agent caffeine. Nucl Acids Res. 32: 1967-1972, 2004.
[CrossRef]
[PubMed]
Borner C. The Bcl-2 protein family: Sensors and checkpoints for life-or-death decisions. Mol Immunol. 39: 615-647, 2003.
[CrossRef]
Claudio PP, Cui J, Ghafouri M, Mariano C, White MK, Safak M, Sheffield JB, Giordano A, Khalili K, Amini S, Sawaya BE. Cdk9 phosphorylates p53 on serine 392 independently of CKII. J Cell Physiol. 208: 602-612, 2006.
[CrossRef]
[PubMed]
Cortez D. Caffeine inhibits checkpoint responses without inhibiting the ataxia-telangiectasia-mutated (ATM) and ATM- and Rad3-related (ATR) protein kinases. J Biol Chem. 278: 37139-37145, 2003.
[CrossRef]
[PubMed]
Criswell T, Leskov K, Miyamoto S, Luo G, Boothman DA. Transcription factors activated in mammalian cells after clinically relevant doses of ionizing radiation. Oncogene. 22: 5813-5827, 2003.
[CrossRef]
[PubMed]
Deplanque G, Ceraline J, Mah-Becherel MC, Cazenave JP, Bergerat JP, Klein-Soyer C. Caffeine and the G2/M block override: A concept resulting from a misleading cell kinetic delay, independent of functional p53. Int J Cancer. 94: 363-369, 2001.
[CrossRef]
[PubMed]
Feng Y, Wu J, Feng X, Tao D, Hu J, Qin J, Li X, Xiao W, Gardner K, Judge SIV, Li QQ, Gong J. Timing of apoptosis onset depends on cell cycle progression in peripheral blood lymphocytes and lymphocytic leukemia cells. Oncol Rep. 17: 1437-1444, 2007.
[PubMed]
Helt CE, Cliby WA, Keng PC, Bambara RA, O'Reilly MA. Ataxia telangiectasia mutated (ATM) and ATM and Rad3-related protein exhibit selective target specificities in response to different forms of DNA damage. J Biol Chem. 280: 1186-1192, 2005.
[CrossRef]
[PubMed]
Hill R, Leidal AM, Madureira PA, Gillis LD, Waisman DM, Chiu A, Lee PW. Chromium-mediated apoptosis: involvement of DNA-dependent protein kinase (DNA-PK) and differential induction of p53 target genes. DNA Repair (Amst). 7: 1484-1499, 2008.
[CrossRef]
[PubMed]
Hoekstra MF. Responses to DNA damage and regulation of cell cycle checkpoints by the ATM protein kinase family. Curr Opin Genet Dev. 7: 170-175, 1997.
[CrossRef]
Jamil S, Mojtabavi S, Hojabrpour P, Cheah S, Duronio V. An essential role for MCL-1 in ATR-mediated CHK1 phosphorylation. Mol Biol Cell. 19: 3212-3220, 2005.
[CrossRef]
[PubMed]
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51: 6304-6311, 1991.
[PubMed]
Kaufmann WK, Heffernan TP, Beaulieu LM, Doherty S, Frank AR, Zhou Y, Bryant MF, Zhou T, Luche DD, Nikolaishvili-Feinberg N, Simpson DA, Cordeiro-Stone M. Caffeine and human DNA metabolism: the magic and the mystery. Mutat Res. 532: 85-102, 2003.
[CrossRef]
Khanna KK, Lavin MF, Jackson SP, Mulhern TD. ATM, a central controller of cellular responses to DNA damage. Cell Death Differ. 8: 1052-1065, 2001.
[CrossRef]
[PubMed]
Lavin MF, Shiloh Y. The genetic defect in ataxia-telangiectasia. Annu Rev Immunol. 15: 177-202, 1997.
[CrossRef]
[PubMed]
Lavin MF, Khanna KK, Beamish H, Spring K, Watters D, Shiloh Y. Relationship of the ataxia-telangiectasia protein ATM to phosphoinositide 3-kinase. Trends Biochem Sci. 20: 382-383, 1995.
[CrossRef]
Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA. 97 :10389-10394, 2000.
[CrossRef]
[PubMed]
Maya R, Balass M, Kim S-T, Shkedy D, Martinez Leal J-F, Shifman O, Moas M, Buschmann T, Ronai Z, Shiloh Y, Kastan MB, Katzir E et al. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Devel. 15: 1067-1077, 2001.
[CrossRef]
Meng AG, Jiang LL. Induction of G2/M arrest by pseudolaric acid B is mediated by activation of the ATM signaling pathway. Acta Pharmacol Sinica. 30: 442-450, 2009.
[CrossRef]
[PubMed]
Pandita TK. A multifaceted role for ATM in genome maintenance. Exp Rev Mol Med. 5: 1-21, 2003.
[CrossRef]
Sabisz M, Skladanowski A. Modulation of cellular response to anticancer treatment by caffeine: inhibition of cell cycle checkpoints, DNA repair and more. Curr Pharmacol Biotechnol. 9: 325-336, 2008.
[CrossRef]
[PubMed]
Sakaguchi K, Sakamoto H, Lewis MS, Anderson CW, Erickson JW, Appella E, Xie D. Phosphorylation of serine 392 stabilizes the tetramer formation of tumor suppressor protein p53. Biochemistry. 36: 10117-10124, 1997.
[CrossRef]
[PubMed]
Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59: 4375-4382, 1999.
[PubMed]
Saito S, Goodarzi AA, Higashimoto Y, Noda Y, Lees-Miller SP, Appella E, Anderson CW. ATM mediates phosphorylation at multiple p53 sites, including Ser(46), in response to ionizing radiation. J Biol Chem. 277: 12491-12494, 2002.
[CrossRef]
[PubMed]
Schwartz GK. CDK inhibitors: cell cycle arrest versus apoptosis. Cell Cycle. 1: 122-123, 2002.
[CrossRef]
Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 91: 325-334, 1997.
[CrossRef]
Skvara H, Thallinger C, Wacheck V, Monia BP, Pehamberger H, Jansen B, Selzer E. Mcl-1 blocks radiation-induced apoptosis and inhibits clonogenic cell death. Anticancer Res. 25: 2697-2703, 2005.
[PubMed]
Steinmann KE, Belinsky GS, Lee D, Schlegel R. Chemically induced premature mitosis: differential response in rodent and human cells and the relationship to cyclin B synthesis and p34cdc2/cyclin B complex formation. Proc Natl Acad Sci USA. 88: 6843-6837, 1991.
[CrossRef]
Tibbetts RS, Brumbaugh KM, Williams JM, Sarkaria JN, Cliby WA, Shieh SY, Taya Y, Prives C, Abraham RT. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 13: 152-157, 1999.
[CrossRef]
Tichy A, Zaskodova D, Rezacova M, Vavrova J, Vokurkova D, Pejchal J, Vilasova Z, Cerman J, Osterreicher J. Gamma-radiation-induced ATM-dependent signalling in human T-lymphocyte leukemic cells, MOLT-4. Acta Biochim Pol 54: 281-287, 2007.
[PubMed]
Tichy A, Zaskodova D, Pejchal J, Rezacova M, Osterreicher J, Vavrova J, Cerman J. Gamma irradiation of human leukaemic cells HL-60 and MOLT-4 induces decrease in Mcl-1 and Bid, release of cytochrome c, and activation of caspase-8 and caspase-9. Int J Radiat Biol. 84: 523-530, 2008.
[CrossRef]
[PubMed]
Vavrova J, Marekova Rezacova M, Vokurkova D, Szkanderova S, Psutka J. Caffeine induces a second wave of apoptosis after low dose-rate gamma radiation of HL-60 cells. Radiat Environ Biophys. 42: 193-199, 2003.
[CrossRef]
[PubMed]
Vavrova J, Rezacova M, Vokurkova D, Psutka J. Cell cycle alteration, apoptosis and response of leukemic cell lines to gamma radiation with high- and low-dose rate. Physiol Res. 53: 335-342, 2004.
[PubMed]
|
BACK
|



